The Importance of Orthostatic Increase in Pulse Wave Velocity in the Diagnosis of Early Vascular Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Head-Up Tilt Test (HUTT) Procedure
2.3. Hemodynamic Measurements
2.4. Statistical Analysis
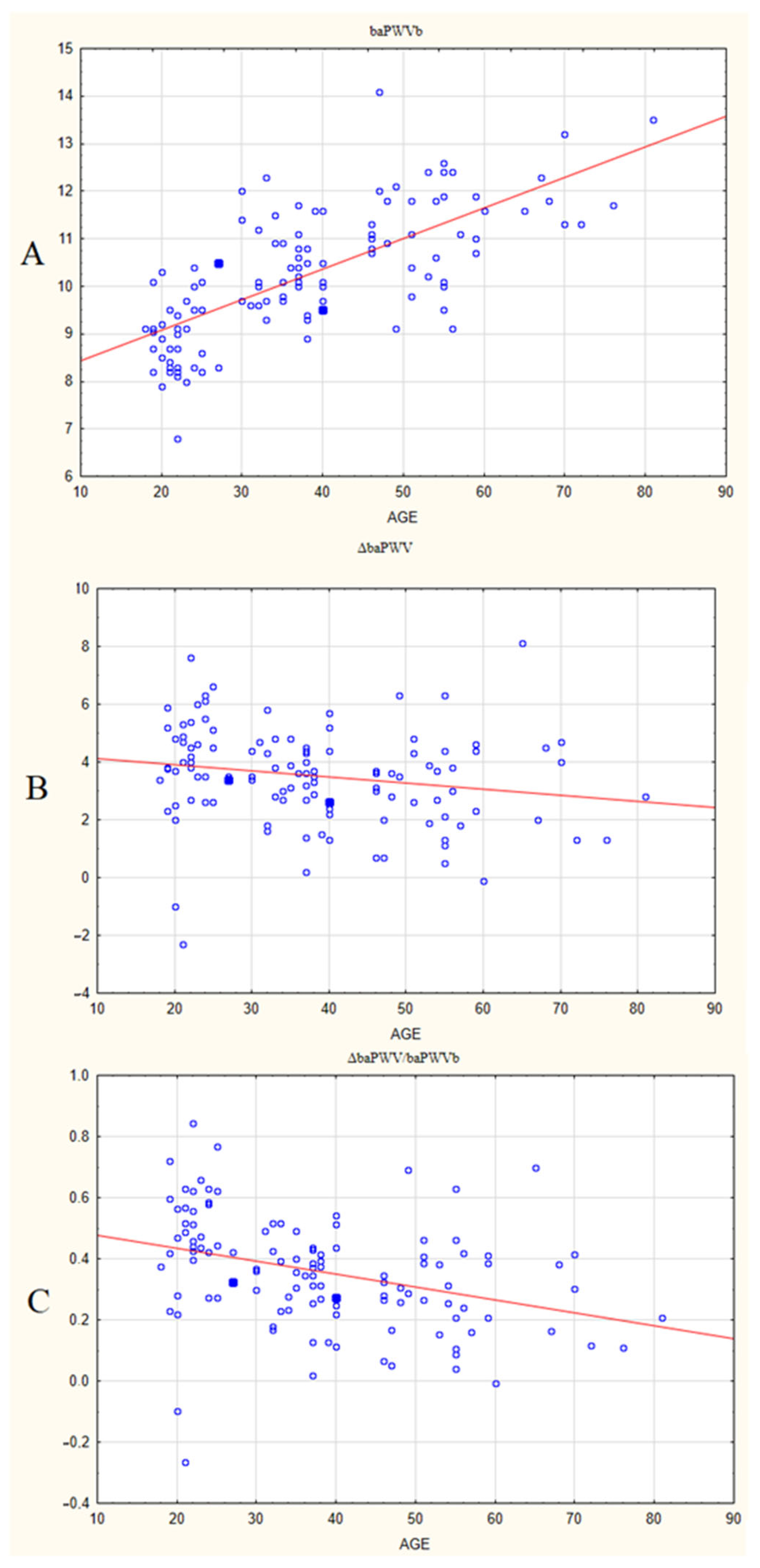
3. Results
4. Discussion
- -
- -
- chronic low-grade systemic inflammation and insulin resistance both affect endothelial function and vascular remodeling.
5. Conclusions
6. Limitations
7. Future Directions
- (1)
- The impact and contribution of lifestyle-related preclinical conditions underlying accelerated aging (such as chronic distress, chronic low-grade inflammation and insulin resistance) on the ‘static’ and ‘dynamic’ components of cardiovascular disadaptation to orthostasis presented by the baPWVb and ΔbaPWV/baPWVb indices.
- (2)
- It is expedient to perform a long-term (longitudinal, prospective) study to evaluate how the newly proposed indicator of vascular aging will change with time, and how the ΔbaPWV/baPWVb index will correlate with preclinical orthostatic abnormalities and endothelial function, as well as with chronic inflammation and oxidative stress, in a healthy population.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, S.N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic Stiffness, Blood Pressure Progression, and Incident Hypertension. JAMA 2012, 308, 875. [Google Scholar] [CrossRef] [PubMed]
- AlGhatrif, M.; Strait, J.B.; Morrell, C.H.; Canepa, M.; Wright, J.; Elango, P.; Scuteri, A.; Najjar, S.S.; Ferrucci, L.; Lakatta, E.G. Longitudinal trajectories of arterial stiffness and the role of blood pressure: The Baltimore Longitudinal Study of Aging. Hypertension 2013, 62, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E. Arterial stiffness as a risk factor for clinical hypertension. Nat. Rev. Cardiol. 2018, 15, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Beros, A.; Sluyter, J.; Hughes, A.; Hametner, B.; Wassertheurer, S.; Scragg, R. Arterial stiffness and incident chronic kidney disease: A large population-based cohort study. J. Nephrol. 2024, 37, 1241–1250. [Google Scholar] [CrossRef]
- Stone, K.; Fryer, S.; Meyer, M.L.; Kucharska-Newton, A.; Faulkner, J.; Zieff, G.; Paterson, C.; Credeur, D.; Matsushita, K.; Hughes, T.M.; et al. The aortic-femoral arterial stiffness gradient: An atherosclerosis risk in communities (ARIC) study. J. Hypertens. 2021, 39, 1370–1377. [Google Scholar] [CrossRef]
- Zhang, Y.; Lacolley, P.; Protogerou, A.D.; Safar, M.E. Arterial Stiffness in Hypertension and Function of Large Arteries. Am. J. Hypertens. 2020, 33, 291–296. [Google Scholar] [CrossRef]
- Liao, J.; Farmer, J. Arterial stiffness as a risk factor for coronary artery disease. Curr. Atheroscler. Rep. 2014, 16, 387. [Google Scholar] [CrossRef]
- Li, J.; Hidru, T.H.; Lin, Y.; Wang, X.; Lin, L.; Chen, S.; Xia, Y.; Yang, X.; Wu, S. Arterial stiffness is associated with cancer mortality: Insight from Kailuan study. Cancer Med. 2023, 12, 16580–16590. [Google Scholar] [CrossRef]
- Tynjälä, A.; Forsblom, C.; Harjutsalo, V.; Groop, P.H.; Gordin, D.; FinnDiane Study Group. Arterial Stiffness Predicts Mortality in Individuals With Type 1 Diabetes. Diabetes Care 2020, 43, 2266–2271. [Google Scholar] [CrossRef]
- Bulpitt, C.J.; Rajkumar, C.; Cameron, J.D. Vascular Compliance as a Measure of Biological Age. J. Am. Geriatr. Soc. 1999, 47, 657–663. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, N.; Herman, A.B.; Gorospe, M.; Lee, J.S. Factors and Pathways Modulating Endothelial Cell Senescence in Vascular Aging. Int. J. Mol. Sci. 2022, 23, 10135. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yan, J.; Zhao, Y.; Yu, Z.; Tian, S.; Khan, A.H.; Zhu, Y.; Wu, A.; Zhang, C.; Tian, X.-L. Vascular Aging: Assessment and Intervention. Clin. Interv. Aging 2023, 18, 1373–1395. [Google Scholar] [CrossRef] [PubMed]
- Reutersberg, B.; Düppers, P.; Menges, A.L.; Schrimpf, C.; Zimmermann, A.; Pelisek, J. Age-related vascular changes exemplified by the carotid artery. Gefasschirurgie 2022, 27, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.J.; Osei-Owusu, P. Elastin haploinsufficiency accelerates age-related structural and functional changes in the renal microvasculature and impairment of renal hemodynamics in female mice. Front. Physiol. 2023, 14, 1141094. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alvarez, V.; Linares Sánchez, M.; López Alvarez, F.; Suárez Nieto, C.; Mäkitie, A.A.; Olsen, K.D.; Ferlito, A. Evaluation of Intima-Media Thickness and Arterial Stiffness as Early Ultrasound Biomarkers of Carotid Artery Atherosclerosis. Cardiol. Ther. 2022, 11, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Nordenstam, F.; Norman, M.; Caidahl, K.; Wickström, R. Arterial Stiffness and Carotid Intima-Media Thickness in Children Exposed to Smokeless Tobacco in Fetal Life. J. Am. Heart Assoc. 2024, 13, e9128. [Google Scholar] [CrossRef]
- The Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Zi-Sheng, A.; Jue, L.; Zhong-Min, L.; Hui-Min, F.; Zhang, D.F.; Yun, Z.; Zhang, L.J.; Zhu, W.Q.; Bao, Y. Reference value of brachial-ankle pulse wave velocity for the eastern Chinese population and potential influencing factors. Braz. J. Med. Biol. Res. 2011, 44, 1000–1005. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Cunha, P.M.G.M.; de Oliveria Vitorino, P.V.; Souza, A.L.L.; Deus, G.D.; Feitosa, A.; Barbosa, E.C.D.; Gomes, M.M.; Jardim, P.C.B.V.; Barroso, W.K.S. Vascular Aging and Arterial Stiffness. Arq. Bras. Cardiol. 2022, 119, 604–615. [Google Scholar] [CrossRef]
- Petrák, O.; Češka, R. Vascular age. Vnitr. Lek. 2020, 65, 770–774. [Google Scholar] [CrossRef]
- Litwin, M.; Feber, J. Origins of Primary Hypertension in Children: Early Vascular or Biological Aging? Hypertension 2020, 76, 1400–1409. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef]
- Aizawa, K.; Gates, P.E.; Mawson, D.M.; Elyas, S.; Casanova, F.; Gooding, K.M.; Adingupu, D.D.; Strain, W.D.; Shore, A.C. Carotid-femoral pulse wave velocity acquisition methods and their associations with cardiovascular risk factors and subclinical biomarkers of vascular health. J. Hypertens. 2022, 40, 658–665. [Google Scholar] [CrossRef]
- Liu, B.; Li, Q.; Qiu, P. Comparison between invasive and non-invasive blood pressure in young, middle and old age. Blood Press. 2016, 25, 155–161. [Google Scholar] [CrossRef]
- Ng, X.N.; Tsai, J.P.; Wang, C.H.; Hsu, B.G. Carotid-Femoral Pulse Wave Velocity Could Be a Marker to Predict Cardiovascular and All-Cause Mortality of Hemodialysis Patients. J. Clin. Med. 2023, 12, 2509. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Cunha, P.G.; Lacolley, P.; Nilsson, P.M. Concept of Extremes in Vascular Aging: From Early Vascular Aging to Supernormal Vascular Aging. Hypertension 2019, 74, 218–228. [Google Scholar] [CrossRef]
- Nilsson, P.M. Early Vascular Aging in Hypertension. Front. Cardiovasc. Med. 2020, 7, 6. [Google Scholar] [CrossRef]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.-M.; Charlton, P.H.; Grillo, A.; et al. Vascular ageing: Moving from bench towards bedside. Eur. J. Prev. Cardiol. 2023, 30, 1101–1117. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Laurent, S.; Cunha, P.G.; Olsen, M.H.; Rietzschel, E.; Franco, O.H.; Ryliškytė, L.; Strazhesko, I.; Vlachopoulos, C.; Chen, C.-H.; et al. Characteristics of healthy vascular ageing in pooled population-based cohort studies: The global Metabolic syndrome and Artery REsearch Consortium. J. Hypertens. 2018, 36, 2340–2349. [Google Scholar] [CrossRef]
- Ferreira, I.; Beijers, H.J.; Schouten, F.; Smulders, Y.M.; Twisk, J.W.; Stehouwer, C.D. Clustering of metabolic syndrome traits is associated with maladaptive carotid remodeling and stiffening: A 6-year longitudinal study. Hypertension 2012, 60, 542–549. [Google Scholar] [CrossRef]
- Westerbacka, J.; Yki-Järvinen, H. Arterial stiffness and insulin resistance. Semin. Vasc. Med. 2002, 2, 157–164. [Google Scholar] [CrossRef]
- Aroor, A.R.; Jia, G.; Sowers, J.R. Cellular mechanisms underlying obesity-induced arterial stiffness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R387–R398. [Google Scholar] [CrossRef]
- Zanoli, L. Arterial stiffness is a vascular biomarker of chronic inflammation. Biomark. Med. 2019, 13, 1335–1337. [Google Scholar] [CrossRef]
- Van Hout, M.J.; Dekkers, I.A.; Westenberg, J.J.; Schalij, M.J.; Widya, R.L.; de Mutsert, R.; Rosendaal, F.R.; de Roos, A.; Scholte, A.J.; Lamb, H.J. Normal and reference values for cardiovascular magnetic resonance-based pulse wave velocity in the middle-aged general population. J. Cardiovasc. Magn. Reson. 2021, 23, 46. [Google Scholar] [CrossRef]
- Xing, C.; Xie, X.; Wu, Y.; Xu, L.; Guan, X.; Li, F.; Zhan, X.; Yang, H.; Li, J.; Zhou, Q.; et al. Reference values of carotid intima-media thickness and arterial stiffness in Chinese adults based on ultrasound radio frequency signal: A nationwide, multicenter study. Chin. Med. J. 2024, 137, 1802–1810. [Google Scholar] [CrossRef]
- Lu, Y.; Kiechl, S.J.; Wang, J.; Xu, Q.; Kiechl, S.; Pechlaner, R. Global distributions of age- and sex-related arterial stiffness: Systematic review and meta-analysis of 167 studies with 509,743 participants. eBioMedicine 2023, 92, 104619. [Google Scholar] [CrossRef]
- Tan, M.P.; Duncan, G.W.; Parry, S.W. Head-up Tilt Table Testing: A state-of-the-art review. Minerva Med. 2009, 100, 329–338. [Google Scholar]
- Dorogovtsev, V.N.; Yankevich, D.S.; Goswami, N. Effects of an Innovative Head-Up Tilt Protocol on Blood Pressure and Arterial Stiffness Changes. J. Clin. Med. 2021, 10, 1198. [Google Scholar] [CrossRef]
- Rose, K.M.; Holme, I.; Light, K.C.; Sharrett, A.R.; Tyroler, H.A.; Heiss, G. Association between the blood pressure response to a change in posture and the 6-year incidence of hypertension: Prospective findings from the ARIC study. J. Hum. Hypertens. 2002, 16, 771–777. [Google Scholar] [CrossRef]
- Thomas, R.J.; Liu, K.; Jacobs, D.R.; Bild, D.E.; Kiefe, C.I.; Hulley, S.B. Positional change in blood pressure and 8-year risk of hypertension: The CARDIA Study. Mayo Clin. Proc. 2003, 78, 951–958. [Google Scholar] [CrossRef]
- Dorogovtsev, V.N.; Yankevich, D.S.; Gaydashev, A.E.; Martyushev-Poklad, A.V.; Podolskaya, J.A.; Borisov, I.V.; Grechko, A.V. Preclinical Orthostatic Abnormalities May Predict Early Increase in Vascular Stiffness in Different Age Groups: A Pilot Study. Diagnostics 2023, 13, 3243. [Google Scholar] [CrossRef]
- Sunagawa, K.; Sato, T.; Kawada, T. Integrative sympathetic baroreflex regulation of arterial pressure. Ann. N. Y. Acad. Sci. 2001, 940, 314–323. [Google Scholar] [CrossRef]
- Kamiya, A.; Kawada, T.; Sugimachi, M. Systems physiology of the baroreflex during orthostatic stress: From animals to humans. Front. Physiol. 2014, 5, 256. [Google Scholar] [CrossRef]
- László, Z.; Rössler, A.; Hinghofer-Szalkay, H.G. Cardiovascular and hormonal changes with different angles of head-up tilt in men. Physiol. Res. 2001, 50, 71–82. [Google Scholar] [CrossRef]
- Humphrey, J.D. Mechanisms of Vascular Remodeling in Hypertension. Am. J. Hypertens. 2021, 34, 432–441. [Google Scholar] [CrossRef]
- Lortz, J.; Halfmann, L.; Burghardt, A.; Steinmetz, M.; Radecke, T.; Jánosi, R.A.; Rassaf, T.; Rammos, C. Rapid and automated risk stratification by determination of the aortic stiffness in healthy subjects and subjects with cardiovascular disease. PLoS ONE 2019, 14, e0216538. [Google Scholar] [CrossRef]
- Fendrik, K.; Biró, K.; Endrei, D.; Koltai, K.; Sándor, B.; Tóth, K.; Késmárky, G. Oscillometric measurement of the ankle-brachial index and the estimated carotid-femoral pulse wave velocity improves the sensitivity of an automated device in screening peripheral artery disease. Front. Cardiovasc. Med. 2023, 10, 1275856. [Google Scholar] [CrossRef]
- Incognito, A.V.; Samora, M.; Shepherd, A.D.; Cartafina, R.A.; Guimarães, G.M.N.; Daher, M.; Vianna, L.C.; Millar, P.J. Sympathetic arterial baroreflex hysteresis in humans: Different patterns during low- and high-pressure levels. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H787–H792. [Google Scholar] [CrossRef]
- Stauss, H.M. Baroreceptor reflex function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R284–R286. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.S.; Shibata, S.; Okada, Y.; Levine, B.D.; Fu, Q. Neural-humoral responses during head-up tilt in healthy young white and black women. Front. Physiol. 2014, 5, 86. [Google Scholar] [CrossRef]
- Rössler, A.; László, Z.; Haditsch, B.; Hinghofer-Szalkay, H.G. Orthostatic stimuli rapidly change plasma adrenomedullin in humans. Hypertension 1999, 34, 1147–1151. [Google Scholar] [CrossRef]
- Goswami, N.; Blaber, A.P.; Hinghofer-Szalkay, H.; Convertino, V.A. Lower Body Negative Pressure: Physiological Effects, Applications, and Implementation. Physiol. Rev. 2019, 99, 807–851. [Google Scholar] [CrossRef]
- Broadbent, J.; Reichmuth, J.; Trozic, I.; Kneihsl, M.; Rössler, A.; Green, D.A.; Rodriguez, J.; Hinghofer-Szalkay, H.; Fazekas, F.; Goswami, N. Adrenomedullin and galanin responses to orthostasis in older persons. Eur. J. Clin. Investig. 2017, 47, 812–818. [Google Scholar] [CrossRef]
- Giunta, S.; Xia, S.; Pelliccioni, G.; Olivieri, F. Autonomic nervous system imbalance during aging contributes to impair endogenous anti-inflammaging strategies. GeroScience 2023, 46, 113–127. [Google Scholar] [CrossRef]
- Seals, D.R.; Esler, M.D. Human ageing and the sympathoadrenal system. J. Physiol. 2000, 528 Pt 3, 407–417. [Google Scholar] [CrossRef]
- Johansson, M.; Fedorowski, A.; Jordan, J.; Engström, G.; Nilsson, P.M.; Hamrefors, V. Orthostatic blood pressure adaptations, aortic stiffness, and central hemodynamics in the general population: Insights from the Malmö Offspring Study (MOS). Clin. Auton. Res. 2023, 33, 29–40. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; He, L.; Chen, H.; Li, Y. Study on the Relationship Between Orthostatic Hypotension and Heart Rate Variability, Pulse Wave Velocity Index, and Frailty Index in the Elderly: A Retrospective Observational Study. Front. Cardiovasc. Med. 2020, 7, 603957. [Google Scholar] [CrossRef]
- Takahashi, M.; Miyai, N.; Nagano, S.; Utsumi, M.; Oka, M.; Yamamoto, M.; Shiba, M.; Uematsu, Y.; Nishimura, Y.; Takeshita, T.; et al. Orthostatic Blood Pressure Changes and Subclinical Markers of Atherosclerosis. Am. J. Hypertens. 2015, 28, 1134–1140. [Google Scholar] [CrossRef]
| Parameters | Group 1 | Group 2 | Group 3 | Inter-Group Differences | KW | ||
|---|---|---|---|---|---|---|---|
| Average Age | n = 40 | n = 40 | n = 40 | ||||
| [Age Range], Years | 22 [20; 24] | 37 [33; 38] | 55 [49; 59] | p1–2 | p1–3 | p2–3 | |
| HRb b/min | 62 [57; 67.5] | 68 [62.5; 77.5] | 64.5 [58; 72] | 0.004 | 0.39 | 0.29 | 10.01 p = 0.007 |
| HRt b/min | 74 [68.5; 78] | 73 [69.5; 85] | 67.5 [62.5; 75] | 0.059 | 0.063 | 0.015 | 8.97 p = 0.011 |
| SBPb mmHg | 120.3 [109.7; 126] | 125.1 [119; 130.6] | 125.6 [115; 136.6] | 0.05 | 0.1 | 0.058 | 6.9 p = 0.03 |
| SBPt mmHg | 123.1 [108.5; 128.5] | 126.3 [113.2; 134.5] | 120.2 [112.8; 131.1] | 0.25 | 0.061 | 0.059 | 3.0 p = 0.223 |
| DBPb mmHg | 72.7 [66.2; 75.7] | 81.5 [73.6; 86.8] | 82.5 [73; 89.8] | 0.001 | 0.001 | 0.054 | 25.75 p < 0.0001 |
| DBPt mmHg | 79.9 [74; 82.5] | 85.9 [79.4; 90.7] | 84.3 [77.5; 90.8] | 0.003 | 0.051 | 0.09 | 11.45 p = 0.003 |
| baPWVb, m/s | 8.9 [8.3; 9.5] | 10.1 [9.7; 11.0] | 11.3 [10.7; 11.9] | 0.001 | 0.001 | 0.001 | 66.7 p < 0.0001 |
| baPWVt, m/s | 12.9 [11.9; 14.2] | 13.8 [13.0; 14.5] | 14.3 [13.3; 15.4] | 0.16 | 0.001 | 0.26 | 13.3 p = 0.0013 |
| ΔbaPWV, m/s | 4.0 [3.45; 5.15] | 3.55 [2.7; 4.35] | 3.05 [1.95; 4.35] | 0.023 | 0.007 | 0.34 | 8.83 p = 0.012 |
| ΔbaPWV/ baPWVb | 0.45 [0.41; 0.58] | 0.35 [0.25; 0.42] | 0.27 [0.16; 0.39] | 0.000075 | 0.000006 | 0.073 | 26.1 p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorogovtsev, V.; Yankevich, D.; Martyushev-Poklad, A.; Borisov, I.; Grechko, A.V. The Importance of Orthostatic Increase in Pulse Wave Velocity in the Diagnosis of Early Vascular Aging. J. Clin. Med. 2024, 13, 5713. https://doi.org/10.3390/jcm13195713
Dorogovtsev V, Yankevich D, Martyushev-Poklad A, Borisov I, Grechko AV. The Importance of Orthostatic Increase in Pulse Wave Velocity in the Diagnosis of Early Vascular Aging. Journal of Clinical Medicine. 2024; 13(19):5713. https://doi.org/10.3390/jcm13195713
Chicago/Turabian StyleDorogovtsev, Victor, Dmitry Yankevich, Andrey Martyushev-Poklad, Ilya Borisov, and Andrey V. Grechko. 2024. "The Importance of Orthostatic Increase in Pulse Wave Velocity in the Diagnosis of Early Vascular Aging" Journal of Clinical Medicine 13, no. 19: 5713. https://doi.org/10.3390/jcm13195713







