Effectiveness of Interspinous Process Devices in Managing Adjacent Segment Degeneration Following Lumbar Spinal Fusion: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
- Comparative Effectiveness: Evaluating the outcomes of IPD implantation compared to traditional lumbar fusion and other fusion techniques, specifically regarding adjacent segment health and overall patient outcomes.
- Safety Profile: Analysing the incidence and types of adverse events associated with IPDs versus fusion surgeries, to determine the relative safety of these devices.
- Long-term Outcomes: Assessing the durability and long-term efficacy of IPDs in preventing or delaying the progression of ASD, given the potential for these devices to provide sustained benefits or, conversely, long-term complications.
- Biomechanical Impact: Investigating the biomechanical effects of IPDs on spinal kinematics and load distribution, including any changes in the adjacent segments’ motion and stress patterns.
2. Materials and Methods
2.1. Search Strategy
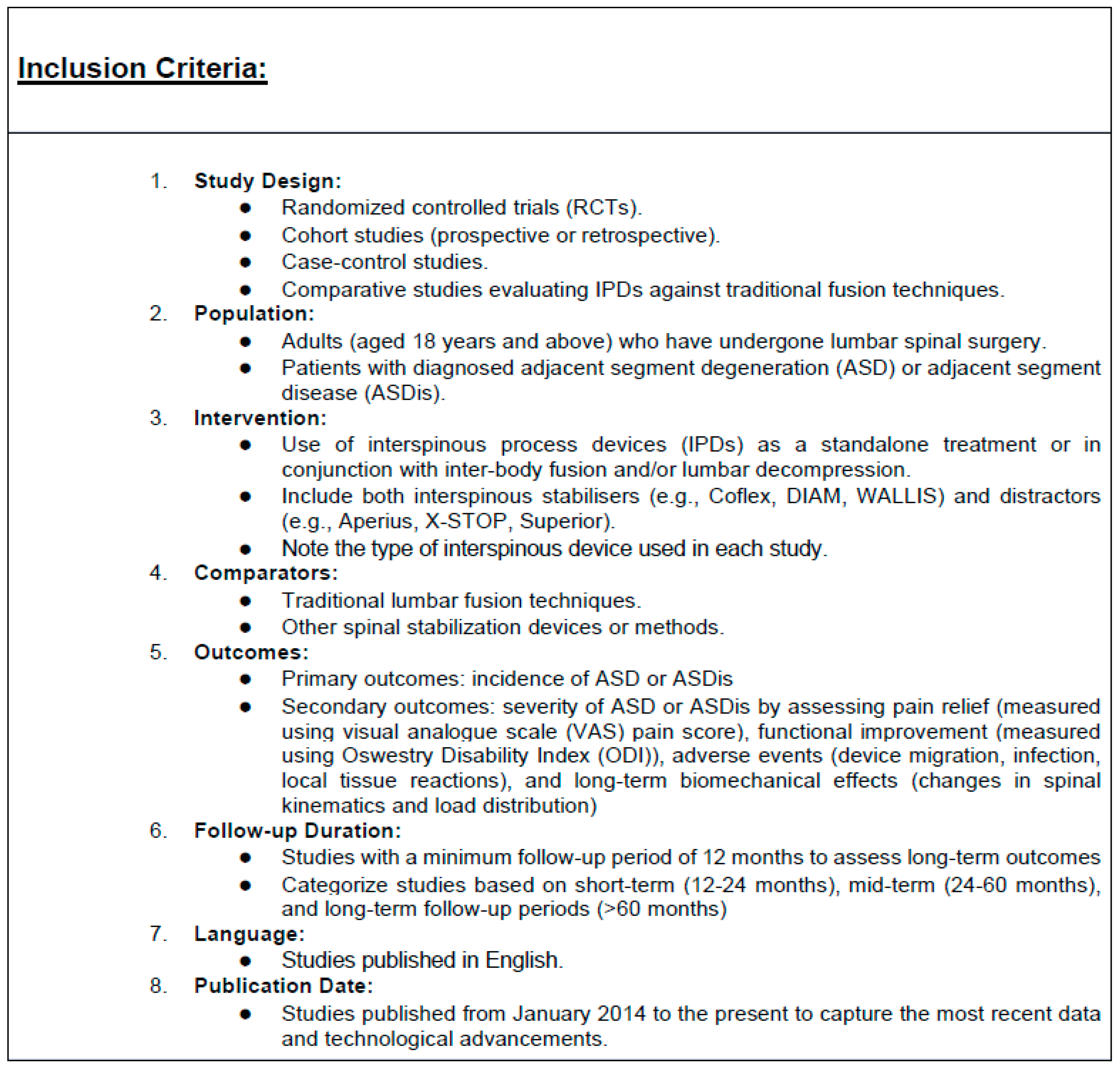
2.2. Selection Criteria
2.3. Data Extraction
2.4. Criteria for ASD
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Power Analysis
3.4. Risk of Bias within Studies
3.5. Meta-Analysis Results
3.5.1. Incidence of ASD
3.5.2. ODI Score
3.5.3. Range of Motion
3.5.4. VAS Lower Back Pain Score
3.5.5. VAS Leg Pain Score
3.5.6. Intraoperative Blood Loss
3.5.7. Operation Time
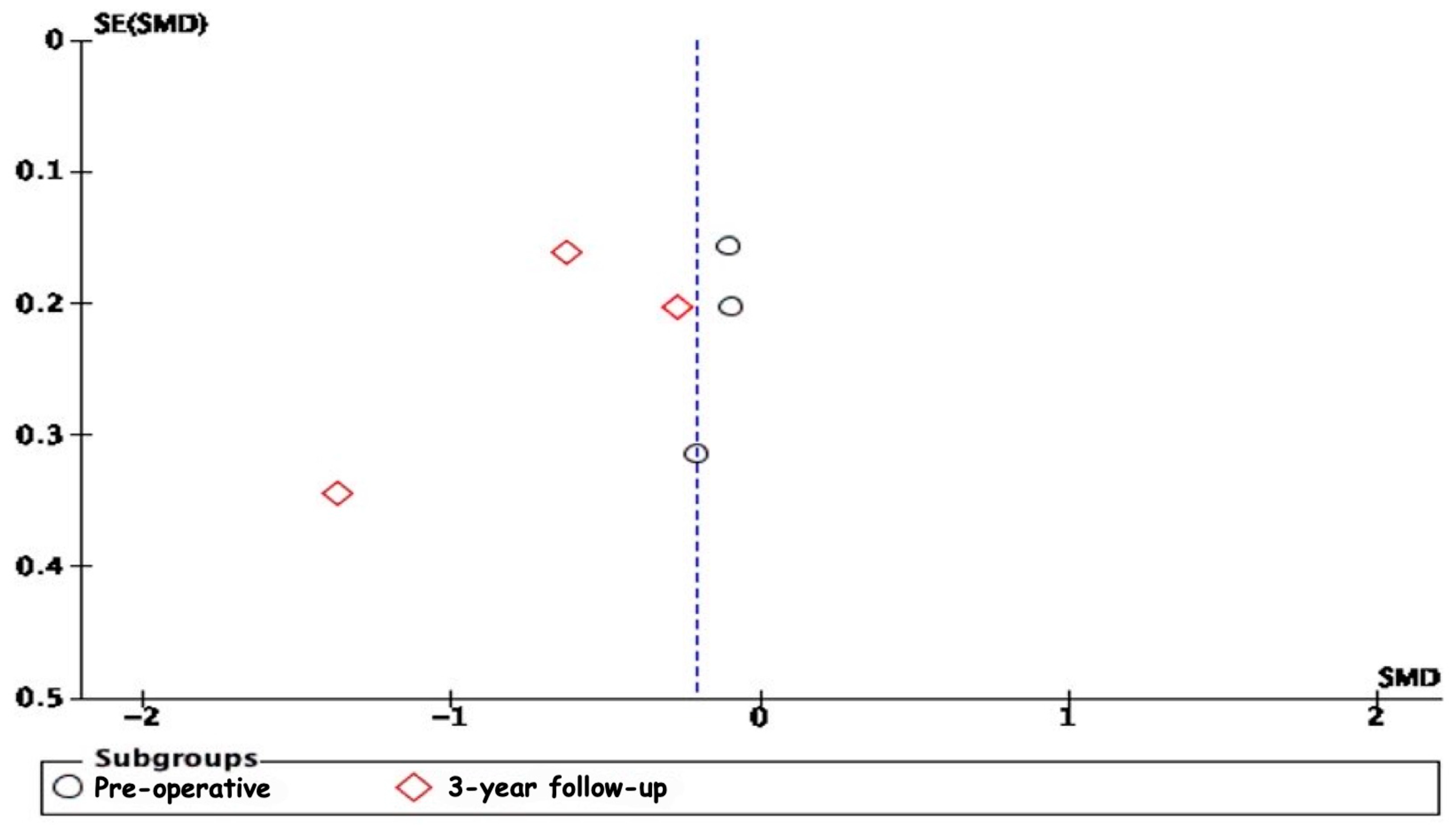
3.5.8. Publication Bias
3.6. Sensitivity Analysis
4. Discussion
4.1. Functional Outcomes
4.2. Preservation of Range of Motion
4.3. Pain Relief
4.4. Intraoperative Benefits
4.5. Safety and Emerging Evidence
4.6. Considerations for Future Research
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | Adjacent Segment Degeneration |
| ASDis | Adjacent Segment Disease |
| IPD | Interspinous Process Devices |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| MeSH | Medical Subject Headings |
| PICO | Patient, Intervention, Comparison, and Outcome |
| VAS | Visual Analog Scale |
| ODI | Oswestry Disability Index |
| RCT | Randomised Controlled Trial |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| PLIF | Posterior Lumbar Interbody Fusion |
| ROM | Range of Motion |
| JOA | Japanese Orthopaedic Association |
| FW | foraminal width |
| RMDQ | Roland–Morris Disability Questionnaire |
| LL | Lumbar Lordosis |
| SS | Sacral Slope |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds Ratio |
| CI | Confidence Interval |
| TLIF | Transforaminal Lumbar Interbody Fusion |
| SMD | Standard Median Deviation |
| GASM | General Adjacent Segment Mobility |
References
- Pannell, W.C.; Savin, D.D.; Scott, T.P.; Wang, J.C.; Daubs, M.D. Trends in the surgical treatment of lumbar spine disease in the United States. Spine J. 2015, 15, 1719–1727. [Google Scholar] [CrossRef]
- Yoshihara, H.; Yoneoka, D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J. 2015, 15, 265–271. [Google Scholar] [CrossRef]
- Park, J.Y.; Chin, D.K.; Cho, Y.E. Accelerated L5-S1 Segment Degeneration after Spinal Fusion on and above L4-5: Minimum 4-Year Follow-Up Results. J. Korean Neurosurg. Soc. 2009, 45, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Wawrose, R.A.; LeVasseur, C.M.; Byrapogu, V.K.; Dombrowski, M.E.; Donaldson, W.F.; Shaw, J.D.; Lee, J.Y.; Anderst, W.J.; Aiyangar, A.K. In vivo changes in adjacent segment kinematics after lumbar decompression and fusion. J. Biomech. 2020, 102, 109515. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.R.; Kumar, M.N.; Deb, A. Adjacent Disc Stress Following Floating Lumbar Spine Fusion: A Finite Element Study. Asian Spine J. 2017, 11, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, G.; Wang, J.C.; Bhatia, N.N.; Hsu, W.K.; Dawson, E.G. Adjacent segment degeneration in the lumbar spine. J. Bone Jt. Surg. Am. 2004, 86, 1497–1503. [Google Scholar] [CrossRef]
- Xia, X.P.; Chen, H.L.; Cheng, H.B. Prevalence of adjacent segment degeneration after spine surgery: A systematic review and meta-analysis. Spine 2013, 38, 597–608. [Google Scholar] [CrossRef]
- Zhang, C.; Berven, S.H.; Fortin, M.; Weber, M.H. Adjacent Segment Degeneration Versus Disease after Lumbar Spine Fusion for Degenerative Pathology: A Systematic Review with Meta-Analysis of the Literature. Clin. Spine Surg. 2016, 29, 21–29. [Google Scholar] [CrossRef]
- Mannion, A.F.; Leivseth, G.; Brox, J.I.; Fritzell, P.; Hägg, O.; Fairbank, J.C. ISSLS Prize winner: Long-term follow-up suggests spinal fusion is associated with increased adjacent segment disc degeneration but without influence on clinical outcome: Results of a combined follow-up from 4 randomized controlled trials. Spine 2014, 39, 1373–1383. [Google Scholar] [CrossRef]
- Wang, J.C.; Spenciner, D.; Robinson, J.C. SPIRE spinous process stabilization plate: Biomechanical evaluation of a novel technology. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2005. J. Neurosurg. Spine 2006, 4, 160–164. [Google Scholar] [CrossRef]
- Karahalios, D.G.; Kaibara, T.; Porter, R.W.; Kakarla, U.K.; Reyes, P.M.; Baaj, A.A.; Yaqoobi, A.S.; Crawford, N.R. Biomechanics of a lumbar interspinous anchor with anterior lumbar interbody fusion. J. Neurosurg. Spine 2010, 12, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.E.; Khalifeh, K.; Hara, J.; Ozgur, B. Interspinous Process (ISP) Devices in Comparison to the Use of Traditional Posterior Spinal Instrumentation. Cureus 2021, 13, e13886. [Google Scholar] [CrossRef] [PubMed]
- Gazzeri, R.; Galarza, M.; Alfieri, A. Controversies about interspinous process devices in the treatment of degenerative lumbar spine diseases: Past, present, and future. Biomed. Res. Int. 2014, 2014, 975052. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Drumm, J.; Häussler, K.; Mack, C.; Steudel, W.I.; Kettler, A. Biomechanical effect of different lumbar interspinous implants on flexibility and intradiscal pressure. Eur. Spine J. 2008, 17, 1049–1056. [Google Scholar] [CrossRef]
- Richter, A.; Schütz, C.; Hauck, M.; Halm, H. Does an interspinous device (Coflex) improve the outcome of decompressive surgery in lumbar spinal stenosis? One-year follow-up of a prospective case control study of 60 patients. Eur. Spine J. 2010, 19, 283–289. [Google Scholar] [CrossRef]
- Bae, I.S.; Bak, K.H.; Chun, H.J. Interspinous Process Fixation Device Versus Extended Pedicle Screw Fixation for Symptomatic Adjacent Segment Disease: 3-Year Retrospective Study. World Neurosurg. 2020, 139, e144–e150. [Google Scholar] [CrossRef]
- Kim, K.R.; Lee, C.K.; Kim, I.S. Efficacy of Interspinous Device on Adjacent Segment Degeneration after Single Level Posterior Lumbar Interbody Fusion: A Minimum 2-Year Follow-Up. Br. J. Neurosurg. 2021, 35, 757–765. [Google Scholar] [CrossRef]
- Yue, Z.J.; Liu, R.Y.; Lu, Y.; Dong, L.L.; Li, Y.Q.; Lu, E.B. Middle-Period Curative Effect of Posterior Lumbar Intervertebral Fusion (PLIF) and Interspinous Dynamic Fixation (Wallis) for Treatment of L45 Degenerative Disease and Its Influence on Adjacent Segment Degeneration. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4481–4487. [Google Scholar]
- Lo, H.J.; Chen, H.M.; Kuo, Y.J.; Yang, S.W. Effect of Different Designs of Interspinous Process Devices on the Instrumented and Adjacent Levels after Double-Level Lumbar Decompression Surgery: A Finite Element Analysis. PLoS ONE 2020, 15, e0244571. [Google Scholar] [CrossRef]
- Mo, Z.; Li, D.; Zhang, R.; Chang, M.; Yang, B.; Tang, S. Comparative Effectiveness and Safety of Posterior Lumbar Interbody Fusion, Coflex, Wallis, and X-Stop for Lumbar Degenerative Diseases: A Systematic Review and Network Meta-Analysis. Clin. Neurol. Neurosurg. 2018, 172, 74–81. [Google Scholar] [CrossRef]
- Cho, H.-J.; Ko, Y.S.; Won, Y.I.; Lee, C.-H.M.; Yang, S.H.; Kim, C.H.; Chung, C.K. The Efficacy of Lumbar Hybrid Fusion for the Prevention of Adjacent Segment Disease: Fact or Artifact? A Meta-Analysis. Clin. Spine Surg. 2021, 34, 260–268. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Imagama, S.; Kawakami, N.; Matsubara, Y.; Tsuji, T.; Ohara, T.; Katayama, Y.; Ishiguro, N.; Kanemura, T. Radiographic Adjacent Segment Degeneration at 5 Years after L4/5 Posterior Lumbar Interbody Fusion with Pedicle Screw Instrumentation: Evaluation by Computed Tomography and Annual Screening with Magnetic Resonance Imaging. Clin. Spine Surg. 2016, 29, E442–E451. [Google Scholar] [CrossRef]
- Park, P.; Garton, H.J.; Gala, V.C.; Hoff, J.T.; McGillicuddy, J.E. Adjacent Segment Disease after Lumbar or Lumbosacral Fusion: Review of the Literature. Spine 2004, 29, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ryu, D.S.; Paik, H.K.; Ahn, S.S.; Kang, M.S.; Kim, K.H.; Park, J.Y.; Chin, D.K.; Kim, K.S.; Cho, Y.E.; et al. Paraspinal Muscle, Facet Joint, and Disc Problems: Risk Factors for Adjacent Segment Degeneration after Lumbar Fusion. Spine J. 2016, 16, 867–875. [Google Scholar] [CrossRef]
- Siewe, J.; Bredow, J.; Oppermann, J.; Koy, T.; Delank, S.; Knoell, P.; Eysel, P.; Sobottke, R.; Zarghooni, K.; Röllinghoff, M. Evaluation of Efficacy of a New Hybrid Fusion Device: A Randomized, Two-Centre Controlled Trial. BMC Musculoskelet. Disord. 2014, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Aota, Y.; Kumano, K.; Hirabayashi, S. Postfusion Instability at the Adjacent Segments after Rigid Pedicle Screw Fixation for Degenerative Lumbar Spinal Disorders. J. Spinal Disord. 1995, 8, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hai, Y.; Meng, X.; Yang, J.; Yin, P. Topping-Off Surgery vs. Posterior Lumbar Interbody Fusion for Degenerative Lumbar Disease: A Comparative Study of Clinical Efficacy and Adjacent Segment Degeneration. J. Orthop. Surg. Res. 2019, 14, 197. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Wang, K.; Zhou, J.; Wang, J.; Zhu, Y.; Liu, H. Topping-Off Technique Prevents Aggravation of Degeneration of Adjacent Segment Fusion Revealed by Retrospective and Finite Element Biomechanical Analysis. J. Orthop. Surg. Res. 2015, 10, 10. [Google Scholar] [CrossRef]
- Chen, X.L.; Guan, L.; Liu, Y.Z.; Yang, J.C.; Wang, W.L.; Hai, Y. Interspinous Dynamic Stabilization Adjacent to Fusion Versus Double-Segment Fusion for Treatment of Lumbar Degenerative Disease with a Minimum Follow-Up of Three Years. Int. Orthop. 2016, 40, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xiong, W.; Li, L.; Li, F. Adjacent Segmental Degeneration Following Wallis Interspinous Stabilization Implantation: Biomechanical Explanations and the Value of Magnetic Resonance Imaging. Medicine 2017, 96, e7056. [Google Scholar] [CrossRef]
- Liao, H.-C.; Wu, Y.-C.; Wang, P.-W.; Chung, M.-H.; Hueng, D.-Y.; Chen, K.-Y.; Tseng, K.-Y. Topping-Off Surgery Versus Transforaminal Lumbar Intervertebral Fusion for Combined One-Level Spondylolisthesis and Adjacent Lumbar Disc Herniation: A Comparative Study of Clinical Efficacy and Radiographic Outcomes with a Two-Year Follow-Up. In Vivo 2023, 37, 1838–1846. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Y.; Mei, W.; Xu, J.; Zhan, S. Biomechanical Changes of Degenerated Adjacent Segment and Intact Lumbar Spine After Lumbosacral Topping-Off Surgery: A Three-Dimensional Finite Element Analysis. BMC Musculoskelet. Disord. 2020, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Høy, K.; Grycel, B.; Andersen, T.; Bünger, C. Does Transforaminal Lumbar Interbody Fusion Produce Leg Pain?—Results from a RCT. J. Orthop. Surg. 2019, 27, 2309499019869469. [Google Scholar] [CrossRef] [PubMed]
- Fuster, S.; Martínez-Anda, J.J.; Castillo-Rivera, S.A.; Vargas-Reverón, C.; Tornero, E. Dynamic Fixation Techniques for the Prevention of Adjacent Segment Disease: A Retrospective Controlled Study. Asian Spine J. 2022, 16, 401–410. [Google Scholar] [CrossRef]
- Nachanakian, A.; El Helou, A.; Alaywan, M. The Interspinous Spacer: A New Posterior Dynamic Stabilization Concept for Prevention of Adjacent Segment Disease. Adv. Orthop. 2013, 2013, 637362. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Sayed, D.; Malinowski DO, M.N.; Rowe, J.J.; Jameson, J.B.; Liang, K.; Sclafani, J.A. A Review of Emerging Evidence for Utilization of a Percutaneous Interspinous Process Decompression Device to Treat Symptomatic Lumbar Adjacent-Segment Degeneration. Pain Med. 2019, 20 (Suppl. S2), S9–S13. [Google Scholar] [CrossRef]
- Schulte, L.M.; O’Brien, J.R.; Matteini, L.E.; Yu, W.D. Change in Sagittal Balance with Placement of an Interspinous Spacer. Spine 2011, 36, E1302–E1305. [Google Scholar] [CrossRef]
- Meyer, B.; Baranto, A.; Schils, F.; Collignon, F.; Zoega, B.; Tan, L.; LeHuec, J.C.; NICE Trial Study Group. Percutaneous Interspinous Spacer vs Decompression in Patients with Neurogenic Claudication: An Alternative in Selected Patients? Neurosurgery 2018, 82, 621–629. [Google Scholar] [CrossRef]
- Holzer, E.M.; Aghayev, E.; O’riordan, D.; Fekete, T.F.; Jeszenszky, D.J.; Haschtmann, D.; Porchet, F.; Kleinstueck, F.S.; Pigott, T.; Munting, E.; et al. Validation of a Surgical Invasiveness Index in Patients with Lumbar Spinal Disorders Registered in the Spine Tango Registry. Eur. Spine J. 2021, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Hosogane, N.; Fujita, N.; Okada, E.; Suzuki, S.; Tsuji, O.; Nagoshi, N.; Nakamura, M.; Matsumoto, M.; Watanabe, K. The Patient Demographics, Radiographic Index and Surgical Invasiveness for Mechanical Failure (PRISM) Model Established for Adult Spinal Deformity Surgery. Sci. Rep. 2020, 10, 9341. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Yamashita, T.; Matsumoto, T.; Nagamoto, Y.; Sugiura, T.; Takahashi, Y.; Maeno, T.; Iwasaki, M. Adjacent Segment Disease After Posterior Lumbar Interbody Fusion: A Case Series of 1000 Patients. Glob. Spine J. 2018, 8, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Roussouly, P.; Nnadi, C. Sagittal Plane Deformity: An Overview of Interpretation and Management. Eur. Spine J. 2010, 19, 1824–1836. [Google Scholar] [CrossRef]
- Kim, W.J.; Ma, C.H.; Kim, S.H.; Min, Y.S.; Lee, J.W.; Chang, S.H.; Park, K.H.; Park, K.Y.; Song, D.G.; Choy, W.S. Prevention of Adjacent Segmental Disease after Fusion in Degenerative Spinal Disorder: Correlation Between Segmental Lumbar Lordosis Ratio and Pelvic Incidence-Lumbar Lordosis Mismatch for a Minimum 5-Year Follow-Up. Asian Spine J. 2019, 13, 654–662. [Google Scholar] [CrossRef] [PubMed]




| Author | Study Design | Number of Patients (Male (M)/Female (F)) | Intervention Used | Control | Incidence of ASD/ASDis | Outcome Measures | Follow-Up | Post-Operative Complications |
|---|---|---|---|---|---|---|---|---|
| Dongyue Li et al. 2019 [29] | Retrospective cohort study | 99 (46 M/53 F) | PLIF + Coflex | PLIF | ASD | VAS, ODI, ROM, Lumbar MRI | 3 years | Intraspinal haematoma, subcutaneous incision infection |
| Zhenqi Zhu et al. 2015 [30] | Retrospective cohort study | 45 (25 M/20 F) | PLIF + Wallis | PLIF | ASD | VAS, JOA | 1 year | NA |
| Xiao-Long Chen et al. 2016 [31] | Retrospective cohort study | 164 (92 M/72 F) | PLIF + Coflex | PLIF | ASD | VAS, ODI, ROM, FW, LL, SS, Lumbar MRI, CT | 3 years | NA |
| Kwang Ryeol Kim et al. 2020 [17] | Retrospective cohort study | 51 (21 M/30 F) | PLIF + DIAM | PLIF | ASD | VAS, RMDQ, ROM, MRI | 2 years | NA |
| Zhiguo Zhou et al. 2017 [32] | Retrospective cohort study | 38 (21 M/17 F) | Discectomy + Wallis | Discectomy | ASD | VAS, ODI. MRI | 2 years | NA |
| Hsiang-Chih Lioa et al. 2023 [33] | Retrospective cohort study | 40 (12 M/28 F) | TLIF + ROCKER (Paonan, Taipei, Taiwan) | TLIF | ASD | VAS, ODI, ROM | 2 years | Infection, device breakage, pain at surgical site |
| In-Suk Bae et al. 2020 [16] | Retrospective cohort study | 109 (62 M/47 F) | PLIF + SPIRE (Medtronic Sofamor Danek, Dublin, Ireland) | PLIF | ASD | VAS, ODI, CT | 3 years | Cage migration, dural tears, revision surgery, screw mispositioning, rod breakage |
| Study | Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | Quality Score |
|---|---|---|---|---|---|---|---|---|---|
| Dongyue Li et al. 2019 [29] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Zhenqi Zhu et al. 2015 [30] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
| Xiao-Long Chen et al. 2016 [31] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Kwang Ryeol Kim et al. 2020 [17] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
| Zhiguo Zhou et al. 2017 [32] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
| Hsiang-Chih Lioa et al. 2023 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| In-Suk Bae et al. 2020 [16] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangal, H.; Felzensztein Recher, D.; Shafafy, R.; Itshayek, E. Effectiveness of Interspinous Process Devices in Managing Adjacent Segment Degeneration Following Lumbar Spinal Fusion: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 5160. https://doi.org/10.3390/jcm13175160
Mangal H, Felzensztein Recher D, Shafafy R, Itshayek E. Effectiveness of Interspinous Process Devices in Managing Adjacent Segment Degeneration Following Lumbar Spinal Fusion: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(17):5160. https://doi.org/10.3390/jcm13175160
Chicago/Turabian StyleMangal, Harris, David Felzensztein Recher, Roozbeh Shafafy, and Eyal Itshayek. 2024. "Effectiveness of Interspinous Process Devices in Managing Adjacent Segment Degeneration Following Lumbar Spinal Fusion: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 17: 5160. https://doi.org/10.3390/jcm13175160














