Differences in Vascular Density between Detached and Nondetached Areas in Eyes with Rhegmatogenous Retinal Detachment
Abstract
:1. Introduction
2. Patients and Methods
2.1. Subjects and Testing Protocol
2.2. Surgical Techniques
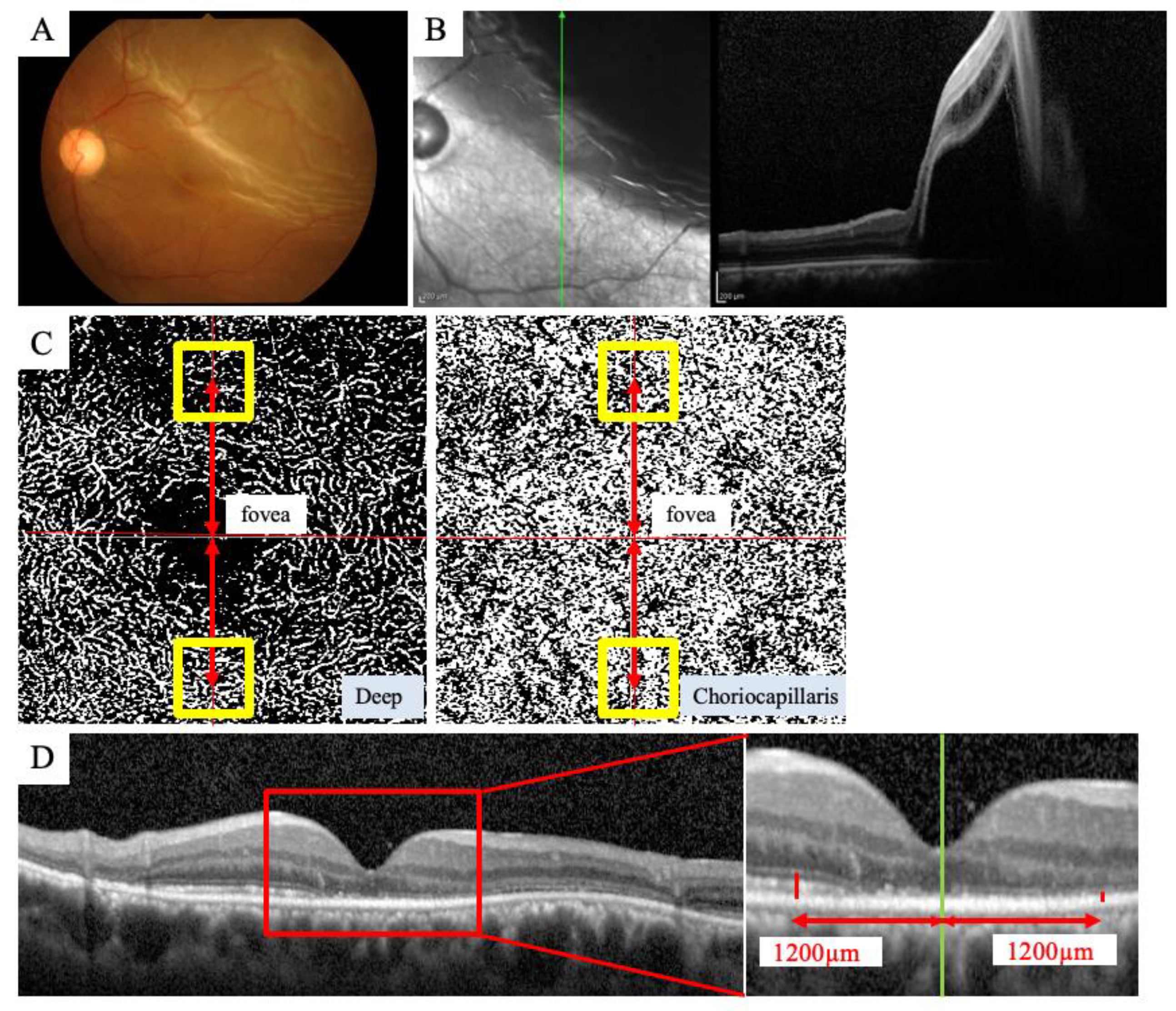
2.3. OCTA Imaging
2.4. SD-OCT Imaging
2.5. Statistical Analyses
3. Results
3.1. Clinical Characteristics of the Subjects
3.2. Comparison of VD in the DCP and CCP between the Detached Area and Nondetached Areas
3.3. Comparison of ELM-EZ and EZ-RPE Thickness between Detached Area and Nondetached Areas
3.4. Correlation of VD and Retinal Thickness between Detached Area and Nondetached Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RRD | rhegmatogenous retinal detachment |
| RPE | retinal pigment epithelium |
| OCT | optical coherence tomography |
| SD | spectral domain |
| EZ | ellipsoid zone |
| ELM | external limiting membrane |
| OCTA | OCT angiography |
| SCP | superficial capillary plexus |
| DCP | deep capillary plexus |
| CCP | choriocapillaris plexus |
| VD | vessel density |
| BCVA | best-corrected visual acuity |
| IOP | intraocular pressure |
| LogMAR | logarithm of the minimum angle of resolution |
| SS | swept-source |
References
- Machemer, R. The importance of fluid absorption, traction, intraocular currents, and chorioretinal scars in the therapy of rhegmatogenous retinal detachments. XLI Edward Jackson memorial lecture. Am. J. Ophthalmol. 1984, 98, 681–693. [Google Scholar] [CrossRef]
- Yang, L.; Bula, D.; Arroyo, J.G.; Chen, D.F. Preventing retinal detachment-associated photoreceptor cell loss in Bax-deficient mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.P.; Charteris, D.G.; Sethi, C.S.; Fisher, S.K. Animal models of retinal detachment and reattachment: Identifying cellular events that may affect visual recovery. Eye 2002, 16, 375–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo, J.G.; Yang, L.; Bula, D.; Chen, D.F. Photoreceptor apoptosis in human retinal detachment. Am. J. Ophthalmol. 2005, 139, 605–610. [Google Scholar] [CrossRef]
- Eibenberger, K.; Georgopoulos, M.; Rezar-Dreindl, S.; Schmidt-Erfurth, U.; Sacu, S. Development of Surgical Management in Primary Rhegmatogenous Retinal Detachment Treatment from 2009 to 2015. Curr. Eye Res. 2018, 43, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Vrabec, T.R.; Baumal, C.R. Demarcation laser photocoagulation of selected macula-sparing rhegmatogenous retinal detachments. Ophthalmology 2000, 107, 1063–1067. [Google Scholar] [CrossRef]
- Jung, J.J.; Cheng, J.; Pan, J.Y.; Brinton, D.A.; Hoang, Q.V. Anatomic, Visual, and Financial Outcomes for Traditional and Nontraditional Primary Pneumatic Retinopexy for Retinal Detachment. Am. J. Ophthalmol. 2019, 200, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Iwase, T.; Yamamoto, K.; Ra, E.; Murotani, K.; Matsui, S.; Terasaki, H. Association Between Photoreceptor Regeneration and Visual Acuity Following Surgery for Rhegmatogenous Retinal Detachment. Investig. Ophthalmol. Vis. Sci. 2016, 57, 889–898. [Google Scholar] [CrossRef] [Green Version]
- Park, D.H.; Choi, K.S.; Sun, H.J.; Lee, S.J. Factors associated with visual outcome after macula-off rhegmatogenous retinal detachment surgery. Retina 2018, 38, 137–147. [Google Scholar] [CrossRef]
- Hasegawa, T.; Ueda, T.; Okamoto, M.; Ogata, N. Relationship between presence of foveal bulge in optical coherence tomographic images and visual acuity after rhegmatogenous retinal detachment repair. Retina 2014, 34, 1848–1853. [Google Scholar] [CrossRef]
- Woo, J.M.; Yoon, Y.S.; Woo, J.E.; Min, J.K. Foveal Avascular Zone Area Changes Analyzed Using OCT Angiography after Successful Rhegmatogenous Retinal Detachment Repair. Curr. Eye Res. 2018, 43, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.H.; Cho, H.; Kim, D.R.; Kang, M.H.; Shin, Y.U.; Seong, M. Changes in Retinal Vessel and Retinal Layer Thickness After Vitrectomy in Retinal Detachment via Swept-Source OCT Angiography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resch, M.D.; Balogh, A.; Lászik, G.; Nagy, Z.Z.; Papp, A. Association between retinal vessel density and postoperative time after primary repair of rhegmatogenous retinal detachment. PLoS ONE 2021, 16, e0258126. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, V.; Ortisi, E.; Scollo, D.; Reibaldi, M.; Russo, A.; Pizzo, A.; Faro, G.; Macchi, I.; Fallico, M.; Toro, M.D.; et al. Vascular changes after vitrectomy for rhegmatogenous retinal detachment: Optical coherence tomography angiography study. Acta Ophthalmol. 2019, 98, e563–e569. [Google Scholar] [CrossRef]
- McKay, K.M.; Vingopoulos, F.; Wang, J.C.; Papakostas, T.D.; Silverman, R.F.; Marmalidou, A.; Lains, I.; Eliott, D.; Vavvas, D.G.; Kim, L.A.; et al. Retinal Microvasculature Changes After Repair of Macula-off Retinal Detachment Assessed with Optical Coherence Tomography Angiography. Clin. Ophthalmol. 2020, 14, 1759–1767. [Google Scholar] [CrossRef]
- Laíns, I.; Wang, J.C.; Cui, Y.; Katz, R.; Vingopoulos, F.; Staurenghi, G.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog. Retin Eye Res. 2021, 84, 100951. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Sun, X.; Ma, Y.; Sun, T. Macular perfusion changes assessed with optical coherence tomography angiography after vitrectomy for rhegmatogenous retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Machemer, R.; Aaberg, T.M.; Freeman, H.M.; Irvine, A.R.; Lean, J.S.; Michels, R.M. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am. J. Ophthalmol. 1991, 112, 159–165. [Google Scholar] [CrossRef]
- Shoji, T.; Yoshikawa, Y.; Kanno, J.; Ishii, H.; Ibuki, H.; Ozaki, K.; Kimura, I.; Shinoda, K. Reproducibility of Macular Vessel Density Calculations Via Imaging With Two Different Swept-Source Optical Coherence Tomography Angiography Systems. Transl. Vis. Sci. Technol. 2018, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.M.G.; Teo, K.Y.C.; Tun, S.B.B.; Busoy, J.M.; Veluchamy, A.B.; Spaide, R.F. Differential reperfusion patterns in retinal vascular plexuses following increase in intraocular pressure an OCT angiography study. Sci. Rep. 2020, 10, 16505. [Google Scholar] [CrossRef] [PubMed]
- Lyssek-Boroń, A.; Wylęgała, A.; Krysik, K.; Janiszewska-Bil, D.; Wylęgała, E.; Grabarek, B.O.; Dobrowolski, D. Assessment of Vascular Changes in Patients after Pars Plana Vitrectomy Surgery Due to Macula-Off Rhegmatogenous Retinal Detachment. J. Clin. Med. 2021, 10, 5054. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Ghasemi Falavarjani, K.; Akil, H.; Sadda, S.R. Impact of image quality on OCT angiography based quantitative measurements. Int. J. Retin. Vitr. 2017, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Czakó, C.; István, L.; Ecsedy, M.; Récsán, Z.; Sándor, G.; Benyó, F.; Horváth, H.; Papp, A.; Resch, M.; Borbándy, Á.; et al. The effect of image quality on the reliability of OCT angiography measurements in patients with diabetes. Int. J. Retina Vitr. 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaderli, S.T.; Karalezli, A.; Sul, S. Microvascular retinal alterations in rhegmatogenous retinal detachment after pneumatic retinopexy. Acta Ophthalmol. 2021, 99, 383–389. [Google Scholar] [CrossRef]
- Ricker, L.J.; Kijlstra, A.; de Jager, W.; Liem, A.T.; Hendrikse, F.; La Heij, E.C. Chemokine levels in subretinal fluid obtained during scleral buckling surgery after rhegmatogenous retinal detachment. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Quintyn, J.C.; Brasseur, G. Subretinal fluid in primary rhegmatogenous retinal detachment: Physiopathology and composition. Surv. Ophthalmol. 2004, 49, 96–108. [Google Scholar] [CrossRef]
- Cardillo Piccolino, F. Vascular changes in rhegmatogenous retinal detachment. Ophthalmologica 1983, 186, 17–24. [Google Scholar] [CrossRef]
- Iwase, T.; Kobayashi, M.; Yamamoto, K.; Yanagida, K.; Ra, E.; Terasaki, H. Changes in Blood Flow on Optic Nerve Head After Vitrectomy for Rhegmatogenous Retinal Detachment. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6223–6233. [Google Scholar] [CrossRef] [Green Version]
- Ra, E.; Ito, Y.; Kawano, K.; Iwase, T.; Kaneko, H.; Ueno, S.; Yasuda, S.; Kataoka, K.; Terasaki, H. Regeneration of Photoreceptor Outer Segments After Scleral Buckling Surgery for Rhegmatogenous Retinal Detachment. Am. J. Ophthalmol. 2017, 177, 17–26. [Google Scholar] [CrossRef]
- Lo, A.C.; Woo, T.T.; Wong, R.L.; Wong, D. Apoptosis and other cell death mechanisms after retinal detachment: Implications for photoreceptor rescue. Ophthalmologica 2011, 226 (Suppl. 1), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dodo, Y.; Suzuma, K.; Ishihara, K.; Yoshitake, S.; Fujimoto, M.; Yoshitake, T.; Miwa, Y.; Murakami, T. Clinical relevance of reduced decorrelation signals in the diabetic inner choroid on optical coherence tomography angiography. Sci. Rep. 2017, 7, 5227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guérin, C.J.; Lewis, G.P.; Fisher, S.K.; Anderson, D.H. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Investig. Ophthalmol. Vis. Sci. 1993, 34, 175–183. [Google Scholar]




| Clinical Characteristics of the RRD Eyes | |
|---|---|
| Age (y) | 55.8 ± 12.3 |
| Sex (male/female) | 10/3 |
| Duration between symptoms and surgery (days) | 9.1 ± 10.1 |
| Axial length (mm) | 25.4 ± 1.6 |
| Preoperative IOP (mmHg) | 14.2 ± 2.2 |
| Preoperative BCVA (Log MAR) | 0.33 ± 0.44 |
| Preoperative BCVA (Log MAR) | 0.01 ± 0.14 |
| Surgical procedure (PPV/buckle) | 11/2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, M.; Iwase, T. Differences in Vascular Density between Detached and Nondetached Areas in Eyes with Rhegmatogenous Retinal Detachment. J. Clin. Med. 2022, 11, 2881. https://doi.org/10.3390/jcm11102881
Sato M, Iwase T. Differences in Vascular Density between Detached and Nondetached Areas in Eyes with Rhegmatogenous Retinal Detachment. Journal of Clinical Medicine. 2022; 11(10):2881. https://doi.org/10.3390/jcm11102881
Chicago/Turabian StyleSato, Mariko, and Takeshi Iwase. 2022. "Differences in Vascular Density between Detached and Nondetached Areas in Eyes with Rhegmatogenous Retinal Detachment" Journal of Clinical Medicine 11, no. 10: 2881. https://doi.org/10.3390/jcm11102881
APA StyleSato, M., & Iwase, T. (2022). Differences in Vascular Density between Detached and Nondetached Areas in Eyes with Rhegmatogenous Retinal Detachment. Journal of Clinical Medicine, 11(10), 2881. https://doi.org/10.3390/jcm11102881






