Constrictive Pericarditis and Protein-Losing Enteropathies: Exploring the Heart–Gut Axis
Abstract
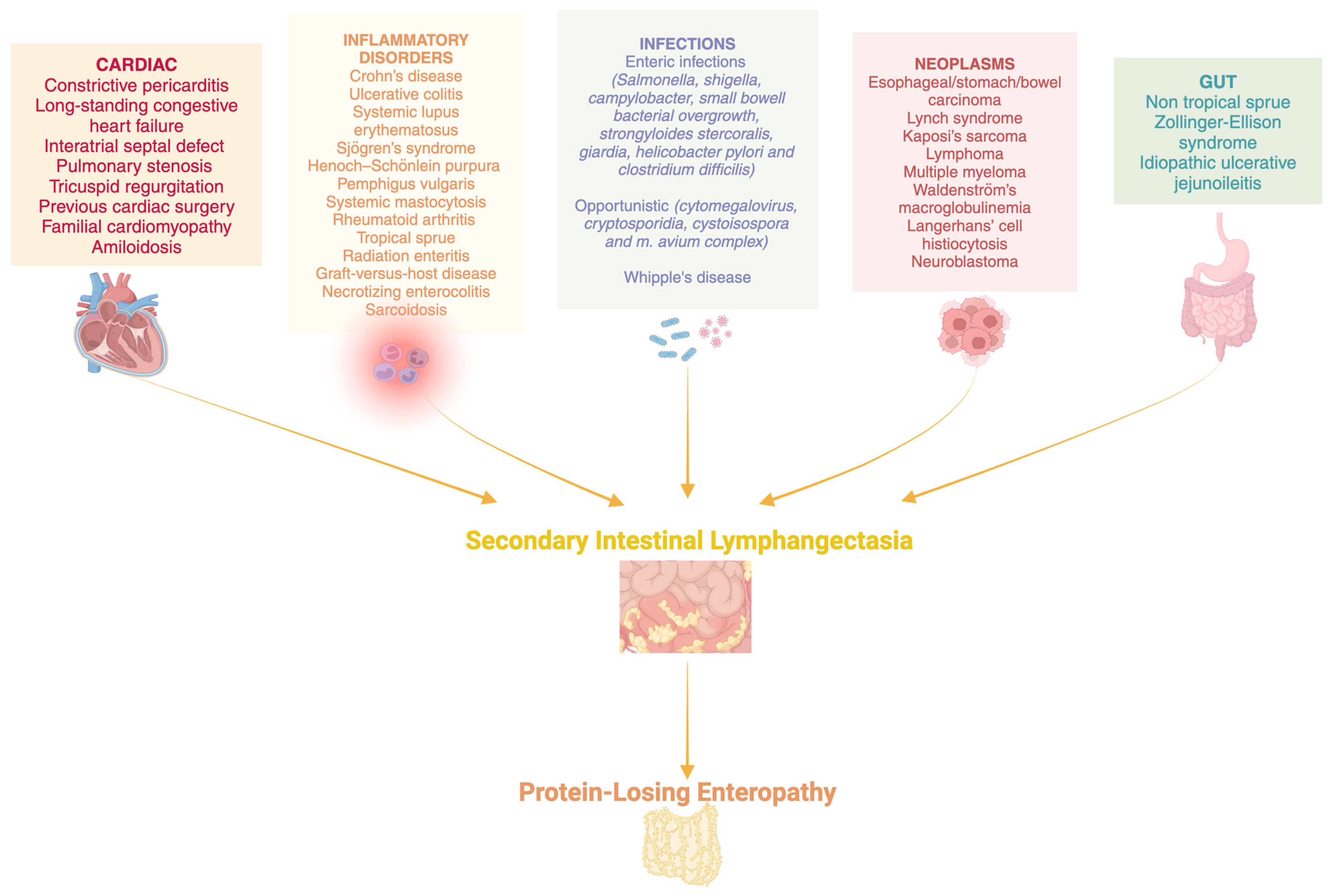
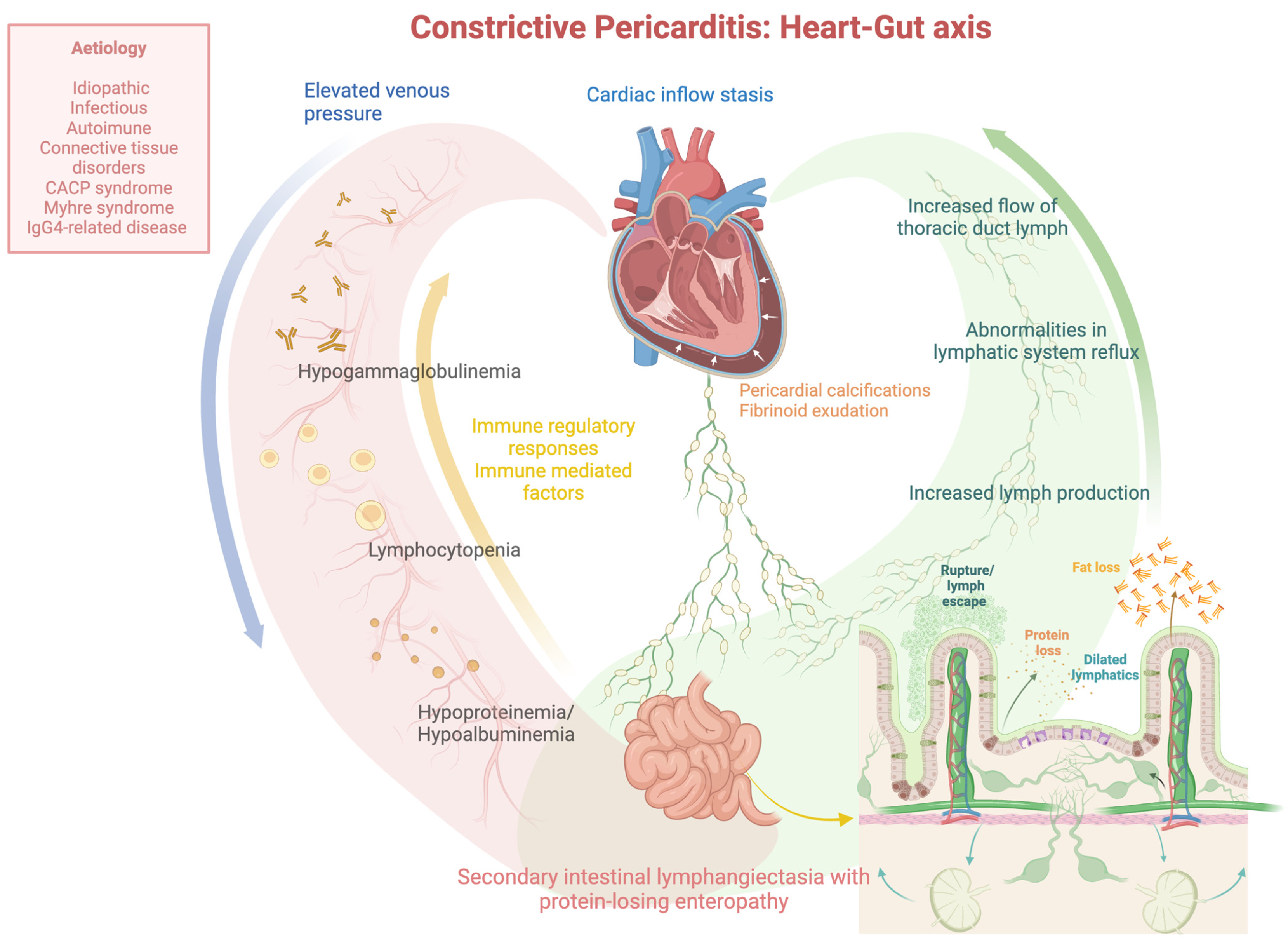
:1. Introduction
2. Materials and Methods
3. Results
3.1. Pediatric Cases
| AUTHOR/YEAR | Kumpe, D.A., 1975 (Ref. [9]) | Nelson, D.L., 1975 (Ref. [16]) | Savilahti, E., 1985 (Ref. [17]) | Peters, B., 2016 (Ref. [19]) | Schmitt, E.G., 2021 (Ref. [3]) | Xi, Y., 2022 (Ref. [20]) |
|---|---|---|---|---|---|---|
| AGE (years)/GENDER | 14-M | 15-M | 5-F | 10-F | 14-F | 14-M |
| COMORBIDITIES | N | cutaneous anergy, immunologic deficiency | diabetes, autoimmune jejunitis, unexplained immunodeficiency | congenital camptodactyly: coxa vara, osteopenia and flattened joints (X-ray) | N | pulmonary tuberculosis |
| ONSET OF SYMPTOMS | 1 month | 1 year | 3 months | NA | 3 weeks | NA |
| ENDOSCOPY | Y | Y | Y | NA | NA | NA |
| VIDEOCAPSULE | N | N | N | N | N | N |
| PLE LOCATION | Jejunum and ileum | Jejunum | Jejunum | NA | NA | NA |
| INTESTINAL HISTOLOGY | intestinal lymphangiectasia | dilated submucosal lymphatic vessels and paracortical lymphocytic depletion (lymph node biopsies) | total villous atrophy, reduced crypt cell proliferation | NA | NA | NA |
| LIVER HISTOLOGY | no cirrhosis | N | N | liver fibrosis | N | liver fibrosis |
| X-RAY—CHEST | pulmonary venous distention, interstitial pulmonary oedema, pleural effusions | N | N | N | mediastinum mass | N |
| X-RAY—BARIUM ENEMA | diffusely thickened mucosal folds | upper GI: mucosal edema | N | N | N | N |
| STOOL ALPHA1-ANTITRYPSIN CLEARANCE | N | N | N | increased | increased | N |
| RADIOISOTOPIC TECHNIQUES | 51Cr albumin stool clearance, increased | 51Cr albumin stool clearance, increased | N | N | N | Tc-GSA scintigraphy, radionuclide accumulation in the intestine |
| ECG | NA | low voltage in all leads | typical T-wave changes | NA | NA | NA |
| ECOCARDIOGRAPHY | N | N | pericardial effusion | pericardialeffusion | thickened pericardium | decreased cardiac function |
| CT SCAN | N | N | N | N | thickened pericardium | lung consolidation (enlarged mediastinal lymph nodes), ascites, pleural/pericardial effusions |
| MRI | N | N | N | thickened pericardium | thickened pericardium | N |
| RIGHT HEART CATHETERIZATION | Y | Y | N | Y | Y | N |
| PERICARDIECTOMY | Y | Y | N | Y | N | Y |
| CONSTRICTIVE PERICARDITIS—HISTOLOGY | Y | Y | N | Y | N | Y |
| ETIOLOGY | unknown | unknown | underlying autoimmune disease | CACP syndrome | inflammatory myofibroblastic tumor (mediastinum) | tuberculosis |
| RESPONSE TO PERICARDIECTOMY | edema disappeared, radioactive albumin turnover returned to normal, as did his cardiac catheterization data and intestinal biopsy after 6 months | intestinal lymphangiectasia and PLE reversed, immune function gradually improved after 6 months | no surgery: unresponsive to gluten-free diet, total parental nutrition, immunosuppression, death of mycotic sepsis | peripheral edema and joint swellings disappeared in one year | no surgery: despite a temporary positive clinical response to medical treatment, she developed clinical signs of right heart failure on follow-up | asymptomatic, serum albumin levels increased during the subsequent 2 years |
3.2. Adult Cases
4. Comparisons between Adult and Pediatric Cases
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nikolaidis, N.; Tziomalos, K.; Giouleme, O.; Gkisakis, D.; Kokkinomagoulou, A.; Karatzas, N.; Papanikolaou, A.; Tsitourides, I.; Eugenidis, N.; Kontopoulos, A. Protein-losing enteropathy as the principal manifestation of constrictive pericarditis. J. Gen. Intern. Med. 2005, 20, C5–C7. [Google Scholar] [CrossRef]
- Kikuchi, S.; Ohte, N.; Wakami, K.; Goto, T.; Kimura, G. Low cardiac output in a case of constrictive pericarditis with protein-losing enteropathy. Intern. Med. 2013, 52, 75–79. [Google Scholar] [CrossRef]
- Schmitt, E.G.; Dalal, A.S.; Kothari, A.; Kitcharoensakkul, M. The Heart of the Matter: Secondary Hypogammaglobulinemia and Constrictive Pericarditis. Pediatrics 2021, 147, e2020021808. [Google Scholar] [CrossRef]
- Ozen, A.; Lenardo, M.J. Protein-Losing Enteropathy. N. Engl. J. Med. 2023, 389, 733–748. [Google Scholar] [CrossRef]
- Müller, C.; Globits, S.; Glogar, D.; Klepetko, W.; Knoflach, P. Constrictive pericarditis without typical haemodynamic changes as a cause of oedema formation due to protein-losing enteropathy. Eur. Heart J. 1991, 12, 1140–1143. [Google Scholar] [CrossRef]
- Moriyama, H.; Kohno, T.; Nishiyama, T.; Hattori, O.; Maekawa, Y.; Yoshida, K.; Murata, M.; Okamoto, K.; Sano, M.; Shimizu, H.; et al. Constrictive Pericarditis and Protein-Losing Enteropathy: Is Extremely Severe Hypoalbuminemia Reversible by Pericardiectomy? Circ. Heart Fail. 2016, 9, e003666. [Google Scholar] [CrossRef]
- Petersen, V.P.; Hastrup, J. Protein-losing enteropathy in constrictive pericarditis. Acta Med. Scand. 1963, 173, 401–410. [Google Scholar] [CrossRef]
- Chamouard, P.; Nehme-Schuster, H.; Simler, J.M.; Finck, G.; Baumann, R.; Pasquali, J.L. Videocapsule endoscopy is useful for the diagnosis of intestinal lymphangiectasia. Dig. Liver Dis. 2006, 38, 699–703. [Google Scholar] [CrossRef]
- Kumpe, D.A.; Jaffe, R.B.; Waldmann, T.A.; Weinstein, M.A. Constrictive pericarditis and protein losing enteropathy. An imitator of intestinal lymphangiectasis. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1975, 124, 365–373. [Google Scholar] [CrossRef]
- Kaihara, S.; Nishimura, H.; Aoyagi, T.; Kameda, H.; Ueda, H. Protein-losing gastroenteropathy as cause of hypoproteinemia in constrictive pericarditis. Jpn. Heart J. 1963, 4, 386–394. [Google Scholar] [CrossRef]
- Strober, W.; Wochner, R.D.; Carbone, P.P.; Waldmann, T.A. Intestinal lymphangiectasia: A protein-losing enteropathy with hypogammaglobulinemia, lymphocytopenia and impaired homograft rejection. J. Clin. Investig. 1967, 46, 1643–1656. [Google Scholar] [CrossRef]
- Weiden, P.L.; Blaese, R.M.; Strober, W.; Block, J.B.; Waldmann, T.A. Impaired lymphocyte transformation in intestinal lymphangiectasia: Evidence for at least two functionally distinct lymphocyte populations in man. J. Clin. Investig. 1972, 51, 1319–1325. [Google Scholar] [CrossRef]
- Jimenez Diaz, C.; Linazasoro, J.M.; Lopez-Garcia, E.; Ramirez Guedes, J. On hypoalbuminemia in pericarditis: Mechanism and repercussions. Studies with labeled proteins on the rate of loss and renewal of the albumin of the plasma. Rev. Clin. Esp. 1960, 77, 252–256. [Google Scholar]
- Davidson, J.D.; Waldmann, T.A.; Goodman, D.S.; Gordon, R.S., Jr. Protein-losing gastroenteropathy in congestive heart-failure. Lancet 1961, 1, 899–902. [Google Scholar] [CrossRef]
- Plauth, W.H., Jr.; Waldmann, T.A.; Wochner, R.D.; Braunwald, N.S.; Braunwald, E. Protein-losing enteropathy secondary to constrictive pericarditis in childhood. Pediatrics 1964, 34, 636–648. [Google Scholar] [CrossRef]
- Nelson, D.L.; Blaese, R.M.; Strober, W.; Bruce, R.; Waldmann, T.A. Constrictive pericarditis, intestinal lymphangiectasia, and reversible immunologic deficiency. J. Pediatr. 1975, 86, 548–554. [Google Scholar] [CrossRef]
- Savilahti, E.; Pelkonen, P.; Holmberg, C.; Perkkiö, M.; Unsworth, J. Fatal unresponsive villous atrophy of the jejunum, connective tissue disease and diabetes in a girl with intestinal epithelial cell antibody. Acta Paediatr. Scand. 1985, 74, 472–476. [Google Scholar] [CrossRef]
- Siurala, M.; Julkunen, H.; Toivonen, S.; Pelkonen, R.; Saxen, E.; Pitkanen, E. Digestive tract in collagen diseases. Acta Med. Scand. 1965, 178, 13–25. [Google Scholar] [CrossRef]
- Peters, B.; Schuurs-Hoeijmakers, J.H.M.; Fuijkschot, J.; Reimer, A.; van der Flier, M.; Lugtenberg, D.; Hoppenreijs, E.P. Protein-losing enteropathy in camptodactyly-arthropathy-coxa vara-pericarditis (CACP) syndrome. Pediatr. Rheumatol. Online J. 2016, 14, 32. [Google Scholar] [CrossRef]
- Xi, Y.; Chen, Z.; Hao, K.; Liu, X. Case Report: Protein-Losing Enteropathy in Association With Tuberculosis-Related Constrictive Pericarditis. Front. Pediatr. 2022, 10, 875032. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.C.; Kiraly, L.; El Badaoui, H. Constrictive pericarditis: Rare but reversible cause of protein losing enteropathy. Cardiol. Young 2022, 33, 76–78. [Google Scholar] [CrossRef]
- Wilkinson, P.; Pinto, B.; Senior, J.R. Reversible protein-losing enteropathy with intestinal lymphangiectasia secondary to chronic constrictive pericarditis. N. Engl. J. Med. 1965, 273, 1178–1181. [Google Scholar] [CrossRef]
- Meijers, B.K.; Schalla, S.; Eerens, F.; Van Suylen, R.J.; Broers, B.; Cheriex, E.M.; Smedema, J.P. Protein-losing enteropathy in association with constrictive pericarditis. Int. J. Cardiovasc. Imaging 2006, 22, 389–392. [Google Scholar] [CrossRef]
- Strober, W.; Cohen, L.S.; Waldmann, T.A.; Braunwald, E. Tricuspid regurgitation. A newly recognized cause of protein-losing enteropathy, lymphocytopenia and immunologic deficiency. Am. J. Med. 1968, 44, 842–850. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Steinfeld, J.L.; Dutcher, T.F.; Davidson, J.D.; Gordon, R.S., Jr. The role of the gastrointestinal system in “idiopathic hypoproteinemia”. Gastroenterology 1961, 41, 197–207. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Cause of hypoalbuminaemia in patients with gastrointestinal and cardiac disease. Lancet 1962, 1, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Wochner, R.D.; Strober, W. The role of the gastrointestinal tract in plasma protein metabolism. Studies with 51Cr-albumin. Am. J. Med. 1969, 46, 275–285. [Google Scholar] [CrossRef]
- Palmer, H.M.; Cocking, J.B.; Emmanuel, I.G. Irradiation-induced constrictive pericarditis in intestinal lymphangiectasis. Br. Med. J. 1970, 4, 783–784. [Google Scholar] [CrossRef] [PubMed]
- George, T.J.; Arnaoutakis, G.J.; Beaty, C.A.; Kilic, A.; Baumgartner, W.A.; Conte, J.V. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann. Thorac. Surg. 2012, 94, 445–451. [Google Scholar] [PubMed]
| AUTHOR/YEAR | Petersen, V.P., 1963 (Ref. [7]) | Wilkinson, P., 1965 (Ref. [22]) | Müller, C., 1991 (Ref. [5]) | Nikolaidis, N., 2005 (Ref. [1]) | Meijers, B.K., 2006 (Ref. [23]) | Chamouard, P., 2006 (Ref. [8]) | Kikuchi, S., 2013 (Ref. [2]) | Moriyama, H., 2016 (Ref. [6]) |
|---|---|---|---|---|---|---|---|---|
| AGE (years)/GENDER | 25/M | 59/M | 41-M | 76-M | 74-M | 35-F | >70-M | 38-M |
| COMORBIDITIES | cardiac cirrhosis | congestive heart failure | N | tuberculous nephritis history, atrial fibrillation, congestive heart failure, diabetes | past coronary bypass grafting, atrial fibrillation | pleuropericarditis history | N | mitral valve replacement history (severe mitral regurgitation/infective endocarditis) |
| ONSET OF SYMPTOMS | 1 year | 2 years | N | 2 months | NA | 19 years | NA | 6 years |
| ENDOSCOPY | N | Y | Y | Y | Y | Y | Y | Y |
| VIDEOCAPSULE | N | N | N | N | N | mucosal edema, white curved lines associated with a combed aspect | N | N |
| PLE LOCATION | Jejunum | Jejunum | Jejunum | Duodenum | NA | Jejunum | NA | NA |
| INTESTINAL HISTOLOGY | autopsy: thickening/edema of peritoneum and small intestine; enlarged/swollen valves of Kerkring and villi containing foamy lipophages (expansion of the mucosal/submucosal lymphatic vessels) | dilatation of the lymphatic vessels of the villi | normal | markedly dilated lymphatics (intestinal lymphangiectasia) | N | NA | normal | NA |
| LIVER HISTOLOGY | mild portal cirrhosis (cardiac) | no cirrhosis | N | liver fibrosis | N | N | N | N |
| X-RAY—CHEST | pericardial calcifications | cardiomegaly | pleuropericardial adhesions | cardiomegaly, pleural effusions | N | N | pleural effusions | N |
| X-RAY—BARIUM ENEMA | proximal jejunal loops, slightly dilated; coarse mucosal folds | N | normal | N | N | non-specific coarsening of jejunum mucosal folds | N | N |
| FECAL ALPHA1-ANTITRYPSIN CLEARANCE | N | N | increased | N | N | increased | N | N |
| RADIOISOTOPIC TECHNIQUES | 131I-polyvinylpyrrolidone, and 131I-albumin tests: reduced pool of exchangeable/high fractional turnover of serum-albumin | 131I-albumin tests: reduced pool of exchangeable; 51Cr albumin stool clearance, increased | 51Cr albumin stool clearance, increased | Tc-GSA scintigraphy, radionuclide accumulation in the intestine | Tc-GSA scintigraphy, radionuclide accumulation in the intestine | N | Tc-GSA scintigraphy, radionuclide accumulation in the intestine | Tc-GSA scintigraphy, radionuclide accumulation in the intestine |
| ECG | low voltage and inversion of T-waves | wandering atrial pacemaker, low-voltage QRS | NA | atrial fibrillation with occasional premature ventricular complexes | NA | NA | NA | NA |
| ECOCARDIOGRAPHY | N | N | thickened pericardium | N | thickened pericardium | inferior vena cava ectasia | thickened pericardium | thickened pericardium with calcifications, biatrial enlargement, mild mitral valve regurgitation |
| CT SCAN | N | N | N | thickened/calcified pericardium, hepatomegaly | N | slight ascites | thickened pericardium | bilateral pleural effusion and thickened pericardium |
| MRI | N | N | thickened pericardium, tubular-shaped right ventricle | thickened and calcified pericardium, hepatomegaly | N | thickened pericardium | N | N |
| RIGHT HEART CATHETERIZATION | Y | Y | Y (missed diagnosis) | Y | Y | Y | Y | Y |
| PERICARDIECTOMY | Y | Y | Y (subtotal) | Y | Y | Y (subtotal) | Y | Y |
| CONSTRICTIVE PERICARDITIS-HISTOLOGY | Y | no typical features | Y | Y | Y | Y | Y | Y |
| ETIOLOGY | unknown | unknown | unknown | unknown | unknown | unknown | unknown | unknown |
| RESPONSE TO PERICARDIECTOMY | edema and ascites were absent for six years, a slight cardiac inflow-stasis persisted and developed a permanent hypoalbuminemia | congestive heart failure and hypoalbuminemia improved, small bowel histology normalized, but liver function did not completely improve | peripheral edema disappeared and serum protein normalized, despite the persistence of a small enteric protein loss one year after surgery | anemia, hypoproteinemia, hypertriglyceridemia ameliorated | cardiac conditionimproved, PLE resolved, serum albumin increased | serum albumin normalized in two months | leg oedema/pleural effusion disappeared, serum albumin normalized | leg oedema and pleural effusion resolved, albumin. levels normalized in three months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birtolo, L.I.; Shahini, E. Constrictive Pericarditis and Protein-Losing Enteropathies: Exploring the Heart–Gut Axis. J. Clin. Med. 2024, 13, 5150. https://doi.org/10.3390/jcm13175150
Birtolo LI, Shahini E. Constrictive Pericarditis and Protein-Losing Enteropathies: Exploring the Heart–Gut Axis. Journal of Clinical Medicine. 2024; 13(17):5150. https://doi.org/10.3390/jcm13175150
Chicago/Turabian StyleBirtolo, Lucia Ilaria, and Endrit Shahini. 2024. "Constrictive Pericarditis and Protein-Losing Enteropathies: Exploring the Heart–Gut Axis" Journal of Clinical Medicine 13, no. 17: 5150. https://doi.org/10.3390/jcm13175150






