Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Membrane Synthesis
2.3. Characterization of Membrane Surface and Cross Section
2.4. Membrane Porosity, Pore Size and Flux
2.5. Hydrophilicity and Membrane Surface Charge
2.6. Membrane Biofouling Tests
3. Results and Discussion
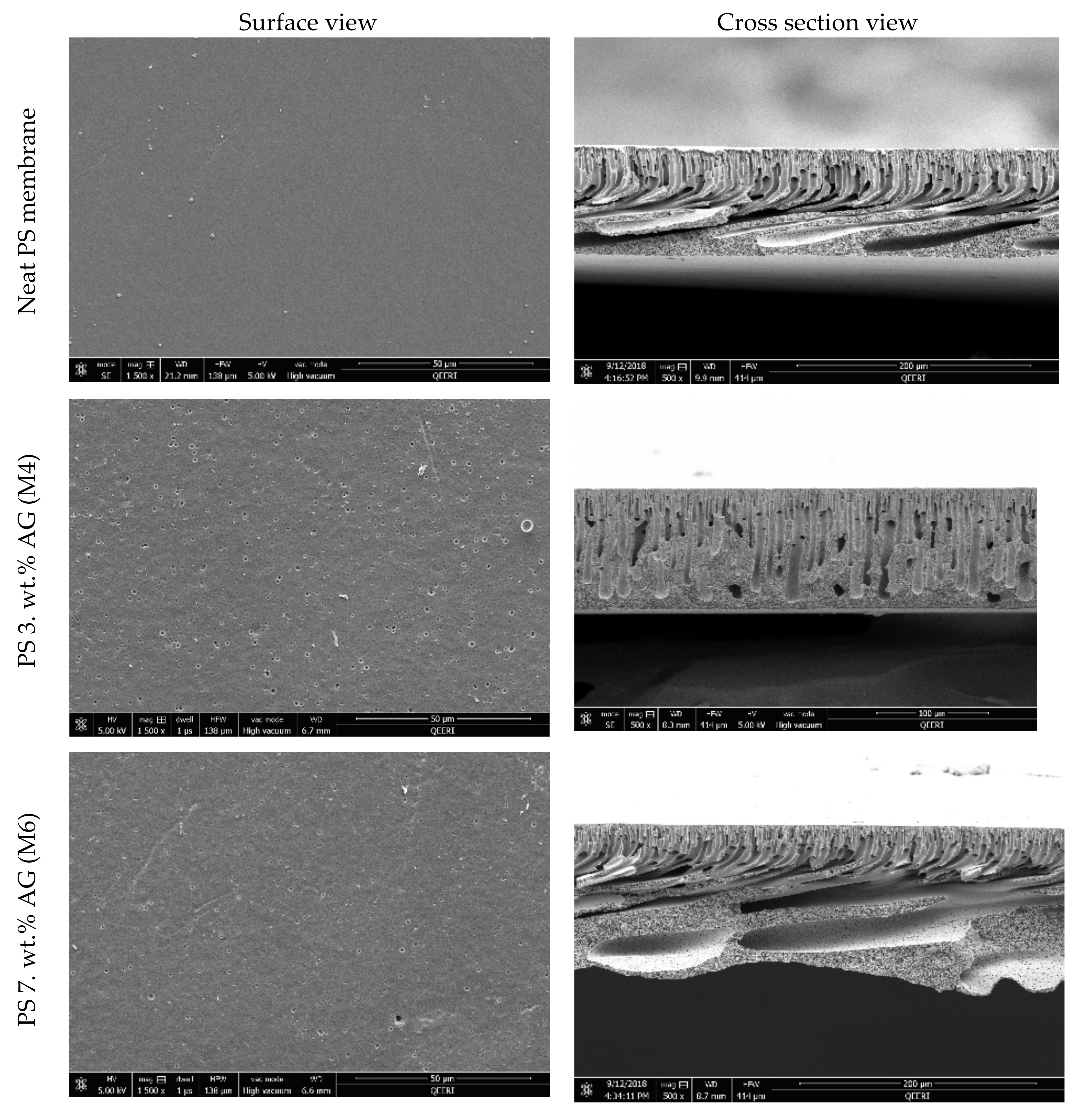
3.1. FESEM Images of the Membrane Surface and Cross Sections
3.2. Membrane Porosity, Pore Size and Flux
3.3. Contact Angle and Surface Charge
3.4. Antibacterial Properties of PS/AG Membranes Against Gram-positive and Gram-negative Bacteria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, S.-K.; Suh, S.-H.; Kim, D.-W. Characteristics of cooling water fouling in a heat exchange system. J. Mech. Sci. Technol. 2008, 22, 1568–1575. [Google Scholar] [CrossRef]
- Flemming, H.-C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Flannery, B.; Gelling, L.B.; Vugia, D.J.; Weintraub, J.M.; Salerno, J.J.; Conroy, M.J.; Stevens, V.A.; Rose, C.E.; Moore, M.R.; Fields, B.S.; et al. Reducing Legionella colonization of water systems with monochloramine. Emerg. Infect. Dis. 2006, 4, 588–596. [Google Scholar] [CrossRef]
- Judd, S. A review of fouling of membrane bioreactors in sewage treatment. Water Sci. Technol. 2004, 49, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Peyghambarzadeh, S.M.; Vatani, A.; Jamialahmadi, M. Experimental study of micro–particle fouling under forced convective heat transfer. Braz. J. Chem. Eng. 2012, 29, 713–724. [Google Scholar] [CrossRef]
- Zahid, A. Reverse Osmosis: Membrane Technology, Water Chemistry & Industrial Applications; Van Nostrand Reinhold: New York, NY, USA, 1993. [Google Scholar]
- Kwak, S.-Y.; Kim, S.H. Hybrid organic/inorganic reverse osmosis (ro) membrane for bactericidal anti-fouling. 1. Preparation and characterization of tio2 nanoparticle self-assembled aromatic polyamide thin-film-composite (tfc) membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Vrouwenvelder, J.S.; Kooij, D.V.D. Diagnosis of fouling problems of NF and RO membrane installations by a quick scan. Desalination 2003, 153, 121–124. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Schaule, G. Biofouling on membranes—A microbiological approach. Desalination 1988, 70, 95–119. [Google Scholar] [CrossRef]
- Bogino, P.C.; Oliva, M.D.L.M.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef]
- Hooshangi, S.; Bentley, W.E. From unicellular properties to multicellular behavior: Bacteria quorum sensing circuitry and applications. Curr. Opin. Biotechnol. 2008, 19, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Geise, G.M.; Lee, H.-S.; Miller, D.J.; Freeman, B.D.; Mcgrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef] [Green Version]
- McGaughey, A.L.; Gustafson, R.D.; Childress, A.E. Effect of long-term operation on membrane surface characteristics and performance in membrane distillation. J. Membr. Sci. 2017, 543, 143–150. [Google Scholar] [CrossRef]
- Bogler, A.; Bar-Zeev, E. Membrane distillation biofouling: Impact of feedwater temperature on biofilm characteristics and membrane performance. Environ. Sci. Technol. 2018, 52, 10019–10029. [Google Scholar] [CrossRef] [PubMed]
- GunTrägårdh. Membrane cleaning. Desalination 1989, 71, 325–335. [Google Scholar] [CrossRef]
- Zondervan, E.; Roffel, B. Evaluation of different cleaning agents used for cleaning ultra filtration membranes fouled by surface water. J. Membr. Sci. 2007, 304, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Polanska, M.; Huysman, K.; Keer, C.V. Investigation of assimilable organic carbon (AOC) in flemish drinking water. Water Res. 2005, 39, 2259–2266. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Bassler, B.L. Small talk: Cell-to-cell communication in bacteria. Cell 2002, 109, 421–424. [Google Scholar] [CrossRef]
- Ueda, A.; Wood, T.K. Connecting quorum sensing, c-di-gmp, pel polysaccharide, and biofilm formation in pseudomonas aeruginosa through tyrosine phosphatase tpba (PA3885). PLOS Pathog. 2009, 5, e1000483. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Hong, S.; Oh, Y.; Seoul, M.; Kweon, J.; Kim, T. Biofouling of reverse osmosis membranes: Microbial quorum sensing and fouling propensity. Desalination 2009, 247, 303–315. [Google Scholar] [CrossRef]
- Whitehead, H.R.; Hunter, G.J.E.; Cox, G.A. Inhibition of bacterial growth by bacteriophage as distinct from lytic action. J. Gen. Microbiol. 1952, 6, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y. Biological control of microbial attachment: A promising alternative for mitigating membrane biofouling. Appl. Microbiol. Biotechnol. 2010, 86, 825–837. [Google Scholar] [CrossRef]
- Araki, M. Advanced slime control process with bacteriophage. Kogyo Yosui 1986, 332, 25–30. [Google Scholar]
- Allie, Z.; Jacobs, E.P.; Maartens, A.; Swart, P. Enzymatic cleaning of ultrafiltration membranes fouled by abattoir effluent. J. Membr. Sci. 2003, 218, 107–116. [Google Scholar] [CrossRef]
- Thayanukul, P.; Kurisu, F.; Kasuga, I.; Furumai, H. Evaluation of microbial regrowth potential by assimilable organic carbon in various reclaimed water and distribution systems. Water Res. 2013, 47, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hilal, N.; Al-Khatib, L.; Atkin, B.P.; Kochkodan, V.; Potapchenko, N. Photochemical modification of membrane surfaces for (bio)fouling reduction: A nano-scale study using AFM. Desalination 2003, 158, 65–72. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Mahmoudi, E.; Johnson, D.J.; Mohammad, A.W.; Atieh, M.A. Characterization and Separation Performance of a Novel Polyethersulfone Membrane Blended with Acacia Gum. Sci. Rep. 2017, 7, 15831. [Google Scholar] [CrossRef] [Green Version]
- Manawi, Y.; Kochkodan, V.; Mohammad, A.; Atieh, M.A. Arabic gum as a novel pore-forming and hydrophilic agent in polysulfone membranes. J. Membr. Sci. 2017, 529, 95–104. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Mousavi, S.A.; Rashidi, A.; Jafari, A.; Nazmara, S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J. Environ. Health Sci. Eng. 2015, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Ng, L.Y.; Ba-Abbad, M.M.; Mohammad, A.W. Novel nanohybrid polysulfone membrane embedded with silver nanoparticles on graphene oxide nanoplates. Chem. Eng. J. 2015, 277, 1–10. [Google Scholar] [CrossRef]
- Smit, J.; Meijer, C.; Decary, F.; Feltkamp-Vroom, T.M. Paraformaldehyde fixation in immunofluorescence and immunoelectron microscopy: Preservation of tissue and cell surface membrane antigens. J. Immunol. Methods 1974, 6, 93–98. [Google Scholar] [CrossRef]
- Lanier, L.; Warner, N. Paraformaldehyde fixation of hematopoietic cells for quantitative flow cytometry (FACS) analysis. J. Immunol. Methods 1981, 47, 25–30. [Google Scholar] [CrossRef]
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.; Pettersson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat. Immunol. 2004, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.E.; Searcy, C. Comparison of mannitol salt agar and blood agar plates for identification and susceptibility testing of Staphylococcus aureus in specimens from cystic fibrosis patients. J. Clin. Microbiol. 2006, 44, 4545–4546. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Wright, J.W. Spot indole test: Evaluation of four reagents. J. Clin. Microbiol. 1982, 15, 589–592. [Google Scholar]
- Yeo, H.-T.; Lee, S.-T.; Han, M.-J. Role of a polymer additive in casting solution in preparation of phase inversion polysulfone membranes. J. Chem. Eng. Jpn. 2000, 33, 180–184. [Google Scholar] [CrossRef]
- Van de Witte, P.; Dijkstra, P.J.; Van den Berg, J.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Wienk, I.; Boom, R.; Beerlage, M.; Bulte, A.; Smolders, C.; Strathmann, H. Recent advances in the formation of phase inversion membranes made from amorphous or semi-crystalline polymers. J. Membr. Sci. 1996, 113, 361–371. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B. Preparation of poly (vinylidene fluoride)(pvdf) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 2005, 46, 7701–7706. [Google Scholar] [CrossRef]
- Duan, L.; Huang, W.; Zhang, Y. High-flux, antibacterial ultrafiltration membranes by facile blending with N-halamine grafted halloysite nanotubes. R. Soc. Chem. Adv. 2015, 5, 6666–6674. [Google Scholar] [CrossRef]
- Montenegro, M.A.; Boiero, M.L.; Valle, L.; Borsarelli, C.D. Gum Arabic: More than an edible emulsifier. In Products and Applications of Biopolymers; InTech: London, UK, 2012. [Google Scholar]
- Qu, P.; Tang, H.; Gao, Y.; Zhang, L.; Wang, S. Polyethersulfone composite membrane blended with cellulose fibrils. BioResources 2010, 5, 2323–2336. [Google Scholar]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, G. Fabrication of high-performance ultrafiltration membranes using zwitterionic carbon nanotubes and polyethersulfone. High Perform. Polym. 2018, 30, 602–611. [Google Scholar] [CrossRef]
- Liao, C.; Zhao, J.; Yu, P.; Tong, H.; Luo, Y. Synthesis and characterization of low content of different SiO2 materials composite poly (vinylidene fluoride) ultrafiltration membranes. Desalination 2012, 285, 117–122. [Google Scholar] [CrossRef]
- Leo, C.; Lee, W.C.; Ahmad, A.; Mohammad, A.W. Polysulfone membranes blended with ZnO nanoparticles for reducing fouling by oleic acid. Sep. Purif. Technol. 2012, 89, 51–56. [Google Scholar] [CrossRef]
- Roux, S.; Jacobs, E.; Van Reenen, A.; Morkel, C.; Meincken, M. Hydrophilisation of polysulphone ultrafiltration membranes by incorporation of branched PEO-block-PSU copolymers. J. Membr. Sci. 2006, 276, 8–15. [Google Scholar] [CrossRef]
- Niu, F.; Su, Y.; Liu, Y.; Wang, G.; Zhang, Y.; Yang, Y. Ovalbumin–gum arabic interactions: Effect of pH, temperature, salt, biopolymers ratio and total concentration. Colloids Surf. B Biointerfaces 2014, 113, 477–482. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef]
- Hu, Q.; Gerhard, H.; Upadhyaya, I.; Venkitanarayanan, K.; Luo, Y. Antimicrobial eugenol nanoemulsion prepared by gum arabic and lecithin and evaluation of drying technologies. Int. J. Biol. Macromol. 2016, 87, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Roymon, M.G. Validation of antibacterial activity of saponin against diarreagenic E. coli isolated from leaves and bark of Acacia arabica. J. Phytol. 2012, 4, 21–23. [Google Scholar]
- Alawi, S.M.A.; Hossain, M.A.; Abusham, A.A. Antimicrobial and cytotoxic comparative study of different extracts of Omani and Sudanese Gum acacia. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 22–26. [Google Scholar] [CrossRef]
- Todkar, S.; Todkar, R.; Kavathekar, V.; Kulkarni, S.; Kulkarni, A. Secondary metabolite profiling of Acacia concinna. Biosci. Biotechnol. Res. Asia 2011, 8, 653–660. [Google Scholar] [CrossRef]
- Ridgway, H.F.; Rigby, M.G.; Argo, D.G. Bacterial adhesion and fouling of reverse osmosis membranes. J.-Am. Water Works Assoc. 1985, 77, 97–106. [Google Scholar] [CrossRef]
- Blenkinsopp, S.; Costerton, J. Understanding bacterial biofilms. Trends Biotechnol. 1991, 9, 138–143. [Google Scholar] [CrossRef]
- Kenyon, E.M.; Hughes, M.F. A concise review of the toxicity and carcinogenicity of dimethylarsinic acid. Toxicology 2001, 160, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Wrigh, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef]
- Lambert, A.P. Bacterial resistance to antibiotics: Modified target sites. Adv. Drug Deliv. Rev. 2005, 57, 1471–1485. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]









| Bacterial Strain | Gram | Origin | Identification |
|---|---|---|---|
| Staphylococcus aureus | Gram-positive | Clinical samples | Isolated on mannitol salted agar and confirmed by biochemical crystal test [37] using Biomic V3 (Giles scientific, USA). |
| Escherichia coli | Gram-negative | Sheep rectal samples | Isolated on selective medium CHROMagar™ (BD–Medysinal FZCO, Dubai, and UAE). Then incubated at 37 °C for 18 h. The single typical E. coli colonies (green color with a smooth surface) were randomly selected and subsequently streaked onto blood agar plates, and then incubated at 37 °C for 18 h to obtain pure single colonies. For further confirmation, the colonies were transferred onto MacConkey agar plates (BD-Medysinal FZCO) and then blood agar plates (BD Medysinal FZCO), followed by an indole spot test (Remel, Thermo Fisher Scientific, Lenexa, KS) for lactose fermenter isolates and biochemical reactions using Crystal ™ Enteric/ nonfermenter id KIT, BD [38]. Results were interpreted by means of Biomic V3 (Giles scientific, USA). |
| Klebsiella pneumonia | Gram-negative | ATCC reference strain | K. pneumonia ATCC, 13883 (Thermoscientific, Kent, UK) |
| Pseudomonas aeruginosa | Gram-negative | ATCC reference strain | P. aeruginosa ATCC, BAA-1744 (Thermoscientific, Kent, UK) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabri, S.; Najjar, A.; Manawi, Y.; Eltai, N.O.; Al-Thani, A.; Atieh, M.A.; Kochkodan, V. Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum. Membranes 2019, 9, 29. https://doi.org/10.3390/membranes9020029
Sabri S, Najjar A, Manawi Y, Eltai NO, Al-Thani A, Atieh MA, Kochkodan V. Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum. Membranes. 2019; 9(2):29. https://doi.org/10.3390/membranes9020029
Chicago/Turabian StyleSabri, Souhir, Ahmad Najjar, Yehia Manawi, Nahla Omer Eltai, Asma Al-Thani, Muataz Ali Atieh, and Viktor Kochkodan. 2019. "Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum" Membranes 9, no. 2: 29. https://doi.org/10.3390/membranes9020029
APA StyleSabri, S., Najjar, A., Manawi, Y., Eltai, N. O., Al-Thani, A., Atieh, M. A., & Kochkodan, V. (2019). Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum. Membranes, 9(2), 29. https://doi.org/10.3390/membranes9020029





