1. Introduction
Gas production and separation play an integral part in mitigating energy demands and supporting various industrial processes worldwide. The importance of efficient gas production and separation processes cannot be overstated, as they directly impact sectors such as power generation, fuel production, chemical manufacturing, and environmental sustainability [
1]. Gas production involves the extraction and purification of gases from various sources, including natural gas fields, biogas plants, and industrial processes. The obtained gases often contain impurities or are in mixed compositions, requiring effective separation techniques to extract the desired components and remove contaminants.
Hydrogen (H
2) gas provides a clean and sustainable fuel alternative to fossil fuels and natural gas [
2]. Hydrogen production is generally performed through the methane stream reforming reaction, which leads to the by-product of carbon monoxide (CO) or carbon dioxide (CO
2) in addition to H
2 [
3]. Another technique is the separation of H
2 from steam either through pressure swing adsorption, cryogenic separation, and membrane separation [
4]. The storage of hydrogen has also been investigated due to its crucial role in the hydrogen economy [
5,
6]. Membrane-based gas separation technology has become increasingly important in recent years due to its minimal energy consumption, cost-effectiveness, and scalability [
7].
Membranes are utilized in a multitude of applications ranging from wastewater treatment [
8] to pollutants removal and processing [
9,
10]. Membranes are utilized to separate different gases from mixtures based on their density, chemical properties, and concentration. There are several types of membranes used in gas production, including polymeric [
11,
12], ceramic [
13,
14], and metallic membranes [
15,
16]. Each type has its unique advantages and limitations in terms of selectivity, permeability, and cost. Perovskite-based membranes have emerged as a promising technology for gas production and separation due to their high selectivity and permeability towards certain gases such as H
2, Oxygen (O
2), and CO
2 [
17,
18]. Perovskite materials have a unique structure that allows the efficient separation of gases based on their specific size and properties. In addition, Perovskite materials are recognized for their stability under harsh operating conditions, such as extreme temperature and elevated pressures, allowing a more durable and longer-lasting operation compared to other types of membranes. Scalability is another advantage of perovskite membranes, as the various fabrication techniques are compatible with large-scale operations.
In this paper, the utilization and role of perovskite membranes in gas production, separation, and capture applications is reviewed and presented. An outlook on the fabrication methods and deposition techniques of perovskite membranes is also summarized. This work highlights the advancement in perovskite membranes and provides a future outlook on their employment as a cost-effective and scalable method for hydrogen production, oxygen enrichment, and carbon dioxide capture.
2. Perovskite Membranes Structure and Fabrication Methods
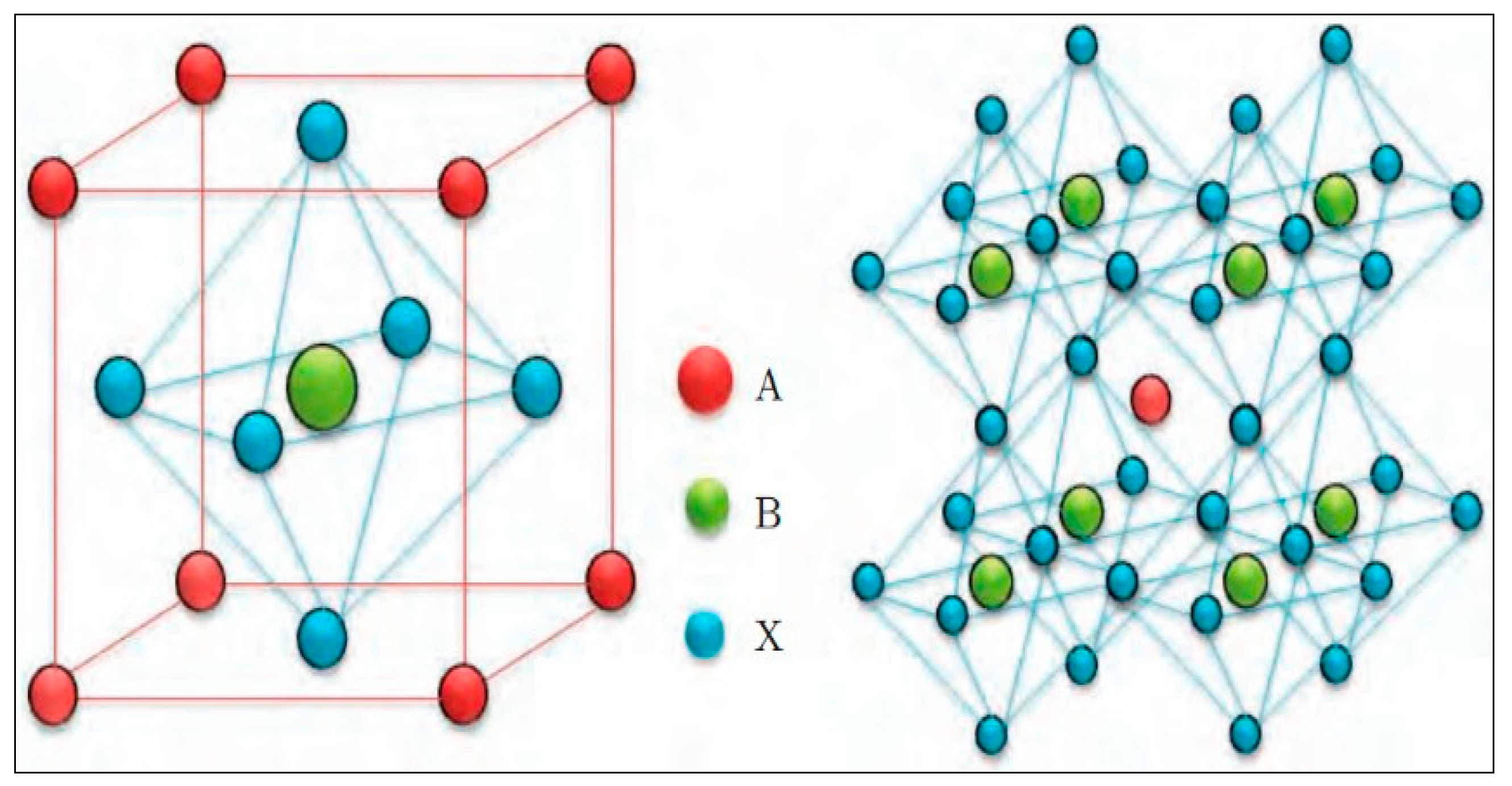
Perovskite materials have the unique cubic structure of ABX
3, where A and B are both positively charged ions (cations) that vary in size, and X is a negatively charged ion (anion). The structure is characterized by the cubic arrangement, where the larger A-site cations occupy the corner of the lattice, and the smaller B-site cations occupy the center of the lattice as shown in
Figure 1. The X-site anions surround the B-site cation at the face-centered position. This arrangement allows the formation of a three-dimensional framework providing stability to the structure. The substitution of the X-site anion with an oxide (generally oxygen) results in a perovskite oxide material with the structure of ABO
3. Both ABX
3 and ABO
3 perovskite structures have unique properties and can be used in various applications. For instance, ABX
3 is mainly used in solar cells [
19,
20], where the A-site cation can be cesium (Cs
+), methylammonium, or formamidinium; B-site cation can be lead (Pb
+2), tin (Sn
+2), or germanium (Ge
+4); and the X-site anion can be either iodide (I
−), chloride (Cl
−), or bromide (Br
−). Whereas ABO
3 is mainly used as catalyst [
21], with A-site cations varying between lanthanum (La), barium (Ba), and strontium (Sr). While B-site cations are transition metals such as molybdenum (Mo), tungsten (W), and zirconium (Zr). The X-site anion is typically an oxygen atom.
Perovskite materials have gained significant attention in various fields, including photovoltaics [
22], optoelectronics [
23], and catalysis [
24], because of their tunable properties. Perovskite materials offer several distinct advantages compared to various membrane materials such as carbon materials, zeolites, MOFs (Metal–Organic Frameworks), and COFs (Covalent Organic Frameworks). For instance, due to the versatile structure of perovskite materials, a wide range of elements and compositions can be incorporated. This versatility enables the tailoring of material properties to suit specific applications, including tunable electronic, optical, and catalytic properties. Perovskite materials exhibit high ion conductivity, particularly in oxide perovskites. This property makes them suitable for various applications in oxygen separation membranes and gas sensors [
25]. Their high ion transport capability enables efficient transport of ions through the material, enhancing the overall membrane performance.
Figure 1.
Perovskite Structure [
26]. (Obtained from an open access source).
Figure 1.
Perovskite Structure [
26]. (Obtained from an open access source).
Perovskite materials often exhibit excellent catalytic activity, especially in perovskite oxides. They can facilitate various catalytic reactions, such as oxygen reduction reactions (ORR), oxygen evolution reactions (OER), and hydrogen evolution reactions (HER). By adjusting the composition and structure, the bandgap of perovskite materials can be tuned over a wide range, allowing efficient absorption of sunlight across the solar spectrum. This tunability is advantageous for designing efficient and cost-effective solar cells [
27]. Perovskite materials can be synthesized using solution-based techniques, which offer the advantage of low-cost and high-throughput production. Solution processing methods, such as spin coating and inkjet printing, enable large-area deposition of perovskite films, making them promising for scalable manufacturing [
28]. Perovskite materials can be easily integrated with existing technologies and fabrication processes. This compatibility with established processes enhances their potential for commercialization and adoption. The complex synthesis procedure of MOFs and COFs presents a challenge for scalability as the high-cost limits large-scale industrial applications [
29]. Zeolites have been widely utilized for large-scale applications. However, some zeolite types may require more complex synthesis methods or costly raw materials [
30].
Table 1 provides a comparison summary of perovskite materials with various membrane materials.
The mechanical performance of perovskite membranes is a vital consideration for their industrial applications. Perovskite materials generally exhibit a degree of flexibility and elasticity, especially when they are fabricated as thin films or nanostructures [
31]. This flexibility is advantageous in applications where the membrane needs to withstand bending or deformation without fracture or delamination. However, the specific mechanical properties can change based on the composition, structure, and processing conditions of the perovskite material [
32]. Perovskite membranes are often deposited on supporting substrates to enhance their mechanical stability. The substrate can provide mechanical reinforcement and prevent the membrane from cracking or breaking under mechanical stress. The choice of substrate material and its compatibility with the perovskite membrane is vital in defining the overall mechanical performance and integrity of the membrane [
33]. The interface between the perovskite membrane and the supporting substrate can significantly alter the mechanical properties. By optimizing the interfacial interactions and engineering suitable interlayers or adhesion promoters, it is possible to improve the adhesion and mechanical stability of the membrane. Various techniques, such as surface modifications and interfacial bonding strategies, can be employed to improve mechanical performance.
Perovskite membranes have the potential to be applied to various gas separation systems beyond H
2, O
2, and CO
2 separation. The advantageous properties of perovskite materials, such as their tunable composition, high ionic conductivity, and selectivity, make them promising candidates for a multitude of gas separation applications. Perovskite membranes have the ability to be employed in natural gas processing for separating methane from other hydrocarbons or impurities [
34]. Efficient methane separation is crucial for applications such as natural gas purification, upgrading, and transportation. Perovskite membranes with suitable composition and surface characteristics can enable the selective separation of methane from complex gas mixtures [
35]. Perovskite membranes can be explored for the separation of various hydrocarbon gases, such as ethane, propane, and butane, from gas mixtures [
36,
37]. This has implications for applications like petrochemical processing and olefin/paraffin separations. The selectivity and transport properties of perovskite membranes can be tailored to achieve the desired separation performance. Perovskite membranes can potentially be used for the recovery and removal of volatile organic compounds (VOC) from gas streams. VOCs are present in industrial emissions and can have harmful environmental impacts. Perovskite membranes with high selectivity and permeability can facilitate the efficient removal and recovery of VOCs, aiding in pollution control and resource conservation [
38].
The elemental composition of perovskite materials allows the multiple utilization of perovskite membranes in various gas separation processes. The separation mechanism of perovskite membranes is closely related to their elemental composition. The choice of elements in the perovskite structure directly impacts the membrane’s selectivity and transport properties. The elemental composition of perovskite, particularly the cationic species, influences the ionic conductivity of the membrane [
39]. For example, the presence of mobile cations, such as alkali metals (e.g., K
+, Na
+), can enhance ionic conductivity by facilitating the movement of ions within the perovskite lattice. The elemental composition of perovskite affects its chemical affinity towards specific molecules or ions. This affinity can influence the selectivity of the membrane for certain components during separation processes. By selecting appropriate cations or anions in the perovskite structure, it is possible to tailor the adsorption and transport properties of the membrane, enabling the selective separation of desired species [
40]. Perovskite membranes can exhibit mixed ionic-electronic conduction, where both ions and electrons contribute to the overall transport process. The elemental composition of the perovskite can influence the balance between ionic and electronic conduction, which impacts the membrane’s overall transport mechanism. For example, doping the perovskite structure with transition metal elements can enhance electronic conductivity, enabling efficient electron transport. The elemental composition of the perovskite affects its crystal structure and the presence of defects within the lattice. Structural defects, such as vacancies or substitutions, can impact the transport properties of the membrane [
41]. For example, the introduction of dopant elements can create oxygen vacancies, which can enhance the oxygen ion transport in perovskite oxide membranes. The elemental composition of perovskite membranes influences their stability and chemical compatibility with the target separation environment. Certain elements may exhibit higher chemical stability, resistance to reactive species, or tolerance towards harsh operating conditions. By selecting appropriate elements in the perovskite composition, it is possible to improve the membrane’s durability and long-term performance.
In addition to the elemental compositions, the structure of perovskite membranes has a crucial effect on their performance. Various structural characteristics, such as composition, crystal structure, morphology, and defect chemistry, can significantly impact the membrane’s transport properties, selectivity, stability, and overall performance. The crystal structure of perovskite membranes affects their transport properties and stability. Variations in the crystal lattice parameters, such as lattice constant, bond lengths, and angles, can impact ion diffusion pathways, electronic band structure, and defect formation [
42]. The crystal structure also determines the type of defects present in the membrane, which can influence the membrane’s ionic or electronic conductivity. The morphology of perovskite membranes, including grain size, porosity, and surface roughness, can influence their performance. Smaller grain sizes and higher porosity can enhance mass transport through the membrane by reducing diffusion pathways and providing larger surface areas for adsorption and reaction. Control over the membrane’s morphology can improve selectivity, flux, and stability. Defects in perovskite membranes, such as vacancies, substitutions, or interstitials, play a crucial role in their performance [
43]. Defects can influence ionic or electronic conductivity, surface reactions, and chemical stability. The type, concentration, and distribution of defects can be tailored to optimize the membrane’s performance for specific applications, such as oxygen ion transport in solid oxide fuel cells or ion selectivity in ion exchange membranes. The interfaces of perovskite membranes, including the membrane–substrate interface and the membrane–environment interface, can significantly impact their performance [
43]. Proper interface engineering can enhance adhesion, minimize interfacial reactions, and improve transport properties. The choice of interlayers or coatings, surface functionalization, or interface modification techniques can optimize the membrane’s performance and stability. The structural stability of perovskite membranes is crucial for long-term performance. Factors such as chemical compatibility, thermal stability, and resistance to reactive species or environmental conditions can affect the membrane’s durability and reliability. Optimizing the structural parameters, composition, and surface characteristics can enhance the membrane’s stability and extend its operational lifespan.
2.1. Perovskite Membrane Fabrication Methods
Perovskite membrane preparation follows a three-step process starting with powder synthesis and calcination, followed by processing to the desired geometry, and finally sintering. The sintering step is the most crucial in the preparation process as it determines the main features of the resulting membranes such as the porosity, grain shape and size, density, and the morphology of the surface. The most facile method of preparing perovskite membranes is by pressing powders in disks followed by sintering [
44]. A well-established process for the manufacturing of thin films and dense membranes is the tape casting method. This method involves creating a slurry by dispersing inorganic powder in a solvent, which can be either water or organic liquids. The viscosity of the slurry controls the thickness of the resulting tape and can be adjusted by adding dispersing agents, binders, and plasticizers. The slurry is then applied onto a flat support to form a tape, where adjustable blades are employed to regulate the thickness of the membrane. For instance, La
0.6Sr
0.4Fe
0.9Ga
0.1O
3 dense disk membranes with a thickness ranging from 0.63 to 1.30 mm [
45]. Extrusion involves the passage of ceramic slurries through a die orifice subjected to high pressure. To achieve the desired shaping, the slurry must possess tailored rheologic properties. For instance, to be able to shape the geometry and press it through the die, a slurry with low viscosity is needed. On the other hand, a tube needs to maintain its annular shape, requiring a sufficiently high viscosity to reinforce the resulting geometry [
46]. The shape of the materials is defined by the measurements and geometry of the die and the cutting length during the shaping process. Once the required shape is achieved, the processed material is subjected to a two-step sintering heat treatment. In the first step, the material is slowly heated to evaporate any organic compounds present in the extruded mixture. Following that, the material is sintered at elevated temperatures to ensure the densification of the membrane. This method has been utilized for the preparation of various perovskite membranes, such as SrCo
0.8Fe
0.2O
3 and La
0.7Ca
0.3Fe
0.85Co
0.15O
3 [
47].
The process of producing perovskite hollow fibers starts by preparing a mixture called dope. In this method, precursor powders are combined with a solution that contains a polymer-based binder and a solvent. The mixture is carefully blended while being continuously stirred. It is essential to carefully regulate parameters such as the particle size of the powder, the ratio of solvent to binder, and the ratio of powder to binder in the final solution. Following the mixing step, the resulting dope solution is then spun to create a hollow fiber. Polyvinylpyrrolidone is commonly employed as an agent to adjust the viscosity in the production process. The dope solution, along with a liquid known as the bore liquid, is injected under pressure through a spinneret. This injection process gives rise to the formation of hollow fiber geometry. The completion of this geometry occurs after a coagulation bath, where the geometry contacts with a liquid bath. Several important parameters require careful control, including the viscosity of the dope solution, injection pressure, selection of bore liquid and coagulant liquid, as well as the air gap (i.e., the distance between the spinneret and the coagulant bath). Subsequently, the hollow fibers are subjected to high-temperature sintering (ranging from 410 to 1000 °C) under atmospheric conditions. During the sintering stage, the polymeric binder is burned off, transforming into carbon, as the polymers react with oxygen from the air at temperatures exceeding 300 °C, leading to the formation of CO2. As a result, the particles come into contact with each other and commence the formation of a perovskite material exhibiting a hollow fiber structure, commonly known as the green fiber.
The fabrication of perovskite thin films on porous substrates is a novel method that has gained major interest recently. However, most of the efforts have not yielded satisfactory outcomes yet. The commonly employed technique revolves around the concept of asymmetric membranes, where the porous substrate allows minimal resistance to gas molecule transport, while the thin film presents a higher resistance. It is understood that gas fluxes through membranes increase as the thickness decreases. This approach proves effective when the coefficient of expansion of the perovskite materials aligns with that of the substrate. Nevertheless, the challenge with perovskites lies in the fact that their coefficients of expansion are predominantly nonlinear [
48]. The dip-coating method has been employed to deposit thin perovskite films on porous flat or tube substrates. However, this approach has resulted in ineffective membranes with significant defects that render them incapable of efficiently separating oxygen from the air. To address these challenges, substrates and films fabricated from the same perovskite material are utilized to match the coefficient of expansion between the layers. Nevertheless, even these perovskite membranes have failed to exhibit satisfactory oxygen separation capabilities. Recently, a novel approach has been investigated, involving the preparation of asymmetric films on porous substrates. The perovskite thin film is first prepared using the tape casting technique, followed by placing the porous substrate on top of it [
49]. This technique yielded remarkable results for the fabrication of membranes from thin dense films of 70
m and 20
m, respectively [
46,
47,
48,
49,
50].
Freeze-cast is another method utilized for the manufacturing of perovskite membranes. This technique shares similarities with the formation of dense films on porous substrates, where the substrates are fabricated through the Freeze-cast method. One significant benefit of Freeze-cast is the capability to produce mechanically stable and vastly porous substrates, where the pores possess organized channels. The method involves the preparation of a stable colloidal suspension, which is then poured into a mold. The suspension is then frozen, followed by the sublimation and sintering of the resulting material. The rapid freezing effect leads to the formation of a solidification front, effectively trapping perovskite particles within the growing crystals. During the sublimation of the slurry solvent/liquid, a structured network of pores is created and retained throughout the following high-temperature sintering processes. Water is commonly employed as a solvent in this environmentally friendly process. The morphology of the pores is influenced by the crystal growth of the solvent, necessitating precise control over the cooling rate [
51].
Table 2 provides a summary of the advantages and drawbacks of the various perovskite membrane fabrication techniques.
2.2. Perovskite Membrane Deposition Methods
The deposition of perovskite on various membranes can be performed through several methods. The following section provides a summary of common perovskite membrane deposition techniques.
2.2.1. Wet Chemical Methods
Wet chemical methods refer to the use of solution-based perovskite precursors that are then deposited on a substrate and transformed into perovskite thin films. The most widely used technique is spin coating. In this method, a solution containing perovskite precursors is spread onto a substrate, which is then rapidly rotated to achieve a uniform coating. Subsequent thermal annealing is usually performed to promote the formation of the perovskite phase [
52]. Another technique is doctor blading, where a blade is used to spread the perovskite precursor solution onto a substrate. The excess solution is scraped off, leaving a thin, uniform film. This method is particularly useful for large-area deposition. Moreover, inkjet printing is a famous technique to deposit perovskite onto membrane substrates. The perovskite precursor solution is loaded into an ink cartridge, and droplets are selectively ejected onto specific locations to form the desired pattern. Inkjet printing is preferred due to the precise control of the thickness of the film thickness and the patterned deposition [
52]. Similarly, slot-die coating involves the continuous deposition of a perovskite precursor solution through a narrow slit onto a moving substrate. The coating thickness is controlled by the gap between the slot die and the substrate, allowing uniform and scalable deposition [
53]. Deposition techniques based on slurry have also been investigated. For instance, in slip casting, a mixture comprising fine powders with submicron dimensions, a liquid medium (such as ethanol or isopropanol), organic binders, and additives is formulated to achieve the desired flow characteristics, suspension stability, and sedimentation properties. When the mixture is poured onto a porous substrate, it permeates the pores of the support, resulting in the formation of a uniform and seamless layer of powder on the surface. The layer is then subjected to drying and subsequent sintering process [
54]. On the other hand, in slurry spraying, powders suspended in a liquid are applied onto a substrate through the use of a spray gun. The resulting layer is then dried and subjected to sintering. This method is suitable for coating complex shapes; however, achieving precise control over the thickness at the edges of a component can be challenging. Another widely utilized technique is the sol–gel method. This process involves the immersion of a substrate in a solution followed by annealing. The thickness is controlled or modified by controlling the number of dips/annealing cycles. A sintering step is lastly conducted to achieve the required microstructure; hence, this method is particularly well-suited for creating such porous geometries [
54].
2.2.2. Plasma Spraying
The plasma spray technique involves the utilization of high DC power voltage electrodes to generate plasma. The particles are melted by the plasma and propelled at high velocities (ranging from 100 to 1200 m/s) through the plasma jet [
55]. Upon contact with the substrate, the particles rapidly solidify, forming a deposition. The temperature of the plasma jet can vary significantly, ranging from 6727 to 19,727 °C [
56]. Depending on the specific method employed, the powders can be deposited onto the substrate under different conditions: atmospheric pressure in the case of atmospheric plasma spray (APS), vacuum conditions (10
−2 to 10
−3 atm) in the case of vacuum plasma spray (VPS), and low pressure (<10
−3 atm) in the low-pressure plasma spray method.
2.2.3. Physical Vapor Deposition
Physical Vapor Deposition (PVD) techniques involve the vaporization of solid perovskite precursor material, followed by condensation on the substrate to form a perovskite thin film [
57]. Some common PVD methods include thermal evaporation that requires precise control of temperature and pressure to accomplish the required film properties. The deposition is accomplished in a vacuum chamber. Electron Beam Evaporation (EBV) is another method, where an electron beam is utilized to vaporize the perovskite precursor material. The high-energy electron beam bombards the material, causing it to evaporate and subsequently deposit onto the substrate. This technique enables precise control over the deposition rate and allows the deposition of complex materials [
58]. Sputtering is commonly used for deposition as well. Sputtering involves bombarding a material’s surface with high-energy ions, leading to the expulsion of atoms from the target. Subsequently, these expelled atoms settle on the substrate, creating a thin film. Both DC sputtering and magnetron sputtering can be used for perovskite membrane deposition [
59]. Recently, Molecular Beam Epitaxy (MBE) has been used as a PVD technique. MBE is a precise deposition technique used for growing high-quality thin films. It involves the evaporation of constituent elements or compounds in an ultra-high vacuum environment, where the vaporized species condense onto the substrate with accurate control over the composition and thickness of the resulting film. MBE is commonly used for the epitaxial growth of perovskite films [
60]. Generally, PVD techniques offer control over film thickness, composition, and crystallinity, making them suitable for the fabrication of perovskite membranes with tailored properties. However, it is worth noting that the application of PVD techniques for perovskite membranes may require specific adaptations and optimizations due to the sensitivity of perovskite materials to high temperatures and reactive environments.
2.2.4. Chemical Vapor Deposition
Chemical Vapor Deposition (CVD) techniques can be employed to fabricate perovskite membranes. In a CVD process, a perovskite precursor with a low vaporization point is usually heated to form active gas phases. The active gas then reacts to form the thin film on the substrate in a vacuum-controlled space referred to as the reaction chamber. Several CVD techniques can be used for the deposition of perovskite materials. For instance, in Atmospheric Pressure Chemical Vapor Deposition (APCVD), the perovskite precursor gases are introduced into a reactor chamber at atmospheric pressure. The substrate, usually a porous support material, is annealed to a certain temperature that allows the precursor gases to react and form a perovskite film. In addition, Low-Pressure Chemical Vapor Deposition (LPCVD) involves operating the reactor at reduced pressures, typically below atmospheric pressure. The lower pressure helps in achieving better control over film growth and allows the use of lower temperatures. The perovskite precursors are added into the chamber, where they decompose and react to form the desired perovskite membrane. Metal–Organic Chemical Vapor Deposition (MOCVD) utilizes metal–organic precursors, such as metal halides or metal alkoxides, to deposit perovskite films. The precursors are introduced into the reactor chamber along with a carrier gas, and they react at elevated temperatures to form the perovskite structure. MOCVD offers precise control over the film’s thickness and morphology. Plasma-Enhanced Chemical Vapor Deposition (PECVD) utilizes the use of plasma to enhance the deposition process. The perovskite precursors are introduced into a reactor chamber, and plasma is generated either by applying an electric field or by using microwave energy. Plasma helps in dissociating and activating the precursor molecules, allowing the deposition of perovskite films. CVD techniques provide flexibility in tailoring the composition, morphology, and thickness of perovskite membranes. The specific deposition parameters, such as temperature, pressure, precursor flow rates, and reaction time, need to be optimized to achieve high-quality perovskite films with desired properties. Additionally, post-deposition treatments like annealing or surface modifications may be applied to further enhance the membrane performance.
Figure 2 shows the advantages and drawbacks of various perovskite deposition techniques.
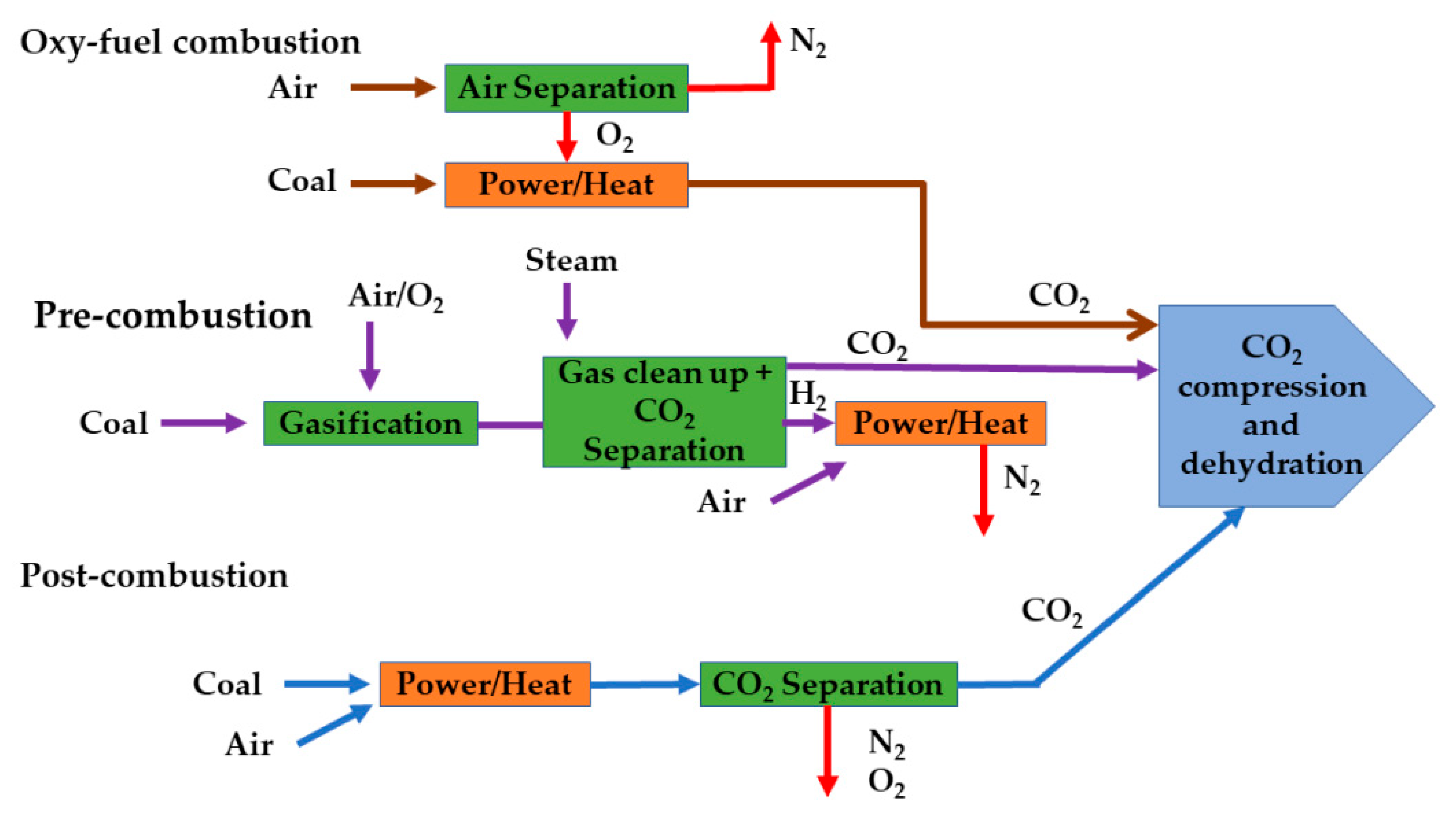
5. Perovskite Membranes in Carbon Dioxide Capture
Reducing carbon dioxide (CO
2) emissions is crucial for mitigating the impacts of climate change and transitioning towards a more sustainable future. CO
2 capture technologies are utilized to reduce emissions. These technologies include pre-combustion, post-combustion, and oxy-fuel combustion [
137,
138]. These methods are highlighted in
Figure 5. Pre-combustion is primarily employed in coal gasification plants, while both post-combustion and oxy-fuel combustion can be applied to both coal- and gas-fired plants, with post-combustion considered to be the most mature compared to its counterparts [
139]. In oxy-fuel combustion, the fuel is burned using pure oxygen rather than atmospheric air. This approach significantly removes nitrogen in the exhaust gas, which has an impact on the successive separation process. Moreover, the technology provides a significant reduction in NOx emissions [
140]. Cryogenic air separation is currently the standard process used in oxy-fuel power plants. Nevertheless, due to excessive energy consumption, a reduction in the overall plant efficiency by 10–12% is reported [
141]. Perovskite-based OTMs are considered the most favorable substitutes for conventional methods.
Important factors are required for the application of perovskite-based membranes in the oxy-fuel combustion process. For instance, having adequate O
2 permeability while maintaining long-term stability under CO
2 atmosphere [
142]. The main issue with perovskite membranes is the presence of alkaline-earth elements such as Ca, Sr, or Ba. When exposed to environments with CO
2 gas, these elements form a carbonate layer that impedes the diffusion of O
2 into the membrane, leading to a diminished flux. The CO
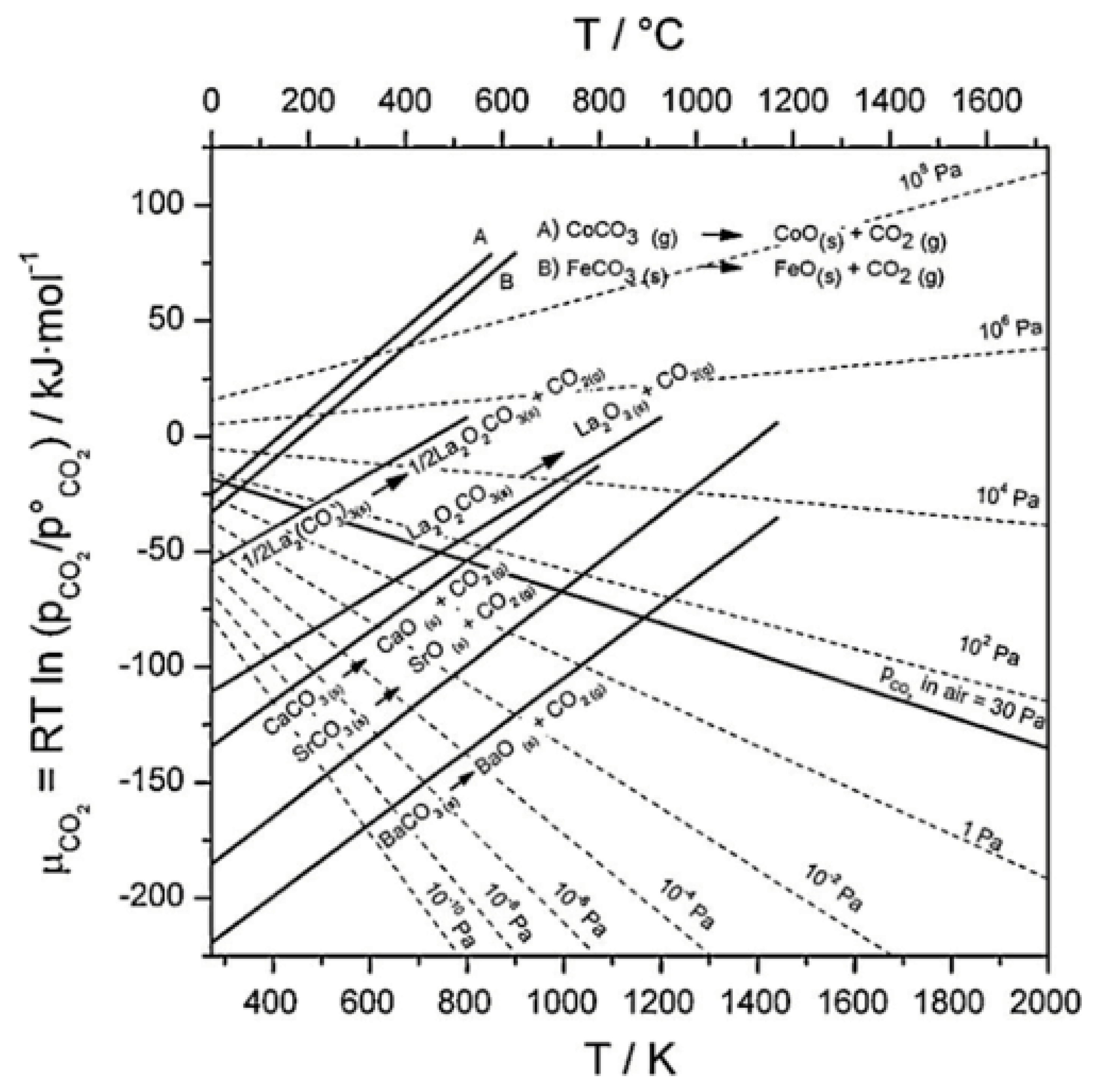
2 resistance of materials containing alkaline-earth elements can be theoretically evaluated with the aid of the Ellingham diagram [
143]. The resistance is evaluated at a certain temperature and partial pressure. In the diagram (seen in
Figure 6 [
144]), the dashed lines on the diagram represent the chemical potential of CO
2 at various CO
2 partial pressures. The solid continuous lines represent the decomposition potential of specific carbonates. To assess the CO
2 resistance, compare the position of the CO
2 chemical potential line with the carbonate decomposition line. If the CO
2 chemical potential line lies below the decomposition line, it suggests that the carbonate is thermodynamically unstable and may decompose.
Materials with high O
2 permeability, such as SrCo
0.8Fe
0.2, demonstrated a significant decrease in flux when subjected to CO
2 environment [
145]. In order to improve the CO
2 resistance of such membranes, various strategies have been employed, including the partial substitution of Sr
2+ with La
3+ [
146], or Co/Fe with Ti
4+ [
145], Zr
4+ [
147], Ta
5+ [
148], or Nb
5+ [
149]. However, this increase in CO
2 resistance is inversely proportional to the O
2 flux [
150]. In addition to site substitution, the utilization of bi-phase OTMs has been explored to improve CO
2 resistance, in spite of their low O
2 permeabilities [
151,
152]. Another method is to increase the O
2 partial pressure in CO
2-dominant environment, where it was reported that the CO
2 resistance is enhanced with the rise in O
2 partial pressure [
150,
152]. For instance, a membrane maintained a high O
2 permeability of 0.84 mL cm
−2 min
−1 when the partial pressure of O
2 was increased [
152]. The impact of sulfur dioxide (SO
2), which is a by-product of flue gas in power plants, on the O
2 permeation of perovskite materials has been examined [
153]. It was stated that the oxygen permeability of La
0.6Sr
0.4Co
0.2Fe
0.8 hollow fiber membranes were diminished when a small quantity of SO
2 was added to the sweep gas, and this poisoning effect was irreversible [
80]. The exposure to SO
2 resulted in the decomposition of the La
0.6Sr
0.4Co
0.2Fe
0.8 material and the formation of SrSO
4, causing severe damage to the membrane.
Figure 6.
Ellingham diagram for various carbonates [
152]. (Obtained from an open access source).
Figure 6.
Ellingham diagram for various carbonates [
152]. (Obtained from an open access source).
6. Challenges, Limitations, and Future Work
6.1. Membranes for Hydrogen Production
The majority of the reported perovskite membranes yield H2 permeation flux lower than the necessitated standard of 1–2 mL cm−2 min−1 at temperatures ranging from 600–700 °C for commercial applications. Additionally, there is a significant variation in the reported hydrogen flux performances, even when considering membranes with the same composition. This variation can be attributed to differences in synthesis, fabrication, and deposition methods as well as test conditions. Furthermore, variations in membrane microstructures resulting from different preparation methods can influence both protonic and electronic conductivities. Under harsh operating conditions, such as elevated temperatures, the reactivity of perovskite-based proton conducting membranes with the sweep gas species (such as water vapor, CO2, etc.) intensifies. This heightened reactivity can result in a decrease in the mechanical, chemical, and structural stability of the perovskite-based membranes, leading to impulsive degradation in flux performance. Despite significant advancements in membrane materials and performance, achieving an ideal membrane that possesses both high H2 permeation flux and excellent stability simultaneously remains challenging.
The primary focus of research on perovskite-based membranes has been on the development of novel composites that offer improved H2 permeation flux and stability. However, research has not focused on the cost effectiveness of the said composites. Existing processing technologies have certain constraints, including challenges in complex powder synthesis and achieving consistent reproducibility at a medium to low level. It is crucial to conduct further assessments on the cost-effectiveness and environmental aspects associated with the current perovskite membrane materials and methods in order to detect the main factors driving the cost.
6.2. Membranes for Oxygen Separation
The primary challenges in the development and commercial implementation of OTMs mainly revolve around fabrication, ensuring long-term reliable operation, and integration of robust systems. It is crucial to minimize material degradation caused by interactions between gases and solids as well as between different solid components. Furthermore, it is essential to develop a thorough comprehension of transport kinetics and establish universally accepted protocols for assessing oxygen flux and permeation. There are substantial gaps in knowledge within these domains, necessitating further investigation and research efforts.
Based on the aforementioned discussions, it can be recommended that A-site cation of the perovskite structure can be doped by strontium, while B-site cation can be doped by Mn, Ni, and Fe to realize the required properties concurrently. Specifically, the La0.7Sr0.3Cr0.7Ti0.3 composition based on titanium exhibits the lowest oxygen nonstoichiometric. Overall, the composition La1−xSrxCr1−yMyTizO3 would be the most promising perovskite composition for OTMs. To achieve a better O2 flux and performance, combining the perovskite phase with the fluorite phase, such as 8YSZ (8 mol% yttria-stabilized zirconia), is recommended to enhance ionic conductivity. Further fundamental research is required in the areas of transport behavior and electrochemical activity, particularly for doped lanthanum chromites, to fully exploit their potential for active OTM application.
6.3. Membranes for Carbon Dioxide Capture
Perovskite membranes have shown potential for CO
2 capture, but several challenges need to be addressed. One major challenge is the stability of perovskite materials subjected to CO
2 as it can cause structural degradation and reduced performance. Achieving high selectivity for CO
2 over other gases is also challenging. Perovskite membranes often exhibit lower permeability and flux compared to other materials, necessitating improvements in gas transport properties. Nevertheless, various strategies can be implemented to alleviate this instability and improve the performance and durability of perovskite membranes in CO
2 separation. These strategies are highlighted in
Figure 7.
The choice of perovskite material composition plays an integral part in enhancing the stability of the membrane in CO2 gas separation. Certain elements or combinations of elements can provide improved chemical stability and resistance to CO2 exposure. For example, incorporating elements with high oxygen affinity or resistance to carbonate formation can mitigate degradation caused by CO2. Surface modifications and coatings can be applied to perovskite membranes to enhance their stability in CO2 gas separation. These coatings can act as protective layers, preventing direct contact between the perovskite material and CO2. For instance, thin oxide films or dense ceramic coatings can provide a barrier against CO2 attack and improve the membrane’s resistance to degradation. Combining perovskite materials with other stable and compatible materials in composite or hybrid structures can help alleviate the instability in CO2 gas separation. By integrating a stable support or protective layer with the perovskite membrane, the overall stability and durability can be improved. This approach can enhance the resistance to CO2-induced degradation and prolong the membrane’s lifespan. Adjusting the operating conditions, such as temperature, pressure, and gas composition, can help alleviate the instability of perovskite membranes in CO2 gas separation. Optimizing these parameters can minimize the adverse effects of CO2 exposure on the perovskite material. Additionally, optimizing the gas composition, such as controlling the presence of impurities or reactive species, can mitigate the degradation caused by CO2. The interface between the perovskite membrane and the surrounding environment can be engineered to improve stability. This includes designing compatible interlayers or coatings that offer chemical resistance and prevent CO2-induced degradation. Surface functionalization methods can also be utilized to improve the stability and performance of the perovskite membrane. Conducting long-term durability studies under realistic operating conditions is crucial to understanding and mitigating the instability of perovskite membranes in CO2 gas separation. By monitoring the membrane’s performance over extended periods and identifying degradation mechanisms, researchers can develop strategies to enhance stability and design more robust membranes.