Tanshinone IIA Inhibits the Endoplasmic Reticulum Stress-Induced Unfolded Protein Response by Activating the PPARα/FGF21 Axis to Ameliorate Nonalcoholic Steatohepatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Establishment of the NASH Model and Treatments
2.3. Liver Surface Microblood Flow Scan
2.4. Biochemical Analysis
2.5. Cytokine Analysis Using ELISAs
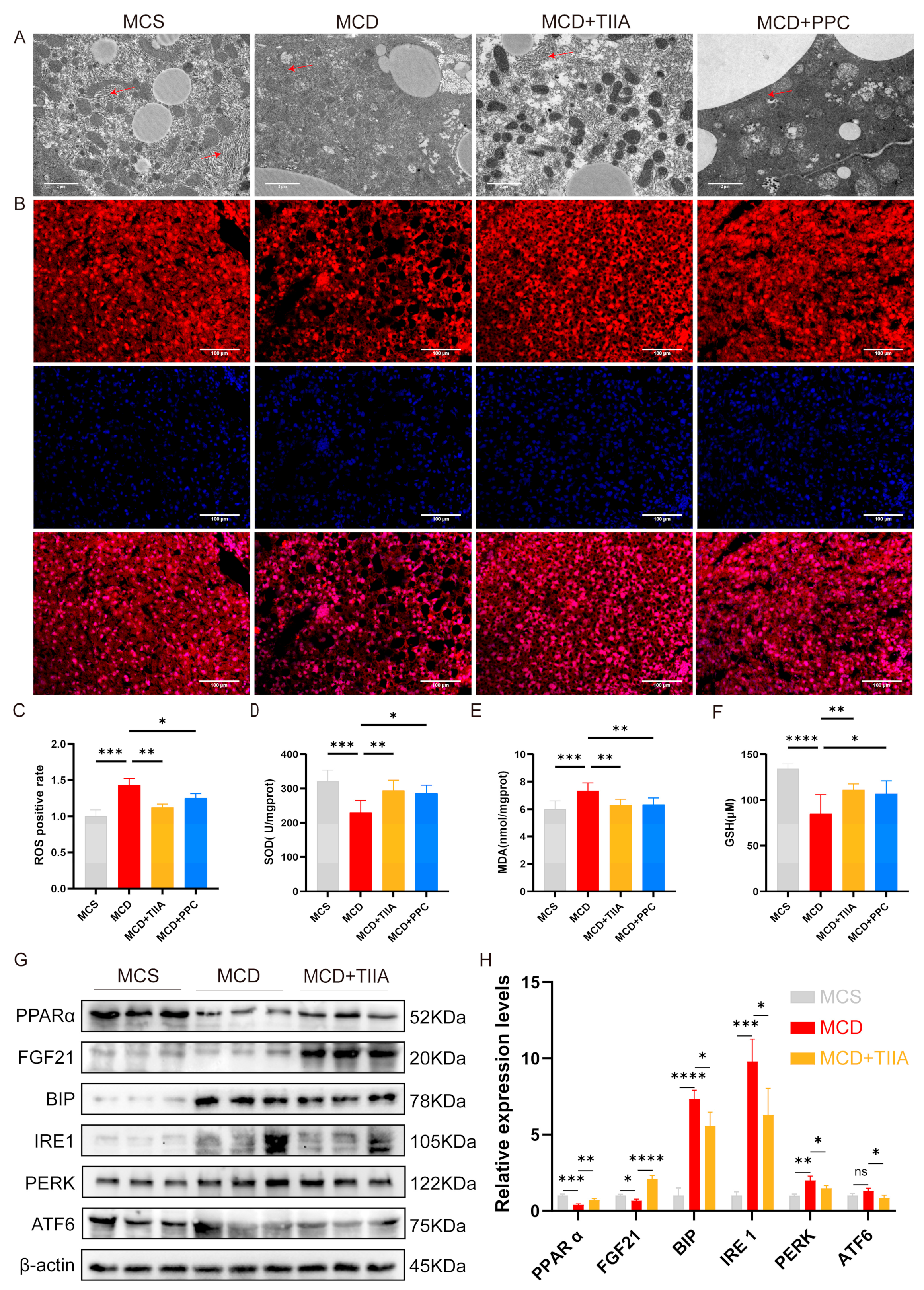
2.6. Detection of Oxidative Stress Indexes
2.7. Histopathological Examination
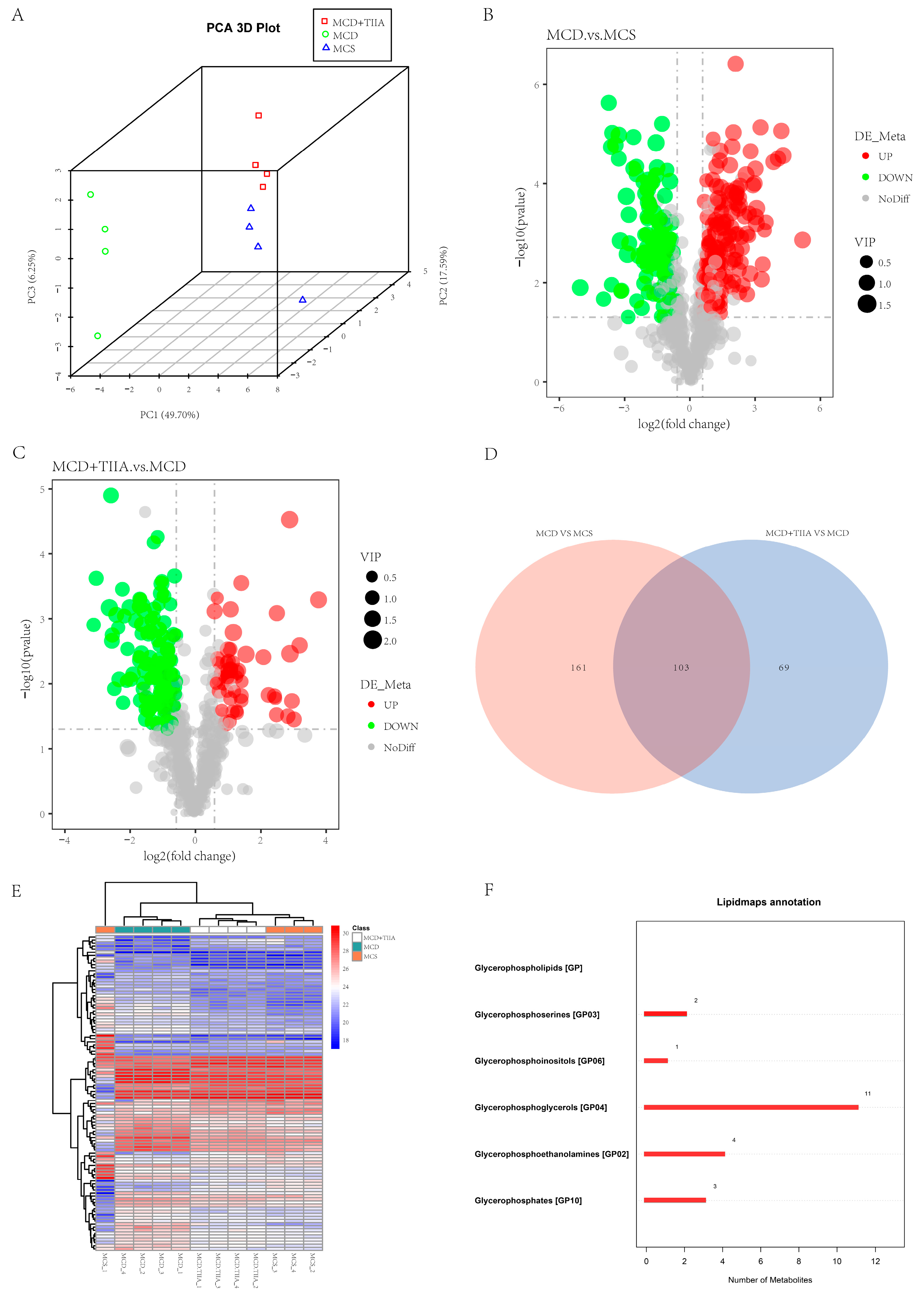
2.8. Liver Lipidomic Analysis
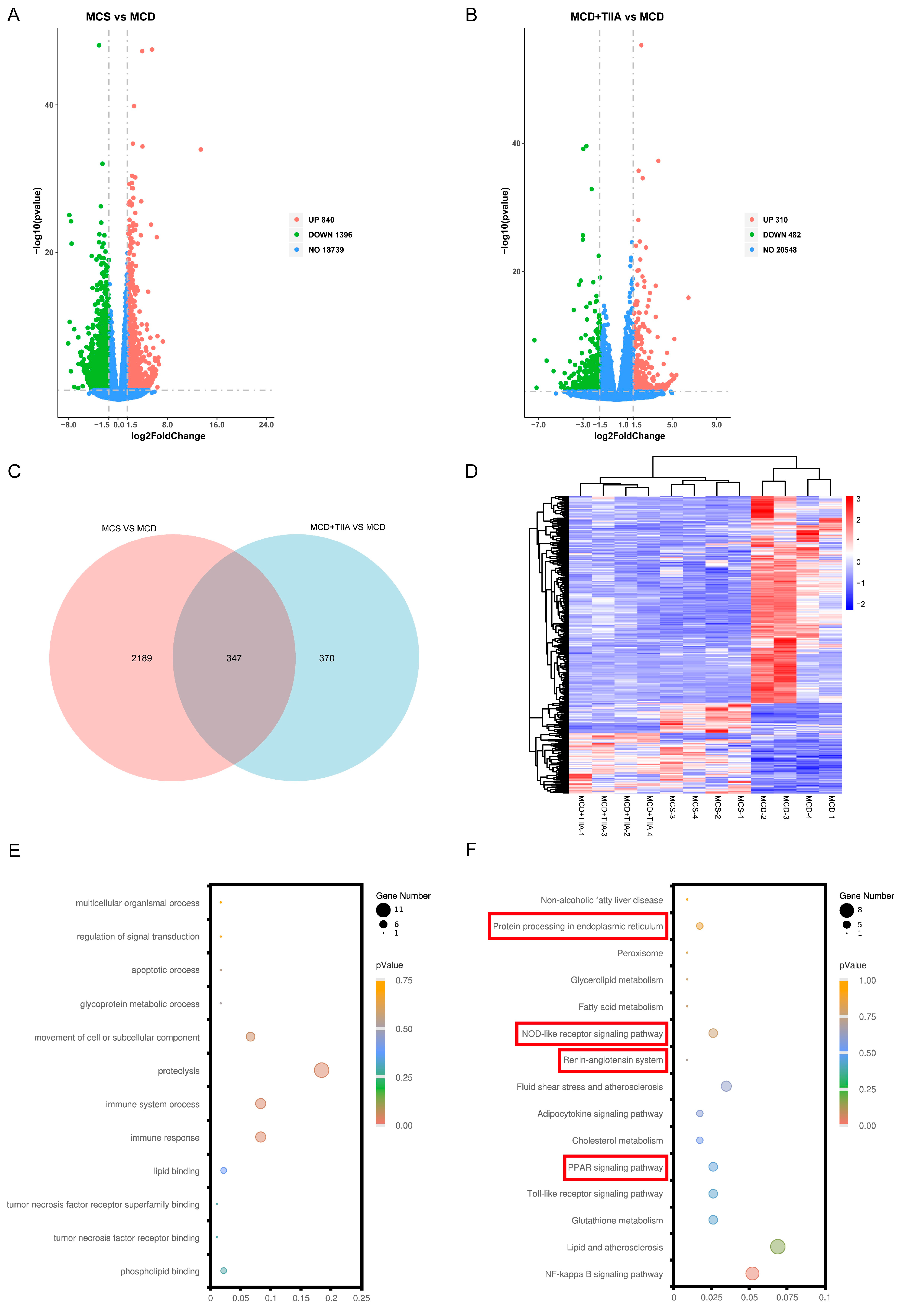
2.9. Liver Transcriptomic Analysis
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. TIIA Improved Lipid Accumulation and Reduced Liver Injury
3.2. TIIA Improved Liver Microcirculation and Fibrosis
3.3. TIIA Extensively Modulated the Hepatic Lipid Profile
3.4. TIIA Regulated ER Function through the PPAR Signalling Pathway
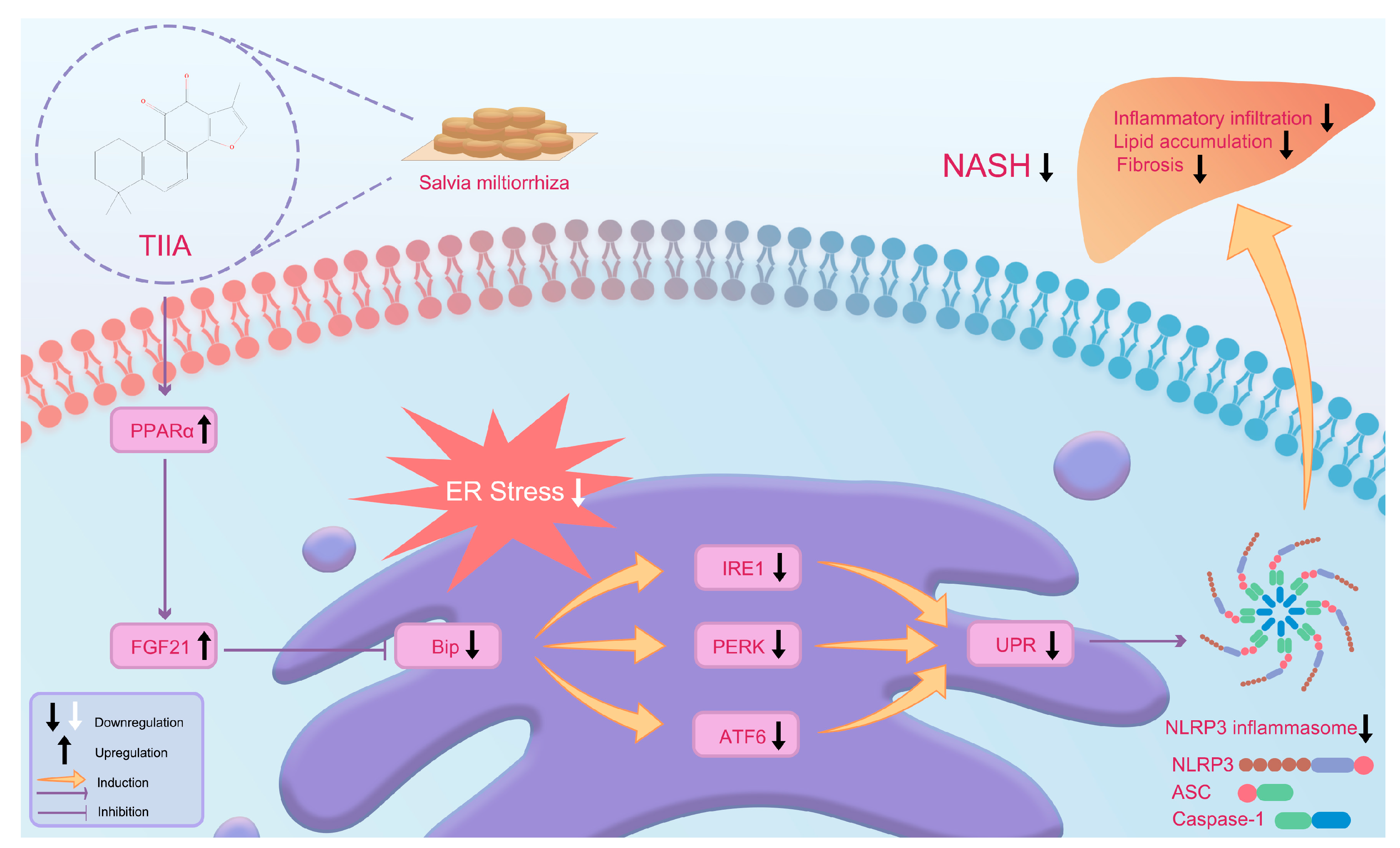
3.5. TIIA Regulated ER Stress by Activating the PPARα/FGF21 Axis
3.6. TIIA Alleviated Inflammation by Inhibiting NLRP3 Inflammasome Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, S.A.; Allen, A.M.; Dubourg, J.; Noureddin, M.; Alkhouri, N. Challenges and opportunities in NASH drug development. Nat. Med. 2023, 29, 562–573. [Google Scholar] [CrossRef]
- Kanwal, F.; Shubrook, J.H.; Younossi, Z.; Natarajan, Y.; Bugianesi, E.; Rinella, M.E.; Harrison, S.A.; Mantzoros, C.; Pfotenhauer, K.; Klein, S.; et al. Preparing for the NASH Epidemic: A Call to Action. Gastroenterology 2021, 161, 1030–1042.e8. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Musso, G.; Saba, F.; Cassader, M.; Gambino, R. Lipidomics in pathogenesis, progression and treatment of nonalcoholic steatohepatitis (NASH): Recent advances. Prog. Lipid Res. 2023, 91, 101238. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, D.; Xu, Y.; Lin, J.; Xu, J.; Wang, K.; Ye, Z.; Luo, Y.; Liu, S.; Yang, H. LncRNA-Gm9795 promotes inflammation in non-alcoholic steatohepatitis via NF-κB/JNK pathway by endoplasmic reticulum stress. J. Transl. Med. 2021, 19, 101. [Google Scholar] [CrossRef]
- Xu, X.; Poulsen, K.L.; Wu, L.; Liu, S.; Miyata, T.; Song, Q.; Wei, Q.; Zhao, C.; Lin, C.; Yang, J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target. Ther. 2022, 7, 287. [Google Scholar]
- Paternostro, R.; Trauner, M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef]
- Guo, C.; Li, Q.; Chen, R.; Fan, W.; Zhang, X.; Zhang, Y.; Guo, L.; Wang, X.; Qu, X.; Dong, H. Baicalein alleviates non-alcoholic fatty liver disease in mice by ameliorating intestinal barrier dysfunction. Food Funct. 2023, 14, 2138–2148. [Google Scholar] [CrossRef]
- Lan, T.; Yu, Y.; Zhang, J.; Li, H.; Weng, Q.; Jiang, S.; Tian, S.; Xu, T.; Hu, S.; Yang, G.; et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology 2021, 74, 686–703. [Google Scholar] [CrossRef]
- Li, H.; Hu, P.; Zou, Y.; Yuan, L.; Xu, Y.; Zhang, X.; Luo, X.; Zhang, Z. Tanshinone IIA and hepatocellular carcinoma: A potential therapeutic drug. Front. Oncol. 2023, 13, 1071415. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Feng, X.; Du, M.; Li, S.; Chang, X.; Liu, P. Tanshinone IIA alleviates atherosclerosis in LDLR(-/-) mice by regulating efferocytosis of macrophages. Front. Pharmacol. 2023, 14, 1233709. [Google Scholar] [CrossRef]
- Shi, M.J.; Dong, B.S.; Yang, W.N.; Su, S.B.; Zhang, H. Preventive and therapeutic role of Tanshinone IIA in hepatology. Biomed. Pharmacother. 2019, 112, 108676. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Liu, H.; Lai, X.; Xing, W. Efficacy and safety of eight types Salvia miltiorrhiza injections in the treatment of unstable angina pectoris: A network meta-analysis. Front. Pharmacol. 2022, 13, 972738. [Google Scholar] [CrossRef]
- Jung, I.; Kim, H.; Moon, S.; Lee, H.; Kim, B. Overview of Salvia miltiorrhiza as a Potential Therapeutic Agent for Various Diseases: An Update on Efficacy and Mechanisms of Action. Antioxidants 2020, 9, 857. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Ji, N.; Du, Y.; Jia, T.; Wei, S.; Wang, W.; Zhang, S.; Chen, W. Tanshinone IIA regulates the TGF-beta1/Smad signaling pathway to ameliorate non-alcoholic steatohepatitis-related fibrosis. Exp. Ther. Med. 2022, 24, 486. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; Jia, T.; Sun, Y.; Du, Y.; Wei, S.; Wang, W.; Zhang, Y.; Chen, W.; Zhang, S. Tanshinone IIA Ameliorates Nonalcoholic Steatohepatitis in Mice by Modulating Neutrophil Extracellular Traps and Hepatocyte Apoptosis. Evid. Based Complement. Alternat. Med. 2022, 2022, 5769350. [Google Scholar] [CrossRef]
- Wang, J.; Hu, R.; Yin, C.; Xiao, Y. Tanshinone IIA reduces palmitate-induced apoptosis via inhibition of endoplasmic reticulum stress in HepG2 liver cells. Fundam. Clin. Pharmacol. 2020, 34, 249–262. [Google Scholar] [CrossRef]
- Farese, R.J.; Walther, T.C. Glycerolipid Synthesis and Lipid Droplet Formation in the Endoplasmic Reticulum. Cold Spring Harb. Perspect. Biol. 2023, 15, a041246. [Google Scholar] [CrossRef]
- Sniegocka, M.; Liccardo, F.; Fazi, F.; Masciarelli, S. Understanding ER homeostasis and the UPR to enhance treatment efficacy of acute myeloid leukemia. Drug Resist. Updat. 2022, 64, 100853. [Google Scholar] [CrossRef]
- Anastasia, I.; Ilacqua, N.; Raimondi, A.; Lemieux, P.; Ghandehari-Alavijeh, R.; Faure, G.; Mekhedov, S.L.; Williams, K.J.; Caicci, F.; Valle, G.; et al. Mitochondria-rough-ER contacts in the liver regulate systemic lipid homeostasis. Cell Rep. 2021, 34, 108873. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Kaplowitz, N.; Lebeaupin, C.; Kroemer, G.; Kaufman, R.J.; Malhi, H.; Ren, J. Endoplasmic reticulum stress in liver diseases. Hepatology 2023, 77, 619–639. [Google Scholar] [CrossRef]
- Celik, C.; Lee, S.; Yap, W.S.; Thibault, G. Endoplasmic reticulum stress and lipids in health and diseases. Prog. Lipid. Res. 2023, 89, 101198. [Google Scholar] [CrossRef]
- Wiseman, R.L.; Mesgarzadeh, J.S.; Hendershot, L.M. Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol. Cell 2022, 82, 1477–1491. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallee, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Alshawsh, M.A.; Alsalahi, A.; Alshehade, S.A.; Saghir, S.; Ahmeda, A.F.; Al, Z.R.; Mahmoud, A.M. A Comparison of the Gene Expression Profiles of Non-Alcoholic Fatty Liver Disease between Animal Models of a High-Fat Diet and Methionine-Choline-Deficient Diet. Molecules 2022, 27, 858. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Wu, J.; Xu, H.; Leung, W.Y.; Fu, K.; Wu, J.; Liu, K.; Man, K.; Yang, X.; et al. Macrophage p38alpha promotes nutritional steatohepatitis through M1 polarization. J. Hepatol. 2019, 71, 163–174. [Google Scholar] [CrossRef]
- Reid, D.T.; Eksteen, B. Murine models provide insight to the development of non-alcoholic fatty liver disease. Nutr. Res. Rev. 2015, 28, 133–142. [Google Scholar] [CrossRef]
- Gallo, M.; Gamiz, F. Choline: An Essential Nutrient for Human Health. Nutrients 2023, 15, 2900. [Google Scholar] [CrossRef]
- Zhang, J.; Zang, X.; Lv, J.; Zhang, Y.; Lv, Z.; Yu, M. Changes in Lipidomics, Metabolomics, and the Gut Microbiota in CDAA-Induced NAFLD Mice after Polyene Phosphatidylcholine Treatment. Int. J. Mol. Sci. 2023, 24, 1502. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Sun, X.; Seidman, J.S.; Zhao, P.; Troutman, T.D.; Spann, N.J.; Que, X.; Zhou, F.; Liao, Z.; Pasillas, M.; Yang, X.; et al. Neutralization of Oxidized Phospholipids Ameliorates Non-alcoholic Steatohepatitis. Cell Metab. 2020, 31, 189–206.e8. [Google Scholar] [CrossRef]
- Xiong, S.; Chng, W.J.; Zhou, J. Crosstalk between endoplasmic reticulum stress and oxidative stress: A dynamic duo in multiple myeloma. Cell. Mol. Life Sci. 2021, 78, 3883–3906. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, K.; Peng, X.; Kan, Y.; Li, H.; Zhu, Y.; Wang, Z.; Li, Z.; Liu, H.Y.; Cai, D. Nuclear Receptor PPARalpha as a Therapeutic Target in Diseases Associated with Lipid Metabolism Disorders. Nutrients 2023, 15, 4772. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; Luo, Y. FGF21 in obesity and cancer: New insights. Cancer Lett. 2021, 499, 5–13. [Google Scholar] [CrossRef]
- Piccinin, E.; Moschetta, A. Hepatic-specific PPARalpha-FGF21 action in NAFLD. Gut 2016, 65, 1075–1086. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouche, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Regnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Inagaki, T. Research Perspectives on the Regulation and Physiological Functions of FGF21 and its Association with NAFLD. Front. Endocrinol. 2015, 6, 147. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.H.; Kim, H.K.; Kim, M.J.; Back, S.H.; Konishi, M.; Itoh, N.; Lee, M.S. Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia 2015, 58, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Takahashi, S.; Zhang, Y.; Krausz, K.W.; Smith, P.B.; Patterson, A.D.; Gonzalez, F.J. Role of fibroblast growth factor 21 in the early stage of NASH induced by methionine- and choline-deficient diet. Biochim. Biophys. Acta 2015, 1852, 1242–1252. [Google Scholar] [CrossRef]
- Lu, X.; Huang, H.; Fu, X.; Chen, C.; Liu, H.; Wang, H.; Wu, D. The Role of Endoplasmic Reticulum Stress and NLRP3 Inflammasome in Liver Disorders. Int. J. Mol. Sci. 2022, 23, 3528. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Q.; Zhou, C.; Xu, Y.; Liu, D.; Fujiwara, N.; Kubota, N.; Click, A.; Henderson, P.; Vancil, J.; et al. Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development. Immunity 2023, 56, 58–77.e11. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.F.; Xu, Q.Q.; Chen, T.Q.; Ming, J.X.; Wang, Y.F.; Mao, L.N.; Zhou, J.J.; Sun, W.G.; Zhou, Q.; Ren, H.; et al. Kinsenoside alleviates inflammation and fibrosis in experimental NASH mice by suppressing the NF-kappaB/NLRP3 signaling pathway. Phytomedicine 2022, 104, 154241. [Google Scholar] [CrossRef]
- Pavillard, L.E.; Marin-Aguilar, F.; Bullon, P.; Cordero, M.D. Cardiovascular diseases, NLRP3 inflammasome, and western dietary patterns. Pharmacol. Res. 2018, 131, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Muriel, P. NLRP3 inflammasome in hepatic diseases: A pharmacological target. Biochem. Pharmacol. 2023, 217, 115861. [Google Scholar] [CrossRef]
- Gaul, S.; Leszczynska, A.; Alegre, F.; Kaufmann, B.; Johnson, C.D.; Adams, L.A.; Wree, A.; Damm, G.; Seehofer, D.; Calvente, C.J.; et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 2021, 74, 156–167. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Tian, J.; Li, J.; Li, X.; Wu, S.; Liu, Y.; Han, J.; Ye, F. CX08005, a Protein Tyrosine Phosphatase 1B Inhibitor, Attenuated Hepatic Lipid Accumulation and Microcirculation Dysfunction Associated with Nonalcoholic Fatty Liver Disease. Pharmaceuticals 2023, 16, 106. [Google Scholar] [CrossRef]
- Sun, X.; Harris, E.N. New aspects of hepatic endothelial cells in physiology and nonalcoholic fatty liver disease. Am. J. Physiol. Cell Physiol. 2020, 318, C1200–C1213. [Google Scholar] [CrossRef]
- Hartl, L.; Rumpf, B.; Domenig, O.; Simbrunner, B.; Paternostro, R.; Jachs, M.; Poglitsch, M.; Marculescu, R.; Trauner, M.; Reindl-Schwaighofer, R.; et al. The systemic and hepatic alternative renin-angiotensin system is activated in liver cirrhosis, linked to endothelial dysfunction and inflammation. Sci. Rep. 2023, 13, 953. [Google Scholar] [CrossRef]
- Lee, K.C.; Wu, P.S.; Lin, H.C. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin. Mol. Hepatol. 2023, 29, 77–98. [Google Scholar] [CrossRef]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases. Drug Des. Devel. Ther. 2020, 14, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-alpha/gamma Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Idowu, M.O.; Parmar, D.; Borg, B.B.; Denham, D.; Loo, N.M.; Lazas, D.; Younes, Z.; Sanyal, A.J. A Phase 2 Double Blinded, Randomized Controlled Trial of Saroglitazar in Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2021, 19, 2670–2672. [Google Scholar] [CrossRef]
- Nakajima, A.; Eguchi, Y.; Yoneda, M.; Imajo, K.; Tamaki, N.; Suganami, H.; Nojima, T.; Tanigawa, R.; Iizuka, M.; Iida, Y.; et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha), versus placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2021, 54, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ding, W.; Wang, M.Q.; Wang, Z.G.; Chen, H.H.; Chen, W.; Yang, Q.; Lu, T.N.; Yang, Q.; He, J.M. Tanshinone IIA ameliorates non-alcoholic fatty liver disease through targeting peroxisome proliferator-activated receptor gamma and toll-like receptor 4. J. Int. Med. Res. 2019, 47, 5239–5255. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi, D.; Liang, Z.; Pan, J.; Zhen, J.; Zheng, C.; Fan, W.; Song, Q.; Pan, M.; Yang, Q.; Zhang, Y. Tanshinone IIA Inhibits the Endoplasmic Reticulum Stress-Induced Unfolded Protein Response by Activating the PPARα/FGF21 Axis to Ameliorate Nonalcoholic Steatohepatitis. Antioxidants 2024, 13, 1026. https://doi.org/10.3390/antiox13091026
Pi D, Liang Z, Pan J, Zhen J, Zheng C, Fan W, Song Q, Pan M, Yang Q, Zhang Y. Tanshinone IIA Inhibits the Endoplasmic Reticulum Stress-Induced Unfolded Protein Response by Activating the PPARα/FGF21 Axis to Ameliorate Nonalcoholic Steatohepatitis. Antioxidants. 2024; 13(9):1026. https://doi.org/10.3390/antiox13091026
Chicago/Turabian StylePi, Dajin, Zheng Liang, Jinyue Pan, Jianwei Zhen, Chuiyang Zheng, Wen Fan, Qingliang Song, Maoxing Pan, Qinhe Yang, and Yupei Zhang. 2024. "Tanshinone IIA Inhibits the Endoplasmic Reticulum Stress-Induced Unfolded Protein Response by Activating the PPARα/FGF21 Axis to Ameliorate Nonalcoholic Steatohepatitis" Antioxidants 13, no. 9: 1026. https://doi.org/10.3390/antiox13091026





