Protective Effect of Epigallocatechin-3-gallate against Hepatic Oxidative Stress Induced by tert-Butyl Hhydroperoxide in Yellow-Feathered Broilers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broilers, Management and Experimental Treatments
2.2. Sample Collection and Processing
2.3. Serum Biochemical Parameters
2.4. Antioxidative Parameters in Plasma and Liver
2.5. Histopathological Analysis
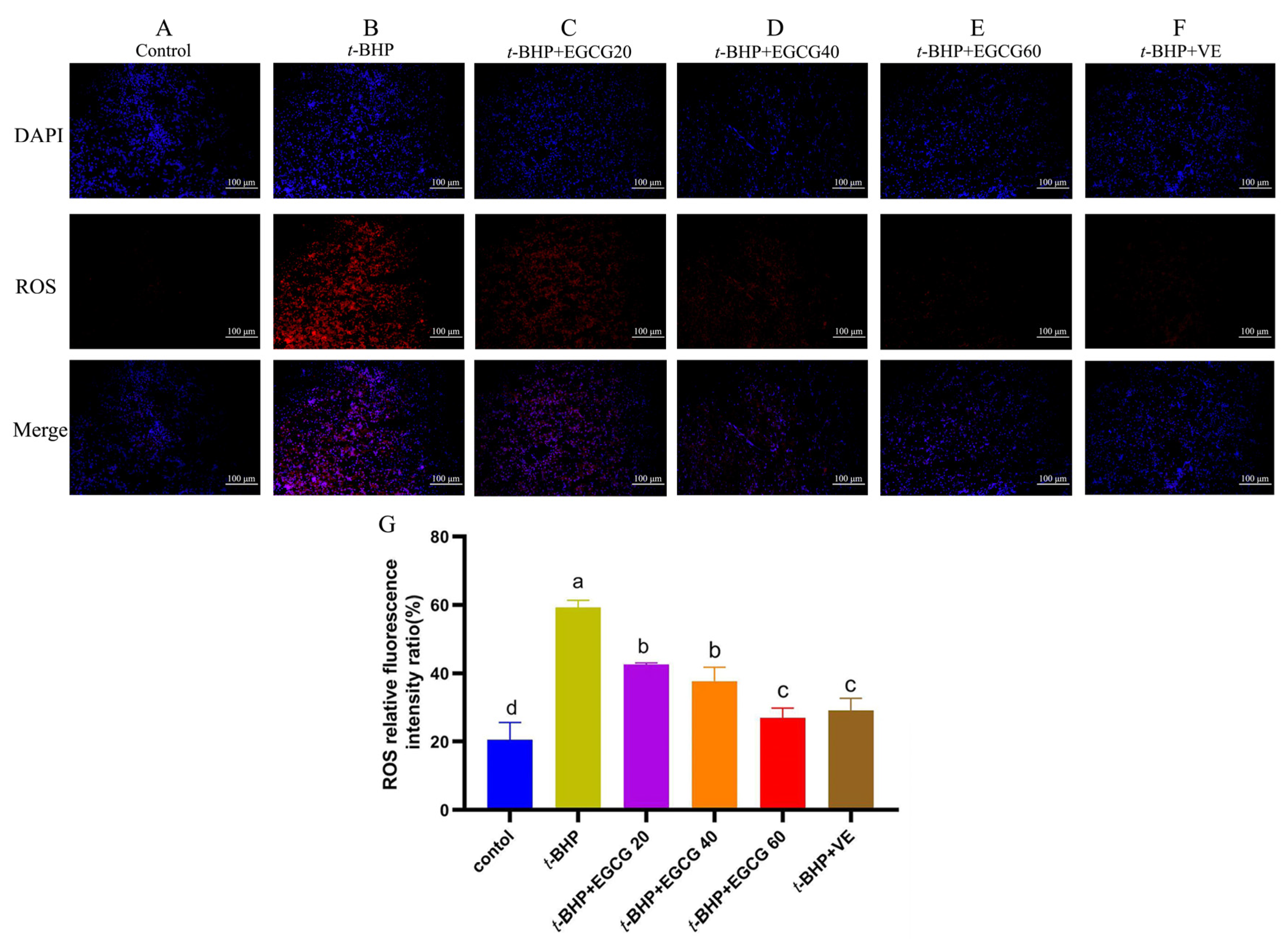
2.6. Detection of Reactive Oxygen Species (ROS)
2.7. Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Body Weight and Relative Weight of Organs in Broilers
3.2. Hepatic Histomorphology
3.3. ROS Accumulation in the Liver
3.4. Redox Status in Tissue
3.5. Gene mRNA Expression Related to Nrf2 and PPARα Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xin, X.F.; Zheng, M.Q.; Wen, J.; Wang, J.M. Situation analysis, future prospects and Countermeasures of broiler industry in China in 2023. Chin. J. Anim. Husb. 2024, 60, 312–317. [Google Scholar]
- Rehman, Z.U.; Meng, C.; Sun, Y.; Safdar, A.; Pasha, R.H.; Munir, M.; Ding, C. Oxidative Stress in Poultry: Lessons from the Viral Infections. Oxid. Med. Cell Longev. 2018, 2018, 5123147. [Google Scholar] [CrossRef]
- Oluwagbenga, E.M.; Fraley, G.S. Heat stress and poultry production: A comprehensive review. Poult. Sci. 2023, 102, 103141. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Endlicher, R.; Drahota, Z.; Cervinkova, Z. Modification of calcium retention capacity of rat liver mitochondria by phosphate and tert-butyl hydroperoxide. Physiol. Res. 2019, 68, 59–65. [Google Scholar] [CrossRef]
- Huang, L.; Gao, W.; He, X.; Yuan, T.; Zhang, H.Q.; Zhang, X.F.; Zheng, W.X.; Wu, Q.L.; Liu, J.; Wang, W.C.; et al. Maternal zinc alleviates tert-butyl hydroperoxide-induced mitochondrial oxidative stress on embryonic development involving the activation of Nrf2/PGC-1alpha pathway. J. Anim. Sci. Biotechnol. 2023, 14, 45. [Google Scholar] [CrossRef]
- Wang, M.W.; Huang, H.J.; Wang, L.; Yin, L.M.; Yang, H.S.; Chen, C.Q.; Zheng, Q.K.; He, S.P. Tannic acid attenuates intestinal oxidative damage by improving antioxidant capacity and intestinal barrier in weaned piglets and IPEC-J2 cells. Front. Nutr. 2022, 9, 1012207. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Li, W.X.; Lu, L.; Zhang, L.Y.; Ji, C.; Lin, X.; Liu, H.C.; Odle, J.; Luo, X.G. Impact of maternal heat stress in conjunction with dietary zinc supplementation on hatchability, embryonic development, and growth performance in offspring broilers. Poult. Sci. 2017, 96, 2351–2359. [Google Scholar] [CrossRef]
- Miao, Z.Q.; Dong, Y.Y.; Qin, X.; Yuan, J.M.; Han, M.M.; Zhang, K.K.; Shi, S.R.; Song, X.Y.; Zhang, J.Z.; Li, J.H. Dietary supplementation of methionine mitigates oxidative stress in broilers under high stocking density. Poult. Sci. 2021, 100, 101231. [Google Scholar] [CrossRef]
- Ye, S.; Yuan, X.; Huang, S.; Zhang, H.; Chen, Z.; Li, J.; Zhang, X.; Zhang, Z. Comparison of genotype imputation strategies using a combined reference panel for chicken population. Animal 2019, 13, 1119–1126. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Dreger, H.; Stangl, K.; Lorenz, M. Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc. Res. 2007, 73, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M. Cellular targets for the beneficial actions of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1642S–1650S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Guo, Q.; Xin, W. Free radical scavenging by green tea polyphenols. Methods Enzymol. 2001, 335, 217–231. [Google Scholar]
- Patel, R.; Maru, G. Polymeric black tea polyphenols induce phase II enzymes via Nrf2 in mouse liver and lungs. Free Radic. Biol. Med. 2008, 44, 1897–1911. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Li, P.F. PPARalpha: An emerging target of metabolic syndrome, neurodegenerative and cardiovascular diseases. Front. Endocrinol. 2022, 13, 1074911. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Wan, X.C.; Shang, Y.Y.; Hu, J.W.; Shao, L.; Chen, W.; Li, D.X. Polyphenol content of plasma and litter after the oral administration of green tea and tea polyphenols in chickens. J. Agric. Food Chem. 2012, 60, 1619–1627. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Wang, J.P.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Zeng, Q.F.; Xuan, Y.; Su, Z.W. Effect of Vanadium and Tea Polyphenols on Intestinal Morphology, Microflora and Short-Chain Fatty Acid Profile of Laying Hens. Biol. Trace Elem. Res. 2016, 174, 419–427. [Google Scholar] [CrossRef]
- Wang, J.P.; Jia, R.; Gong, H.J.; Celi, P.; Zhuo, Y.; Ding, X.M.; Bai, S.P.; Zen, Q.F.; Yin, H.D.; Xu, S.Y.; et al. The effect of oxidative stress on the chicken ovary: Involvement of microbiota and melatonin interventions. Antioxidants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Wang, M.Y.; Luo, W.; Yu, T.X.; Liang, S.Q.; Sun, J.F.; Zhang, Y.; Han, X.; Long, X.H.; Liang, G.; Li, G. Corynoline protects ang II-induced hypertensive heart failure by increasing PPARα and Inhibiting NF-κB pathway. Biomed. Pharmacother. 2022, 150, 113075. [Google Scholar] [CrossRef]
- Hu, Y.X.; Liao, X.D.; Wen, Q.; Lu, L.; Zhang, L.Y.; Luo, X.G. Phosphorus absorption and gene expression levels of related transporters in the small intestine of broilers. Brit. J. Nutr. 2018, 119, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Zerehdaran, S.; Hassani, S.; Gharebash, A.M.; Khanahmadi, A.; Farivar, F. A breeding program for balanced improvement of performance and health in broilers. Pak. J. Biol. Sci. 2009, 12, 79–82. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Lee, E.J.; Ahn, D.U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J. Agric. Food Chem. 2011, 59, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Zhang, L.; Li, J.L.; Gao, F.; Zhou, G.H. Hydrogen Peroxide-Induced Change in Meat Quality of the Breast Muscle of Broilers Is Mediated by ROS Generation, Apoptosis, and Autophagy in the NF-kappaB Signal Pathway. J. Agric. Food Chem. 2017, 65, 3986–3994. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, R.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Induction of nuclear factor-kappaB signal-mediated apoptosis and autophagy by reactive oxygen species is associated with hydrogen peroxide-impaired growth performance of broilers. Animal 2018, 12, 2561–2570. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Han, S.S.; Wang, Y.; Li, D.Y.; Zhao, X.L.; Zhu, Q.; Yin, H.D. Oxidative Stress and Apoptotic Changes in Broiler Chicken Splenocytes Exposed to T-2 Toxin. Biomed. Res. Int. 2019, 2019, 5493870. [Google Scholar] [CrossRef]
- Liu, C.L.; Wang, J.M.; Chu, C.Y.; Cheng, M.T.; Tseng, T.H. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem. Toxicol. 2002, 40, 635–641. [Google Scholar] [CrossRef]
- Nishikawa, H.; Wakano, K.; Kitani, S. Inhibition of NADPH oxidase subunits translocation by tea catechin EGCG in mast cell. Biochem. Biophys. Res. Commun. 2007, 362, 504–509. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Tao, L.; Forester, S.C.; Lambert, J.D. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (−)-epigallocatechin-3-gallate, in oral cells. Mol. Nutr. Food Res. 2014, 58, 665–676. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.T.; Tang, M.L.; Su, L.; Chen, L.; Hu, P.; Wang, H.L.; Wang, M.; Ruan, D.Y. Effects of Epigallocatechin-3-gallate on lead-induced oxidative damage. Toxicology 2008, 249, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, P.; Ling, T.; Wang, Y.; Dong, R.; Zhang, C.; Zhang, L.; Han, M.; Wang, D.; Wan, X. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016, 204, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, X.; Zhang, H.; Song, Y.; Li, T.; Liu, X.; Liu, Y.; Guo, L.; Wang, F.; Yang, T. Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radic. Biol. Med. 2017, 108, 840–857. [Google Scholar] [CrossRef] [PubMed]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef]
- Yang, C.S.; Zhang, J. Studies on the Prevention of Cancer and Cardiometabolic Diseases by Tea: Issues on Mechanisms, Effective Doses, and Toxicities. J. Agric. Food Chem. 2019, 67, 5446–5456. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- Yan, Y.X.; Chen, X.X.; Huang, J.P.; Huan, C.C.; Li, C.M. H2O2-induced oxidative stress impairs meat quality by inducing apoptosis and autophagy via ROS/NF-kappaB signaling pathway in broiler thigh muscle. Poult. Sci. 2022, 101, 101759. [Google Scholar] [CrossRef]
- Qi, G.Y.; Mi, Y.S.; Fan, R.; Li, R.N.; Wang, Y.W.; Li, X.Y.; Huang, S.X.; Liu, X.B. Tea polyphenols ameliorate hydrogen peroxide- and constant darkness-triggered oxidative stress via modulating the Keap1/Nrf2 transcriptional signaling pathway in HepG2 cells and mice liver. RSC Adv. 2017, 7, 32198–32208. [Google Scholar] [CrossRef]
- Muzio, G.; Barrera, G.; Pizzimenti, S. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants 2021, 10, 1734. [Google Scholar] [CrossRef]


| Ingredients | Content (%) |
|---|---|
| Corn | 60.76 |
| Soybean meal (46% crude protein content) | 28.32 |
| Corn gluten meal (61% crude protein content) | 5.15 |
| Soybean oil | 1.00 |
| Lysine-HCl | 0.30 |
| DL-Methionine | 0.26 |
| Monocalcium phosphate | 2.10 |
| Limestone | 0.90 |
| Salt | 0.21 |
| Vitamins and minerals 1 | 1.00 |
| Calculated values (%) 2 | |
| Crude protein | 21.5 |
| Crude fat | 3.40 |
| Crude fiber | 3.39 |
| Calcium | 1.00 |
| Non-phytic acid phosphor | 0.46 |
| Metabolizable energy (kcal/kg of diet) | 3003 |
| Genes | Accession Number | Primer Sequences (5′→3′) | Product Size (bp) |
|---|---|---|---|
| β-actin | NM_205518 | F: TGCGTGACATCAAGGAGAAG R: TGCCAGGGTACATTGTGGTA | 300 |
| CAT | NM_001031215.2 | F: AGCAGGTGCCTTTGGCTATT R: TCCAGCAACAGTGGAGAACC | 121 |
| SOD1 | NM_205064.1 125 | F: CCGGCTTGTCTGATGGAGAT R: TGCATCTTTTGGTCCACCGT | 125 |
| SOD2 | NM_204211.2 | F: CGCTGGCAAAAGGTGATGTT R: GCGAAGGAACCAAAGTCACG | 172 |
| Nrf2 | XM_046921130.1 | F: CATAGAGCAAGTTTGGGAAGAG R: GTTTCAGGGCTCGTGATTGT | 105 |
| PPARα | NM_001001464 | F: CAAACCAACCATCCTGACGAT R: GGAGGTCAGCCATTTTTTGGA | 64 |
| ACACA | AB160952.1 | F: CTCGTTCGGTTAGGGCAGAGG R: CCCGTCCCAGCACCTTGTT | 177 |
| ACAA1 | NM_001197288.1 | F: CCAGCATACTGACAGCCCAA R: TCCCACTTGCACATCAGACC | 170 |
| ME1 | NM_204303.2 | F: AGCATTACGGTTTAGCATTTCGG R: CAGGTAGGCACTCATAAGGTTTC | 240 |
| Treatment | Body Weight (g) | Spleen Index (%) | Liver Index (%) | Bursa of Fabricius Index (%) |
|---|---|---|---|---|
| Control group | 508 ± 21 a | 0.20 ± 0.01 a | 3.36 ± 0.10 | 0.30 ± 0.02 |
| t-BHP group | 442 ± 30 c | 0.16 ± 0.01 c | 3.32 ± 0.03 | 0.25 ± 0.01 |
| t-BHP + EGCG20 | 456 ± 30 c | 0.16 ± 0.01 bc | 3.17 ± 0.06 | 0.27 ± 0.02 |
| t-BHP + EGCG40 | 477 ± 26 bc | 0.16 ± 0.01 bc | 3.2 ± 0.08 | 0.29 ± 0.02 |
| t-BHP + EGCG60 | 507 ± 33 a | 0.17 ± 0.01 b | 3.33 ± 0.09 | 0.32 ± 0.02 |
| t-BHP + VE | 498 ± 38 ab | 0.14 ± 0.01 bc | 3.16 ± 0.06 | 0.28 ± 0.01 |
| p value | 0.001 | 0.002 | 0.324 | 0.269 |
| Treatments | Plasma | Liver | |||||
|---|---|---|---|---|---|---|---|
| T-AOC, nmol/mL | T-SOD, U/mL | CAT, U/mL | MDA, nmol/mL | T-SOD, U/mgprot | CAT, U/mgprot | MDA, nmol/mgprot | |
| Control | 1.59 ± 0.15 a | 534 ± 24 a | 14.93 ± 1.50 a | 1.77 ± 0.32 b | 638 ± 78 a | 3.47 ± 0.69 a | 0.43 ± 0.05 b |
| t-BHP group | 1.27 ± 0.13 b | 451 ± 32 b | 11.76 ± 1.40 c | 2.53 ± 0.64 a | 509 ± 22 d | 1.44 ± 0.39 d | 0.66 ± 0.06 a |
| t-BHP + EGCG20 | 1.56 ± 0.26 a | 488 ± 49 ab | 11.60 ± 1.71 c | 1.89 ± 0.23 b | 590 ± 19 bc | 1.74 ± 0.58 cd | 0.63 ± 0.10 a |
| t-BHP + EGCG40 | 1.61 ± 0.16 a | 495 ± 34 ab | 12.32 ± 1.43 bc | 1.97 ± 0.29 b | 623 ± 27 ab | 2.35 ± 0.52 b | 0.59 ± 0.10 a |
| t-BHP + EGCG60 | 1.56 ± 0.07 a | 536 ± 41 a | 14.53 ± 1.91 a | 1.67 ± 0.34 b | 635 ± 25 a | 2.73 ± 0.22 b | 0.48 ± 0.02 b |
| t-BHP + VE | 1.60 ± 0.09 a | 536 ± 85 a | 14.65 ± 2.08 a | 1.67 ± 0.17 b | 559 ± 13 c | 2.77 ± 0.38 b | 0.49 ± 0.04 b |
| p value | 0.009 | 0.040 | 0.002 | 0.003 | 0.001 | 0.002 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Ni, J.; Wang, W.; Zhu, Y.; Zhang, Y.; Sun, M. Protective Effect of Epigallocatechin-3-gallate against Hepatic Oxidative Stress Induced by tert-Butyl Hhydroperoxide in Yellow-Feathered Broilers. Antioxidants 2024, 13, 1153. https://doi.org/10.3390/antiox13101153
Ma X, Ni J, Wang W, Zhu Y, Zhang Y, Sun M. Protective Effect of Epigallocatechin-3-gallate against Hepatic Oxidative Stress Induced by tert-Butyl Hhydroperoxide in Yellow-Feathered Broilers. Antioxidants. 2024; 13(10):1153. https://doi.org/10.3390/antiox13101153
Chicago/Turabian StyleMa, Xinyan, Junli Ni, Wei Wang, Yongwen Zhu, Yuqing Zhang, and Mingfei Sun. 2024. "Protective Effect of Epigallocatechin-3-gallate against Hepatic Oxidative Stress Induced by tert-Butyl Hhydroperoxide in Yellow-Feathered Broilers" Antioxidants 13, no. 10: 1153. https://doi.org/10.3390/antiox13101153





