Zinc Application Mitigates Copper Toxicity by Regulating Cu Uptake, Activity of Antioxidant Enzymes, and Improving Physiological Characteristics in Summer Squash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Growth Characteristics and Fruit Yield
2.3. Antioxidant Enzymes Activity
2.4. Proline, MDA, and H2O2 Content
2.5. Photosynthetic Pigments Content
2.6. Measurement of Cu, Zn, and Mn Content
2.7. Statistical Analysis
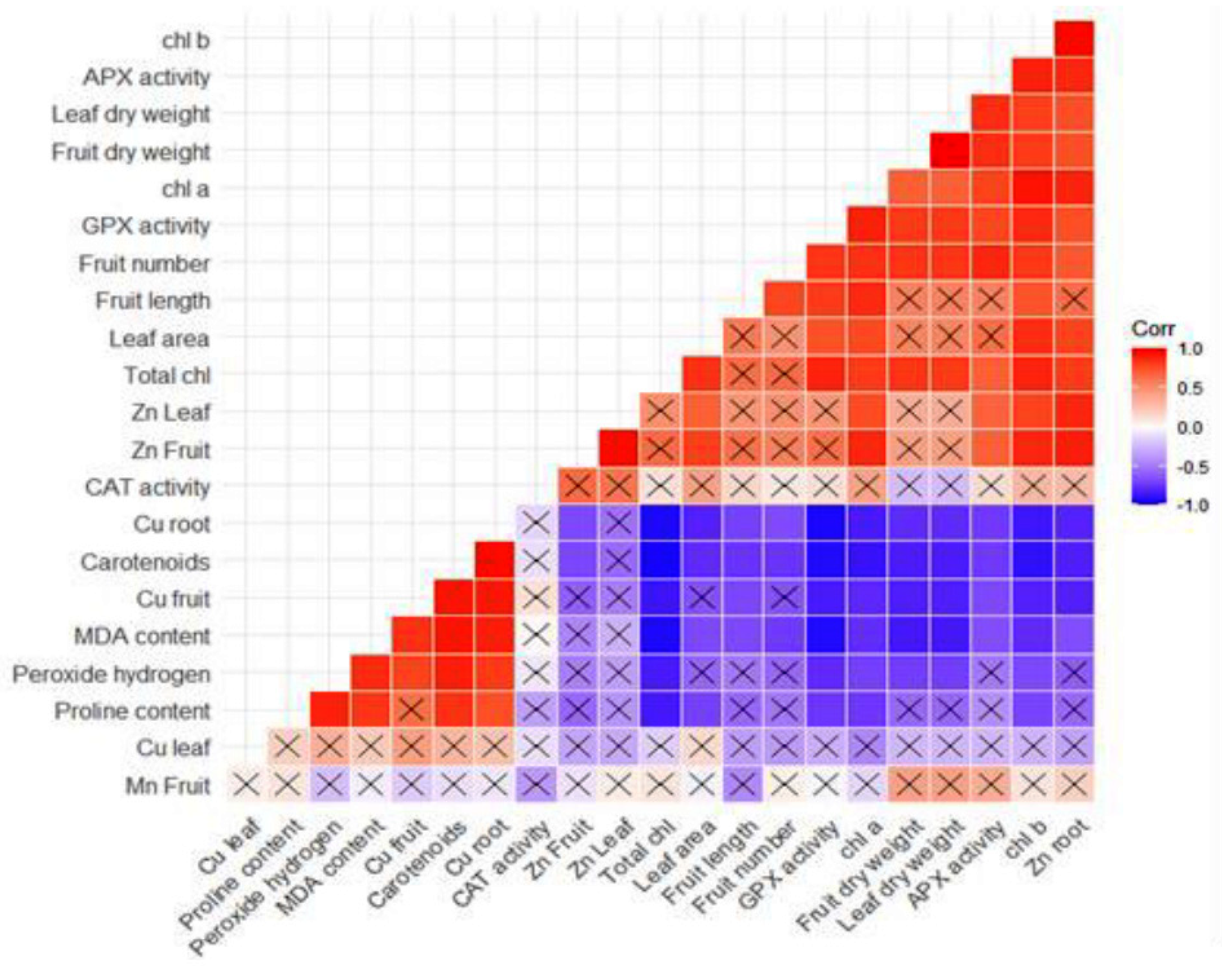
3. Results and Discussion
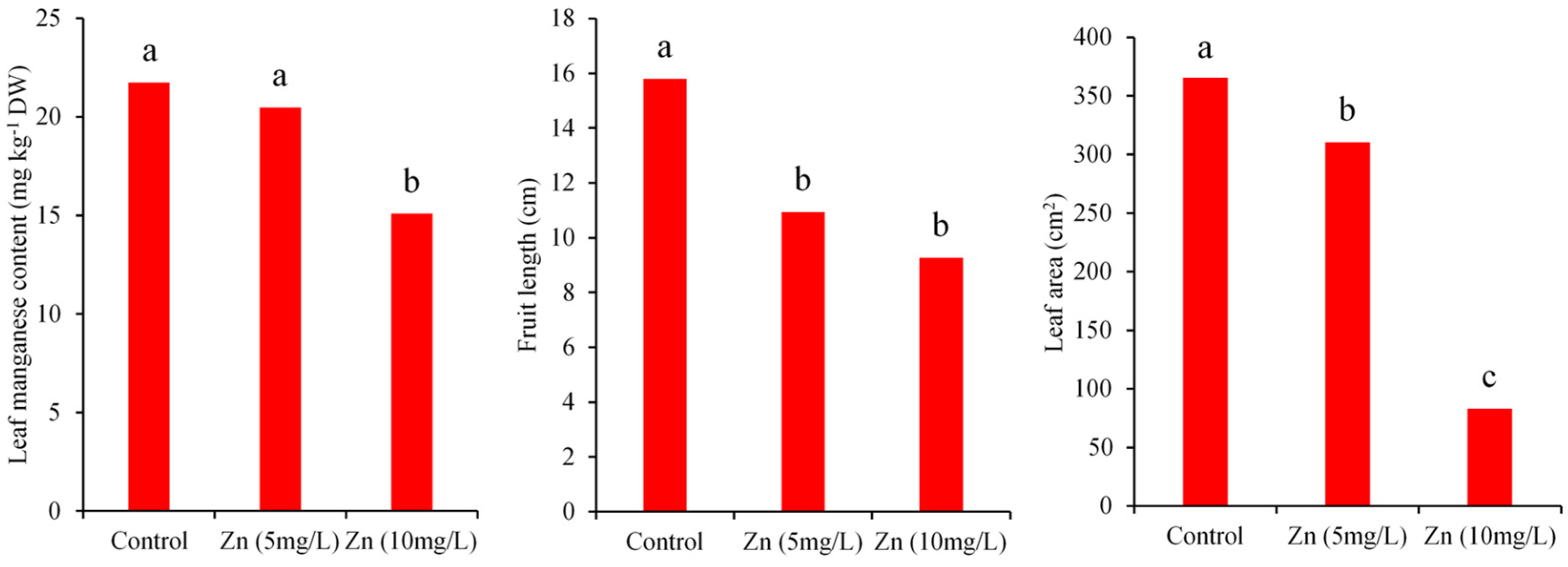
3.1. The Effects of Zinc and Copper on Growth Parameters
3.2. The Effects of Zinc and Copper on Antioxidant Enzymes Activity
3.3. The Effects of Zinc and Copper on the Content of Photosynthetic Pigments
3.4. The Effects of Zinc and Copper on the Content of Photosynthetic Pigments
3.5. The Effects of Zinc and Copper on Cu, Zn, and Mn Concentrations in Roots, Leaves, and Fruits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Babalar, M.; Sarcheshmeh, M.A.A.; Morshedloo, M.R.; Shokrpour, M. Effects of exogenous application of citrulline on prolonged water stress damages in hyssop (Hyssopus officinalis L.): Antioxidant activity, biochemical indices, and essential oils profile. Food Chem. 2020, 333, 127433. [Google Scholar] [CrossRef]
- Ahmadi, H.; Morshedloo, M.R.; Emrahi, R.; Javanmard, A.; Rasouli, F.; Maggi, F.; Kumar, M.; Lorenzo, J.M. Introducing three new fruit-scented mints to farmlands: Insights on drug yield, essential-oil quality, and antioxidant properties. Antioxidants 2022, 11, 866. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Fereydouni, S.; Ahmadi, H.; Hassanpouraghdam, M.B.; Aghaee, A.; Mehrabani, L.V.; Maggi, F. Natural diversity in fatty acids profiles and antioxidant properties of sumac fruits (Rhus coriaria L.): Selection of preferable populations for food industries. Food Chem. 2022, 374, 131757. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.; Cases, R.; Picorel, R.; Yruela, I. Foliar and root Cu supply affect differently Fe-and Zn-uptake and photosynthetic activity in soybean plants. Environ. Exp. Bot. 2007, 60, 145–150. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, W.; Wang, M.; Chen, W.; Li, X.; Li, Y.; Cai, Y. Mechanisms and uncertainties of Zn supply on regulating rice Cd uptake. Environ. Pollut. 2019, 253, 959–965. [Google Scholar] [CrossRef]
- Aydin, S.S.; Gökçe, E.; Büyük, İ.; Aras, S. Characterization of stress induced by copper and zinc on cucumber (Cucumis sativus L.) seedlings by means of molecular and population parameters. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 746, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Tamez, C.; Hernandez-Molina, M.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Uptake, transport, and effects of nano-copper exposure in zucchini (Cucurbita pepo). Sci. Total Environ. 2019, 665, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Turan, V. Calcite in combination with olive pulp biochar reduces Ni mobility in soil and its distribution in chili plant. Int. J. Phytorem. 2022, 24, 166–176. [Google Scholar] [CrossRef]
- Turan, V. Arbuscular mycorrhizal fungi and pistachio husk biochar combination reduces Ni distribution in mungbean plant and improves plant antioxidants and soil enzymes. Physiol. Plant. 2021, 173, 418–429. [Google Scholar] [CrossRef]
- Rasool, B.; Zubair, M.; Khan, M.A.; Ramzani, P.M.A.; Dradrach, A.; Turan, V.; Iqbal, M.; Khan, S.A.; Tauqeer, H.M.; Farhad, M. Synergetic efficacy of amending Pb-polluted soil with P-loaded jujube (Ziziphus mauritiana) twigs biochar and foliar chitosan application for reducing Pb distribution in moringa leaf extract and improving its anti-cancer potential. Water Air Soil Pollut. 2022, 233, 344. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.á.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Maqbool, A. A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef]
- Chaudhry, F.; Sharif, M.; Latif, A.; Qureshi, R. Zinc-copper antagonism in the nutrition of rice (Oryza sativa L.). Plant Soil 1973, 38, 573–580. [Google Scholar] [CrossRef]
- Shahriaripour, R.; Tajabadipour, A. Zinc nutrition of pistachio: Interaction of zinc with other trace elements. Commun. Soil Sci. Plant Anal. 2010, 41, 1885–1888. [Google Scholar] [CrossRef]
- Youssef, M.A.; AL-Huqail, A.A.; Ali, E.F.; Majrashi, A. Organic amendment and mulching enhanced the growth and fruit quality of squash plants (Cucurbita pepo L.) grown on silty loam soils. Horticulturae 2021, 7, 269. [Google Scholar] [CrossRef]
- Allowances, R.D. Recommended Dietary Allowances; National Research Council-National Academy Press: Washington, DC, USA, 1989. [Google Scholar]
- Coolong, T.W.; Randle, W.M.; Toler, H.D.; Sams, C.E. Zinc availability in hydroponic culture influences glucosinolate concentrations in Brassica rapa. Hortscience 2004, 39, 84–86. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Dong, J.; Wu, F.; Zhang, G. Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 2006, 64, 1659–1666. [Google Scholar] [CrossRef]
- Pande, P.; Anwar, M.; Chand, S.; Yadav, V.K.; Patra, D. Optimal level of iron and zinc in relation to its influence on herb yield and production of essential oil in menthol mint. Commun. Soil Sci. Plant Anal. 2007, 38, 561–578. [Google Scholar] [CrossRef]
- Azooz, M.M.; Abou-Elhamd, M.F.; Al-Fredan, M.A. Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (‘Triticum aestivum’ cv. Hasaawi) at early growing stage. Aust. J. Crop Sci. 2012, 6, 688–694. [Google Scholar]
- Yan, Z.W.; Li, H.N.; Shuanggui, T.; Deyi, X.; Weihong, Z.; Jianshuang, Q.; Runqing, Y. Effects of excess copper on the oxidative stress in roots of maize seedlings. Afr. J. Agric. Res. 2011, 6, 4998–5004. [Google Scholar]
- Emrahi, R.; Morshedloo, M.R.; Ahmadi, H.; Javanmard, A.; Maggi, F. Intraspecific divergence in phytochemical characteristics and drought tolerance of two carvacrol-rich Origanum vulgare subspecies: Subsp. hirtum and subsp. gracile. Ind. Crops Prod. 2021, 168, 113557. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Rodríguez, F.E.; Laporte, D.; González, A.; Mendez, K.N.; Castro-Nallar, E.; Meneses, C.; Huidobro-Toro, J.P.; Moenne, A. Copper-induced increased expression of genes involved in photosynthesis, carotenoid synthesis and C assimilation in the marine alga Ulva compressa. BMC Genom. 2018, 19, 829. [Google Scholar] [CrossRef]
- Javed, T.; Ali, M.M.; Shabbir, R.; Anwar, R.; Afzal, I.; Mauro, R.P. Alleviation of copper-induced stress in pea (Pisum sativum L.) through foliar application of gibberellic acid. Biology 2021, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Muslu, A.; Ergün, N. Effects of copper and chromium and high temperature on growth, proline and protein content in wheat seedlings. Bangladesh J. Bot. 2013, 42, 105–112. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ghorbanpour, M.; Brestic, M. Exogenous putrescine changes redox regulations and essential oil constituents in field-grown Thymus vulgaris L. under well-watered and drought stress conditions. Ind. Crops Prod. 2018, 122, 119–132. [Google Scholar] [CrossRef]
- Shu-Hsien, H.; Chih-Wen, Y.; Lin, C.H. Hydrogen peroxide functions as a stress signal in plants. Bot. Bull. Acad. Sin. 2005, 46, 1–10. [Google Scholar]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Kaplan, M. Accumulation of copper in soils and leaves of tomato plants in greenhouses in Turkey. J. Plant Nutr. 1999, 22, 237–244. [Google Scholar] [CrossRef]
- Åkesson, M.T.; Point, C.C.; di Caracalla, V.d.T. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods; WHO: Geneva, Switzerland, 2015. [Google Scholar]


| Concentration (mg L−1) | Micro-Element Providing Salts | Concentration (g L−1) | Macro-Element Providing Salts |
|---|---|---|---|
| 2.86 | H3BO3 | 0.47 | Ca(NO3)2·2H2O |
| 1.81 | MnCl2·4H2O | 0.3 | KNO3 |
| 0.22 | ZnSO4·7H2O | 0.25 | MgSO4,7H2O |
| 0.02 | NaMOO4·2H2O | 0.06 | NH4H2PO4 |
| 0.08 | CuSO4·5H2O | 0.1 | Fe-EDTA |
| Treatments | Proline Content (nmol g−1 FW) | Peroxide Hydrogen (µmol g−1 FW) | MDA Content (µmol g−1 FW) | CAT Activity (Unit g−1 FW min−1) | GPX Activity (Unit g−1 FW min−1) | APX Activity (Unit g−1 FW min−1) |
|---|---|---|---|---|---|---|
| Control | 0.41 ± 0.07 c | 0.33 ± 0.03 e | 1.17 ± 0.13 d | 0.04 ± 0.00 d | 0.29 ± 0.00 a,b | 1.16 ± 0.12 c |
| Zn(0) + Cu(0.1) | 1.36 ± 0.07 a | 0.81 ± 0.01 b | 2.08 ± 0.74 ab | 0.05 ± 0.02 c,d | 0.17 ± 0.02 c | 0.71 ± 0.13 e |
| Zn(0) + Cu(0.2) | 1.52 ± 0.26 a | 1.3 ± 0.17 a | 2.54 ± 0.16 a | 0.09 ± 0.00 b,c | 0.06 ± 0.04 d | 0.67 ± 0.00 e |
| Zn(5) + Cu(0) | 0.32 ± 0.05 c | 0.42 ± 0.14 de | 1.13 ± 0.06 d | 0.08 ± 0.01 c,d | 0.31 ± 0.07 a | 1.4 ± 0.00 b |
| Zn(5) + Cu(0.1) | 0.4 ± 0.03 c | 0.48 ± 0.04 d,e | 1.49 ± 0.02 c,d | 0.1 ± 0.01 b,c | 0.19 ± 0.07 b,c | 0.81 ± 0.01 de |
| Zn(5) + Cu(0.2) | 0.62 ± 0.14 b | 0.55 ± 0.03 c,d | 1.98 ± 0.3 b,c | 0.13 ± 0.00 a,b | 0.16 ± 0.04 c | 0.72 ± 0.03 e |
| Zn(10) + Cu(0) | 0.47 ± 0.05 b,c | 0.41 ± 0.03 d,e | 1.2 ± 0.00 d | 0.14 ± 0.01 a,b | 0.36 ± 0.06 a | 2.23 ± 0.23 a |
| Zn(10) + Cu(0.1) | 0.39 ± 0.04 c | 0.48 ± 0.02 d,e | 1.85 ± 0.01 b,c | 0.14 ± 0.01 a | 0.21 ± 0.01 b,c | 0.95 ± 0.00 d |
| Zn(10) + Cu(0.2) | 0.46 ± 0.01 b,c | 0.69 ± 0.05 b,c | 1.54 ± 0.28 b,c,d | 0.15 ± 0.00 a | 0.27 ± 0.02 a,b | 0.71 ± 0.00 e |
| Treatments | Total Chlorophyll Indicator (SPAD Value) | Chlorophyll b (mg g−1 FW) | Carotenoids Content (mg g−1 FW) |
|---|---|---|---|
| Control | 41.57 ± 1.52 c,d | 5.83 ± 1.30 c | 4.76 ± 0.26 d,f |
| Zn(0) + Cu(0.1) | 28.13 ± 6.00 e | 1.41 ± 0.35 d | 10.55 ± 1.46 b |
| Zn(0) + Cu(0.2) | 19.44 ± 0.51 f | 0.68 ± 0.34 d | 15.84 ± 1.59 a |
| Zn(5) + Cu(0) | 51.33 ± 2.18 a | 9.2 ± 1.31 ab | 3.56 ± 0.40 f,g |
| Zn(5) + Cu(0.1) | 43.33 ± 2.36 b,c | 5.3 ± 0.53 c | 7.28 ± 1.62 c,d |
| Zn(5) + Cu(0.2) | 29.49 ± 2.96 e | 1.73 ± 0.40 d | 10.74 ± 1.10 b |
| Zn(10) + Cu(0) | 47.93 ± 4.68 a,b | 12.93 ± 1.23 a | 2.84 ± 0.70 g |
| Zn(10) + Cu(0.1) | 39.52 ± 0.99 c,d | 7.81 ± 0.90 b,c | 5.71 ± 0.52 e |
| Zn(10) + Cu(0.2) | 37.58 ± 3.40 d | 4.92 ± 0.81 c | 7.91 ± 0.54 c |
| Treatments | Fruit Cu (mg kg−1 DW) | Leaf Cu (mg kg−1 DW) | Root Cu (mg kg−1 DW) | Fruit Zn (mg kg−1 DW) | Leaf Zn (mg kg−1 DW) | Root Zn (mg kg−1 DW) | Fruit Mn (mg kg−1 DW) |
|---|---|---|---|---|---|---|---|
| Control | 0.77 ± 0.16 f | 0.75 ± 0.04 d,e,f | 0.11 ± 0.01 d | 4.91 ± 0.20 e | 3.43 ± 1.11 e | 0.21 ± 0.01 c | 5.6 ± 1.41 a,b |
| Zn(0) + Cu(0.1) | 1.48 ± 0.24 d,e | 1.47 ± 0.07 c,d | 0.15 ± 0.01 c | 4.26 ± 0.37 e | 6.64 ± 2.51 d,e | 0.18 ± 0.01 d | 6.31 ± 0.12 a |
| Zn(0) + Cu(0.2) | 4.01 ± 0.09 a | 3.97 ± 0.35 a | 0.29 ± 0.02 a | 2.95 ± 0.73 e | 7.35 ± 1.01 c,d,e | 0.15 ± 0.02 e | 5.19 ± 1.27 a,b |
| Zn(5) + Cu(0) | 0.56 ± 0.09 e,f | 0.55 ± 0.22 e,f | 0.04 ± 0.02 e | 16.58 ± 2.30 c | 17.3 ± 1.15 b | 0.27 ± 0.02 b | 6.25 ± 0.30 b |
| Zn(5) + Cu(0.1) | 1.26 ± 0.95 c,d | 1.25 ± 0.22 d,e | 0.13 ± 0.01 c,d | 12.27 ± 0.32 d | 11.83 ± 1.76 c | 0.23 ± 0.02 c | 6.08 ± 0.16 a |
| Zn(5) + Cu(0.2) | 3.26 ± 0.82 b | 3.28 ± 0.19 b | 0.19 ± 0.02 b | 5.76 ± 2.50 e | 9.10 ± 1.48 c,d | 0.15 ± 0.01 e | 6.73 ± 0.29 a |
| Zn(10) + Cu(0) | 0.21 ± 0.07 f | 0.21 ± 0.11 f | 0.03 ± 0.02 e | 31.61 ± 2.61 a | 38.8 ± 6.95 a | 0.35 ± 0.02 a | 6.6 ± 0.78 a |
| Zn(10) + Cu(0.1) | 1.14 ± 0.06 d,e,f | 1.16 ± 0.18 d,e | 0.12 ± 0.03 d | 27.74 ± 2.98 b | 35.63 ± 3.16 a | 0.28 ± 0.02 b | 4.76 ± 0.57 b |
| Zn(10) + Cu(0.2) | 2.01 ± 0.43 bc | 2.01 ± 0.14 c | 0.11 ± 0.01 d | 15.77 ± 2.79 c | 11.4 ± 1.51 c | 0.19 ± 0.02 d | 1.95 ± 0.52 c |
| Main Effects of Treatments (Mean Values of Main Effects) | ||||||
|---|---|---|---|---|---|---|
| Traits | Added Concentration of ZnSO4·7H2O to Basal Nutrient Solution | Added Concentration of CuSO4·5H2O to Basal Nutrient Solution | ||||
| Control | 5 mg L−1 | 10 mg L−1 | Control | 0.1 mg L−1 | 0.2 mg L−1 | |
| Proline content (nmol g−1 FW) | 1.1 ± 0.54 a | 0.45 ± 0.16 a | 0.44 ± 0.05 b | 0.4 ± 0.08 c | 0.72 ± 0.49 b | 0.87 ± 0.51 a |
| Peroxide hydrogen (µmol g−1 FW) | 0.81 ± 0.43 a | 0.48 ± 0.1 a | 0.53 ± 0.13 a | 0.38 ± 0.09 c | 0.59 ± 0.17 b | 0.85 ± 0.36 a |
| MDA content (µmol g−1 FW) | 1.93 ± 0.71 a | 1.53 ± 0.4 b | 1.53 ± 0.31 b | 1.17 ± 0.08 c | 1.8 ± 0.45 a | 2.02 ± 0.49 a |
| CAT activity (Unit g−1 FW min−1) | 0.06 ± 0.02 c | 0.1 ± 0.03 b | 0.14 ± 0.01 a | 0.08 ± 0.04 c | 0.1 ± 0.04 b | 0.12 ± 0.03 a |
| GPX activity (Unit g−1 FW min−1) | 0.17 ± 0.11 b | 0.22 ± 0.09 b | 0.28 ± 0.07 a | 0.32 ± 0.07 a | 0.19 ± 0.04 b | 0.16 ± 0.1 b |
| APX activity (Unit g−1 FW min−1) | 0.85 ± 0.25 c | 0.98 ± 0.32 b | 1.3 ± 0.71 a | 1.6 ± 0.5 a | 0.83 ± 0.12 b | 0.7 ± 0.03 c |
| Total chlorophyll indicator (SPAD value) | 29.71 ± 10.14 b | 41.38 ± 9.82 a | 41.68 ± 5.59 a | 46.94 ± 5.07 a | 37 ± 7.59 b | 28.83 ± 8.19 c |
| Chlorophyll a (mg g−1 FW) | 15.6 ± 4.93 b | 17.75 ± 2.92 b | 24.15 ± 5.25 a | 23.25 ± 5.4 a | 18.25 ± 4.66 b | 16.01 ± 4.81 b |
| Chlorophyll b (mg g−1 FW) | 2.64 ± 2.51 c | 5.41 ± 3.32 b | 8.55 ± 3.62 a | 9.32 ± 3.27 a | 4.84 ± 2.85 b | 2.45 ± 1.97 c |
| Carotenoids content (mg g−1 FW) | 10.39 ± 4.92 a | 7.19 ± 3.27 b | 5.49 ± 2.26 c | 3.72 ± 0.94 c | 7.85 ± 2.42 b | 11.5 ± 3.62 a |
| Fruit Cu content (mg kg−1 DW) | 2.09 ± 1.48 a | 1.69 ± 1.37 a | 1.12 ± 0.81 b | 0.51 ± 0.26 c | 1.3 ± 0.52 b | 3.09 ± 0.99 a |
| Leaf Cu content (mg kg−1 DW) | 2.06 ± 1.47 b | 3.34 ± 1.85 a | 1.13 ± 0.79 c | 2.15 ± 2.52 b | 1.29 ± 0.2 c | 3.09 ± 0.89 a |
| Root Cu content (mg kg−1 DW) | 0.18 ± 0.08 a | 0.12 ± 0.07 b | 0.08 ± 0.04 c | 0.06 ± 0.04 c | 0.13 ± 0.02 b | 0.19 ± 0.08 a |
| Fruit Zn content (mg kg−1 DW) | 4.04 ± 0.96 c | 11.54 ± 5.02 b | 25.04 ± 7.55 a | 17.7 ± 11.72 a | 14.76 ± 10.45 b | 8.16 ± 6.14 c |
| Leaf Zn content (mg kg−1 DW) | 5.81 ± 2.32 c | 12.74 ± 3.84 b | 28.61 ± 13.5 a | 19.84 ± 15.84 a | 18.04 ± 13.57 a | 9.28 ± 2.11 b |
| Root Zn content (mg kg−1 DW) | 0.18 ± 0.03 c | 0.22 ± 0.05 b | 0.27 ± 0.07 a | 0.28 ± 0.06 a | 0.23 ± 0.04 b | 0.16 ± 0.02 c |
| Fruit Mn content (mg kg−1 DW) | 5.7 ± 1.07 b | 6.35 ± 0.37 a | 4.44 ± 2.1 b | 6.15 ± 0.93 a | 5.72 ± 0.79 b | 4.62 ± 2.23 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behtash, F.; Abedini, F.; Ahmadi, H.; Mosavi, S.B.; Aghaee, A.; Morshedloo, M.R.; Lorenzo, J.M. Zinc Application Mitigates Copper Toxicity by Regulating Cu Uptake, Activity of Antioxidant Enzymes, and Improving Physiological Characteristics in Summer Squash. Antioxidants 2022, 11, 1688. https://doi.org/10.3390/antiox11091688
Behtash F, Abedini F, Ahmadi H, Mosavi SB, Aghaee A, Morshedloo MR, Lorenzo JM. Zinc Application Mitigates Copper Toxicity by Regulating Cu Uptake, Activity of Antioxidant Enzymes, and Improving Physiological Characteristics in Summer Squash. Antioxidants. 2022; 11(9):1688. https://doi.org/10.3390/antiox11091688
Chicago/Turabian StyleBehtash, Farhad, Fatemeh Abedini, Hosein Ahmadi, Seyed Bahman Mosavi, Ahmad Aghaee, Mohammad Reza Morshedloo, and Jose M. Lorenzo. 2022. "Zinc Application Mitigates Copper Toxicity by Regulating Cu Uptake, Activity of Antioxidant Enzymes, and Improving Physiological Characteristics in Summer Squash" Antioxidants 11, no. 9: 1688. https://doi.org/10.3390/antiox11091688







