Prone Positioning and Molecular Biomarkers in COVID and Non-COVID ARDS: A Narrative Review
Abstract
:1. Background
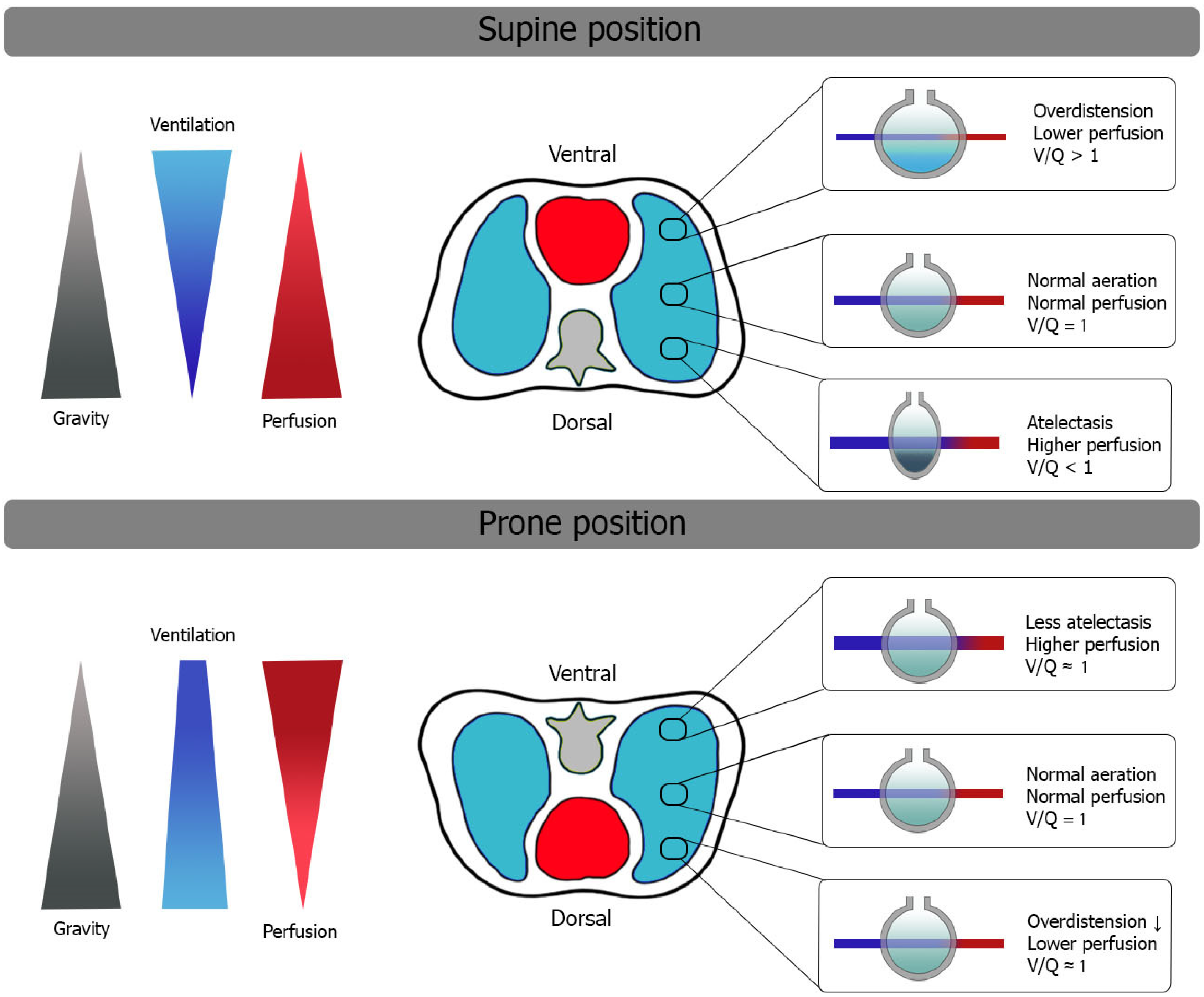
2. Oxygenation and Ventilatory Mechanics in Prone Position
3. Biomarkers in COVID ARDS and Non-COVID ARDS
4. Prone Positioning and Biomarkers in COVID-19 and Non-COVID-19 ARDS
Biomarkers in Prone Position in Relation to Main Outcomes
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Piehl, M.A.; Brown, R.S. Use of extreme position changes in acute respiratory failure. Crit. Care Med. 1976, 4, 13–14. [Google Scholar] [CrossRef]
- Gattinoni, L.; Tognoni, G.; Pesenti, A.; Taccone, P.; Mascheroni, D.; Labarta, V.; Malacrida, R.; Di Giulio, P.; Fumagalli, R.; Pelosi, P.; et al. Effect of Prone Positioning on the Survival of Patients with Acute Respiratory Failure. N. Engl. J. Med. 2001, 345, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.; Gaillard, S.; Lemasson, S.; Ayzac, L.; Girard, R.; Beuret, P.; Palmier, B.; Le, Q.V.; Sirodot, M.; Rosselli, S.; et al. Effects of Systematic Prone Positioning in Hypoxemic Acute Respiratory Failure: A Randomized Controlled Trial. JAMA 2004, 292, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Brusatori, S.; D’albo, R.; Maj, R.; Velati, M.; Zinnato, C.; Gattarello, S.; Lombardo, F.; Fratti, I.; Romitti, F.; et al. Prone position: How understanding and clinical application of a technique progress with time. Anesthesiol. Perioper. Sci. 2023, 1, 3. [Google Scholar] [CrossRef]
- Jozwiak, M.; Teboul, J.-L.; Anguel, N.; Persichini, R.; Silva, S.; Chemla, D.; Richard, C.; Monnet, X. Beneficial Hemodynamic Effects of Prone Positioning in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2013, 188, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.-D.; Combes, A.; Dreyfuss, D.; Forel, J.M.; Guérin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69. [Google Scholar] [CrossRef]
- Gattinoni, L.; Busana, M.; Giosa, L.; Macrì, M.M.; Quintel, M. Prone Positioning in Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 094–100. [Google Scholar] [CrossRef]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef]
- Camporota, L.; Sanderson, B.B.; Chiumello, D.; Terzi, N.; Argaud, L.; Rimmelé, T.; Metuor, R.; Verstraete, A.; Cour, M.; Bohé, J.; et al. Prone Position in COVID-19 and -COVID-19 Acute Respiratory Distress Syndrome: An International Multicenter Observational Comparative Study. Crit. Care Med. 2021, 50, 633–643. [Google Scholar] [CrossRef]
- Johannigman, J.A.; Davis, K.; Miller, S.L.; Campbell, R.S.; Luchette, F.A.; Frame, S.B.; Branson, R.D. Prone positioning for acute respiratory distress syndrome in the surgical intensive care unit: Who, when, and how long? Surgery 2000, 128, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Brazzi, L.; Gattinoni, L. Prone position in acute respiratory distress syndrome. Eur. Respir. J. 2002, 20, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Neyroud, F.; Barnsley, J.; Hunter, E.; Beecham, R.; Radharetnas, M.; Grocott, M.P.W.; Dushianthan, A. Prone Positioning in Mechanically Ventilated COVID-19 Patients: Timing of Initiation and Outcomes. J. Clin. Med. 2023, 12, 4226. [Google Scholar] [CrossRef] [PubMed]
- Munshi, L.; Del Sorbo, L.; Adhikari, N.K.J.; Hodgson, C.L.; Wunsch, H.; Meade, M.O.; Uleryk, E.; Mancebo, J.; Pesenti, A.; Ranieri, V.M.; et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14, S280–S288. [Google Scholar] [CrossRef]
- Spadaro, S.; Scaramuzzo, G.; Volta, C.A. Prone the Lung and Keep It Prone! Chest 2023, 163, 469–470. [Google Scholar] [CrossRef]
- Karlis, G.; Markantonaki, D.; Kakavas, S.; Bakali, D.; Katsagani, G.; Katsarou, T.; Kyritsis, C.; Karaouli, V.; Athanasiou, P.; Daganou, M. Prone Position Ventilation in Severe ARDS due to COVID-19: Comparison between Prolonged and Intermittent Strategies. J. Clin. Med. 2023, 12, 3526. [Google Scholar] [CrossRef]
- Okin, D.; Huang, C.-Y.; Alba, G.A.; Jesudasen, S.J.; Dandawate, N.A.; Gavralidis, A.; Chang, L.L.; Moin, E.E.; Ahmad, I.; Witkin, A.S.; et al. Prolonged Prone Position Ventilation Is Associated With Reduced Mortality in Intubated COVID-19 Patients. Chest 2022, 163, 533–542. [Google Scholar] [CrossRef]
- Parker, E.M.; Bittner, E.A.; Berra, L.; Pino, R.M. Efficiency of Prolonged Prone Positioning for Mechanically Ventilated Patients Infected with COVID-19. J. Clin. Med. 2021, 10, 2969. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Golicnik, A.; Zivanovic, I.; Gorjup, V.; Berden, J. Same but Different—ECMO in COVID-19 and ARDS of Other Etiologies. Comparison of Survival Outcomes and Management in Different ARDS Groups. J. Intensive Care Med. 2023, 38, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Schmidt, M.; Hajage, D.; Combes, A.; Petit, M.; Lebreton, G.; Rilinger, J.; Giani, M.; Le Breton, C.; Duburcq, T.; et al. Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Intensive Care Med. 2022, 48, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.H.; Ramanathan, K.; Ling, R.R.; Yang, I.X.; Tan, C.S.; Schmidt, M.; Shekar, K. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care 2021, 25, 292. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; On behalf of the LUNG SAFE Investigators and the ESICM Trials Group; Laffey, J.G.; Pham, T.; Fan, E. The LUNG SAFE study: A presentation of the prevalence of ARDS according to the Berlin Definition! Crit. Care 2016, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- For the Investigators of the APRONET Study Group; The REVA Network; The Réseau Recherche de la Société Française d’Anesthésie-Réanimation (SFAR-Recherche); The ESICM Trials Group; Guérin, C.; Beuret, P.; Constantin, J.M.; Bellani, G.; Garcia-Olivares, P.; Roca, O.; et al. A prospective international observational prevalence study on prone positioning of ARDS patients: The APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2017, 44, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Stilma, W.; van Meenen, D.M.P.; Valk, C.M.A.; de Bruin, H.; Paulus, F.; Neto, A.S.; Schultz, M.J.; on behalf of the PRoVENT-COVID Collaborative Group. Incidence and Practice of Early Prone Positioning in Invasively Ventilated COVID-19 Patients—Insights from the PRoVENT-COVID Observational Study. J. Clin. Med. 2021, 10, 4783. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Neto, A.S.; Paulus, F. Battling COVID-19-related mortality: From a fight for ventilators to a cry for oxygen. Lancet Respir. Med. 2021, 9, 939–941. [Google Scholar] [CrossRef]
- Constantin, J.-M.; Jabaudon, M.; Lefrant, J.-Y.; Jaber, S.; Quenot, J.-P.; Langeron, O.; Ferrandière, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880. [Google Scholar] [CrossRef]
- Bain, W.; Yang, H.; Shah, F.A.; Suber, T.; Drohan, C.; Al-Yousif, N.; DeSensi, R.S.; Bensen, N.; Schaefer, C.; Rosborough, B.R.; et al. COVID-19 versus Non–COVID-19 Acute Respiratory Distress Syndrome: Comparison of Demographics, Physiologic Parameters, Inflammatory Biomarkers, and Clinical Outcomes. Ann. Am. Thorac. Soc. 2021, 18, 1202–1210. [Google Scholar] [CrossRef]
- Gattinoni, L.; Taccone, P.; Carlesso, E.; Marini, J.J. Prone Position in Acute Respiratory Distress Syndrome. Rationale, Indications, and Limits. Am. J. Respir. Crit. Care Med. 2013, 188, 1286–1293. [Google Scholar] [CrossRef]
- Lamm, W.J.; Graham, M.M.; Albert, R.K. Mechanism by which the prone position improves oxygenation in acute lung injury. Am. J. Respir. Crit. Care Med. 1994, 150, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Nyrén, S.; Mure, M.; Jacobsson, H.; Larsson, S.A.; Lindahl, S.G.E.; Henderson, A.C.; Sá, R.C.; Theilmann, R.J.; Buxton, R.B.; Prisk, G.K.; et al. Pulmonary perfusion is more uniform in the prone than in the supine position: Scintigraphy in healthy humans. J. Appl. Physiol. 1999, 86, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhao, Z.; Chao, Y.; Chen, D.; Chen, H.; Zhang, R.; Liu, S.; Xie, J.; Yang, Y.; Qiu, H.; et al. Effects of early versus delayed application of prone position on ventilation–perfusion mismatch in patients with acute respiratory distress syndrome: A prospective observational study. Crit. Care 2023, 27, 462. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.K.; Hubmayr, R.D. The Prone Position Eliminates Compression of the Lungs by the Heart. Am. J. Respir. Crit. Care Med. 2000, 161, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Rimeika, D.; Nyrén, S.; Wiklund, N.P.; Koskela, L.R.; Tørring, A.; Gustafsson, L.E.; Larsson, S.A.; Jacobsson, H.; Lindahl, S.G.E.; Wiklund, C.U. Regulation of Regional Lung Perfusion by Nitric Oxide. Am. J. Respir. Crit. Care Med. 2004, 170, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, G.; Ball, L.; Pino, F.; Ricci, L.; Larsson, A.; Guérin, C.; Pelosi, P.; Perchiazzi, G. Influence of Positive End-Expiratory Pressure Titration on the Effects of Pronation in Acute Respiratory Distress Syndrome: A Comprehensive Experimental Study. Front. Physiol. 2020, 11, 179. [Google Scholar] [CrossRef]
- Scaramuzzo, G.; Broche, L.; Pellegrini, M.; Porra, L.; Derosa, S.; Tannoia, A.P.; Marzullo, A.; Borges, J.B.; Bayat, S.; Bravin, A.; et al. Regional Behavior of Airspaces During Positive Pressure Reduction Assessed by Synchrotron Radiation Computed Tomography. Front. Physiol. 2019, 10, 719. [Google Scholar] [CrossRef]
- Vollenberg, R.; Matern, P.; Nowacki, T.; Fuhrmann, V.; Padberg, J.-S.; Ochs, K.; Schütte-Nütgen, K.; Strauß, M.; Schmidt, H.; Tepasse, P.-R. Prone Position in Mechanically Ventilated COVID-19 Patients: A Multicenter Study. J. Clin. Med. 2021, 10, 1046. [Google Scholar] [CrossRef]
- Pelosi, P.; D’Andrea, L.; Vitale, G.; Pesenti, A.; Gattinoni, L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994, 149, 8–13. [Google Scholar] [CrossRef]
- Nuckton, T.J.; Alonso, J.A.; Kallet, R.H.; Daniel, B.M.; Pittet, J.-F.; Isner, M.D.E.; Matthay, M.A. Pulmonary Dead-Space Fraction as a Risk Factor for Death in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2002, 346, 1281–1286. [Google Scholar] [CrossRef]
- Fossali, T.; Pavlovsky, B.; Ottolina, D.; Colombo, R.; Basile, M.C.; Castelli, A.; Rech, R.; Borghi, B.; Ianniello, A.; Flor, N.; et al. Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients With COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. Crit. Care Med. 2022, 50, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Marino, A.; Brioni, M.; Cigada, I.; Menga, F.; Colombo, A.; Crimella, F.; Algieri, I.; Cressoni, M.; Carlesso, E.; et al. Lung Recruitment Assessed by Respiratory Mechanics and Computed Tomography in Patients with Acute Respiratory Distress Syndrome. What Is the Relationship? Am. J. Respir. Crit. Care Med. 2016, 193, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, G.; Spadaro, S.; Waldmann, A.D.; Böhm, S.H.; Ragazzi, R.; Marangoni, E.; Alvisi, V.; Spinelli, E.; Mauri, T.; Volta, C.A. Heterogeneity of regional inflection points from pressure-volume curves assessed by electrical impedance tomography. Crit. Care 2019, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- Musch, G.; Layfield, J.D.H.; Harris, R.S.; Melo, M.F.V.; Winkler, T.; Callahan, R.J.; Fischman, A.J.; Venegas, J.G. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J. Appl. Physiol. 2002, 93, 1841–1851. [Google Scholar] [CrossRef]
- Santini, A.; Protti, A.; Langer, T.; Comini, B.; Monti, M.; Sparacino, C.C.; Dondossola, D.; Gattinoni, L. Prone position ameliorates lung elastance and increases functional residual capacity independently from lung recruitment. Intensive Care Med. Exp. 2015, 3, 17. [Google Scholar] [CrossRef]
- Cornejo, R.A.; Díaz, J.C.; Tobar, E.A.; Bruhn, A.R.; Ramos, C.A.; González, R.A.; Repetto, C.A.; Romero, C.M.; Gálvez, L.R.; Llanos, O.; et al. Effects of Prone Positioning on Lung Protection in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2013, 188, 440–448. [Google Scholar] [CrossRef]
- The ICU-RER COVID-19 Collaboration; Scaramuzzo, G.; Gamberini, L.; Tonetti, T.; Zani, G.; Ottaviani, I.; Mazzoli, C.A.; Capozzi, C.; Giampalma, E.; Reggiani, M.L.B.; et al. Sustained oxygenation improvement after first prone positioning is associated with liberation from mechanical ventilation and mortality in critically ill COVID-19 patients: A cohort study. Ann. Intensive Care 2021, 11, 63. [Google Scholar] [CrossRef]
- Hering, R.; Wrigge, H.; Vorwerk, R.; Brensing, K.A.; Schröder, S.; Zinserling, J.; Hoeft, A.; Spiegel, T.V.; Putensen, C. The effects of prone positioning on intraabdominal pressure and cardiovascular and renal function in patients with acute lung injury. Anesth. Analg. 2001, 92, 1226–1231. [Google Scholar] [CrossRef]
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, M.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022, 48, 667–678. [Google Scholar] [CrossRef]
- Huang, S.; Vieillard-Baron, A.; Evrard, B.; Prat, G.; Chew, M.S.; Balik, M.; Clau-Terré, F.; De Backer, D.; Dessap, A.M.; Orde, S.; et al. Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: Post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023, 49, 946–956. [Google Scholar] [CrossRef]
- Guérin, C.; Albert, R.K.; Beitler, J.; Gattinoni, L.; Jaber, S.; Marini, J.J.; Munshi, L.; Papazian, L.; Pesenti, A.; Vieillard-Baron, A.; et al. Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Med. 2020, 46, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Vagginelli, F.; Carlesso, E.; Taccone, P.; Conte, V.; Chiumello, D.; Valenza, F.; Caironi, P.; Pesenti, A. Decrease in Paco2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit. Care Med. 2003, 31, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Protti, A.; Chiumello, D.; Cressoni, M.; Carlesso, E.; Mietto, C.; Berto, V.; Lazzerini, M.; Quintel, M.; Gattinoni, L. Relationship between gas exchange response to prone position and lung recruitability during acute respiratory failure. Intensive Care Med. 2009, 35, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Tomashefski, J.F., Jr. Pulmonary Pathology of Acute Respiratory Distress Syndrome. Clin. Chest Med. 2000, 21, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, P.; Rietdijk, W.; Somhorst, P.; Endeman, H.; Gommers, D. A systematic review of biomarkers multivariately associated with acute respiratory distress syndrome development and mortality. Crit. Care 2020, 24, 243. [Google Scholar] [CrossRef] [PubMed]
- Curley, G.F.; Laffey, J.G.; Zhang, H.; Slutsky, A.S. Biotrauma and Ventilator-Induced Lung Injury. Chest 2016, 150, 1109–1117. [Google Scholar] [CrossRef]
- García-Laorden, M.I.; Lorente, J.A.; Flores, C.; Slutsky, A.S.; Villar, J. Biomarkers for the acute respiratory distress syndrome: How to make the diagnosis more precise. Ann. Transl. Med. 2017, 5, 283. [Google Scholar] [CrossRef]
- Bos, L.D.; Weda, H.; Wang, Y.; Knobel, H.H.; Nijsen, T.M.; Vink, T.J.; Zwinderman, A.H.; Sterk, P.J.; Schultz, M.J. Exhaled breath metabolomics as a noninvasive diagnostic tool for acute respiratory distress syndrome. Eur. Respir. J. 2014, 44, 188–197. [Google Scholar] [CrossRef]
- Calfee, C.S.; Ware, L.B.; Eisner, M.D.; Parsons, P.E.; Thompson, B.T.; Wickersham, N.; Matthay, M.A.; Network, T.N.A. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008, 63, 1083–1089. [Google Scholar] [CrossRef]
- Jabaudon, M.; Blondonnet, R.; Pereira, B.; Cartin-Ceba, R.; Lichtenstern, C.; Mauri, T.; Determann, R.M.; Drabek, T.; Hubmayr, R.D.; Gajic, O.; et al. Plasma sRAGE is independently associated with increased mortality in ARDS: A meta-analysis of individual patient data. Intensive Care Med. 2018, 44, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Radujkovic, A.; Weigand, M.A.; Merle, U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann. Intensive Care 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Sato, N.; Nakae, H.; Yamada, Y.; Makabe, H.; Abe, H.; Imai, S.; Wakabayashi, G.; Inada, K.; Sato, S. Surfactant protein A and D (SP-A, AP-D) levels in patients with septic ARDS. Res. Commun. Mol. Pathol. Pharmacol. 2002, 111, 245–251. [Google Scholar] [PubMed]
- Determann, R.M.; Royakkers, A.A.; Haitsma, J.J.; Zhang, H.; Slutsky, A.S.; Ranieri, V.M.; Schultz, M.J. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm. Med. 2010, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Peukert, K.; Seeliger, B.; Fox, M.; Feuerborn, C.; Sauer, A.; Schuss, P.; Schneider, M.; David, S.; Welte, T.; Putensen, C.; et al. SP-D Serum Levels Reveal Distinct Epithelial Damage in Direct Human ARDS. J. Clin. Med. 2021, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, M.L.; Aman, J.; van Nieuw, A.; Geerten, P.; Groeneveld, A.B.J. Plasma Biomarkers for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Crit. Care Med. 2014, 42, 691–700. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Q.; Zhao, L.; Liu, Z.; Cui, H.; Li, P.; Fan, H.; Guo, L. Circulating Pulmonary-Originated Epithelial Biomarkers for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 6090. [Google Scholar] [CrossRef]
- Ware, L.B.; Koyama, T.; Billheimer, D.D.; Wu, W.; Bernard, G.R.; Thompson, B.T.; Brower, R.G.; Standiford, T.J.; Martin, T.R.; Matthay, M.A. Prognostic and Pathogenetic Value of Combining Clinical and Biochemical Indices in Patients with Acute Lung Injury. Chest 2010, 137, 288–296. [Google Scholar] [CrossRef]
- Calfee, C.S.; Ware, L.B.; Glidden, D.V.; Eisner, M.D.; Parsons, P.E.; Thompson, B.T.; Matthay, M.A. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit. Care Med. 2011, 39, 711–717. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Suter, P.M.; Tortorella, C.; De Tullio, R.; Dayer, J.M.; Brienza, A.; Bruno, F.; Slutsky, A.S. Effect of Mechanical Ventilation on Inflammatory Mediators in Patients With Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA 1999, 282, 54–61. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.A.H.; Garrigos, Z.E.; Marcelin, J.R.; Vijayvargiya, P. COVID-19 Pathogenesis and Clinical Manifestations. Infect. Dis. Clin. N. Am. 2022, 36, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, G.; Nucera, F.; Asmundo, A.; Messina, R.; Mari, M.; Montanaro, F.; Johansen, M.D.; Monaco, F.; Fadda, G.; Tuccari, G.; et al. Cellular and molecular features of COVID-19 associated ARDS: Therapeutic relevance. J. Inflamm. 2023, 20, 11. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Domingo, P.; Mur, I.; Mateo, G.M.; Gutierrez, M.D.M.; Pomar, V.; de Benito, N.; Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; et al. Association between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef]
- Gandini, L.; Fior, G.; Schibler, A.; Obonyo, N.G.; Bassi, G.L.; Suen, J.Y.; Fraser, J.F. Interleukin-6 inhibitors in non-COVID-19 ARDS: Analyzing the past to step into the post-COVID-19 era. Crit. Care 2023, 27, 124. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Martucci, G.; La Via, L.; Cuttone, G.; Dimarco, G.; Pulizzi, C.; Arcadipane, A.; Astuto, M. Hemoperfusion and blood purification strategies in patients with COVID-19: A systematic review. Artif. Organs 2021, 45, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bagshaw, S.M.; Bellomo, R.; Clark, W.R.; Husain-Syed, F.; Kellum, J.A.; Ricci, Z.; Rimmelé, T.; Reis, T.; Ostermann, M. Extracorporeal Blood Purification and Organ Support in the Critically Ill Patient during COVID-19 Pandemic: Expert Review and Recommendation. Blood Purif. 2020, 50, 17–27. [Google Scholar] [CrossRef]
- Hayanga, J.W.A.; Song, T.; Durham, L.; Garrison, L.; Smith, D.; Molnar, Z.; Scheier, J.; Deliargyris, E.N.; Moazami, N. Extracorporeal hemoadsorption in critically ill COVID-19 patients on VV ECMO: The CytoSorb therapy in COVID-19 (CTC) registry. Crit. Care 2023, 27, 243. [Google Scholar] [CrossRef]
- Lertussavavivat, T.; Srisawat, N. Hemoperfusion in COVID-19. Contrib. Nephrol. 2023, 200, 192–200. [Google Scholar] [CrossRef]
- Ricci, Z.; Romagnoli, S.; Reis, T.; Bellomo, R.; Ronco, C. Hemoperfusion in the intensive care unit. Intensive Care Med. 2022, 48, 1397–1408. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Selickman, J.; Vrettou, C.S.; Mentzelopoulos, S.D.; Marini, J.J. COVID-19-Related ARDS: Key Mechanistic Features and Treatments. J. Clin. Med. 2022, 11, 4896. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Fogagnolo, A.; Campo, G.; Zucchetti, O.; Verri, M.; Ottaviani, I.; Tunstall, T.; Grasso, S.; Scaramuzzo, V.; Murgolo, F.; et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit. Care 2021, 25, 74. [Google Scholar] [CrossRef]
- Jovandaric, M.Z.; Dokic, M.; Babovic, I.R.; Milicevic, S.; Dotlic, J.; Milosevic, B.; Culjic, M.; Andric, L.; Dimic, N.; Mitrovic, O.; et al. The Significance of COVID-19 Diseases in Lipid Metabolism Pregnancy Women and Newborns. Int. J. Mol. Sci. 2022, 23, 15098. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Kansakar, U.; Sardu, C.; Varzideh, F.; Avvisato, R.; Wang, X.; Matarese, A.; Marfella, R.; Ziosi, M.; Gambardella, J.; et al. COVID-19 Causes Ferroptosis and Oxidative Stress in Human Endothelial Cells. Antioxidants 2023, 12, 326. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Physiol. 2021, 322, C1–C11. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Sinha, P.; Furfaro, D.; Cummings, M.J.; Abrams, D.; Delucchi, K.; Maddali, M.V.; He, J.; Thompson, A.; Murn, M.; Fountain, J.; et al. Latent Class Analysis Reveals COVID-19–related Acute Respiratory Distress Syndrome Subgroups with Differential Responses to Corticosteroids. Am. J. Respir. Crit. Care Med. 2021, 204, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Blanco, J.; Kacmarek, R.M. Acute respiratory distress syndrome definition: Do we need a change? Curr. Opin. Crit. Care 2011, 17, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Sega, F.V.D.; Fortini, F.; Spadaro, S.; Ronzoni, L.; Zucchetti, O.; Manfrini, M.; Mikus, E.; Fogagnolo, A.; Torsani, F.; Pavasini, R.; et al. Time course of endothelial dysfunction markers and mortality in COVID-19 patients: A pilot study. Clin. Transl. Med. 2021, 11, e283. [Google Scholar] [CrossRef] [PubMed]
- Kallet, R.H. A Comprehensive Review of Prone Position in ARDS. Respir. Care 2015, 60, 1660–1687. [Google Scholar] [CrossRef] [PubMed]
- Nonas, S.A.; Vinasco, L.M.; Ma, S.F.; Jacobson, J.R.; Desai, A.A.; Dudek, S.M.; Flores, C.; Hassoun, P.M.; Sam, L.; Ye, S.Q.; et al. Use of consomic rats for genomic insights into ventilator-associated lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2007, 293, L292–L302. [Google Scholar] [CrossRef] [PubMed]
- Dolinay, T.; Kaminski, N.; Felgendreher, M.; Kim, H.P.; Reynolds, P.; Watkins, S.C.; Karp, D.; Uhlig, S.; Choi, A.M.K. Gene expression profiling of target genes in ventilator-induced lung injury. Physiol. Genom. 2006, 26, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; He, Q.; Edwards, M.G.; Sergew, A.; Riches, D.W.H.; Albert, R.K.; Douglas, I.S. Mitogen-activated Protein Kinase Phosphatase-1 Modulates Regional Effects of Injurious Mechanical Ventilation in Rodent Lungs. Am. J. Respir. Crit. Care Med. 2012, 186, 72–81. [Google Scholar] [CrossRef]
- Rashid, A.; Zeng, C.; Motta-Ribeiro, G.; Dillon, S.T.; Libermann, T.A.; Lessa, M.A.; Bagchi, A.; Hutchinson, J.; Melo, M.F.V. Proteomics of lung tissue reveals differences in inflammation and alveolar-capillary barrier response between atelectasis and aerated regions. Sci. Rep. 2022, 12, 7065. [Google Scholar] [CrossRef]
- Papazian, L.; Gainnier, M.; Marin, V.; Donati, S.; Arnal, J.-M.; Demory, D.; Roch, A.; Forel, J.-M.; Bongrand, P.; Brégeon, F.; et al. Comparison of prone positioning and high-frequency oscillatory ventilation in patients with acute respiratory distress syndrome. Crit. Care Med. 2005, 33, 2162–2171. [Google Scholar] [CrossRef]
- Chan, M.-C.; Hsu, J.-Y.; Liu, H.-H.; Lee, Y.-L.; Pong, S.-C.; Chang, L.-Y.; Kuo, B.I.-T.; Wu, C.-L. Effects of Prone Position on Inflammatory Markers in Patients with ARDS Due to Community-acquired Pneumonia. J. Formos. Med. Assoc. 2007, 106, 708–716. [Google Scholar] [CrossRef]
- Musso, G.; Taliano, C.; De Iuliis, M.; Paschetta, E.; Fonti, C.; Ferraris, A.; Druetta, M.; Vianou, I.S.; Ranghino, F.; Riedo, F.; et al. Mechanical power normalized to aerated lung predicts noninvasive ventilation failure and death and contributes to the benefits of proning in COVID-19 hypoxemic respiratory failure. EPMA J. 2023, 14, 341–379. [Google Scholar] [CrossRef] [PubMed]


| Biomarkers in Prone Position | ||||
| NON-COVID ARDS | Reference | Year | Biomarker | Main Outcome (s) |
| Papazian L | 2005 | Both in blood and in BAL: neutrophils, IL-8; IL-1B; IL-6; TNF-α. | To compare the physiologic (oxygenation) and proinflammatory effects of HFOV, prone positioning (PP), or their combination in severe ARDS | |
| Chan M-C | 2007 | IL-6, IL-10, IL-1B; TNF-α | To evaluate the safety of continuous PP ventilation and its effects on oxygenation and biomarkers. Compared with supine. | |
| C-ARDS | Musso G | 2023 | CRP, Procalcitonin, D-Dimer | 2º outcome: the effect of PP on Mechanical Power. Mechanical Ventilation (MV) parameters, biomarkers, days of MV, and mortality |
| Lavillegrand J-R | 2021 | IL-1B, IL-6, CRP, IL-10, TNF-α, fibrinogen, limphocyte | To compare the immuno-inflammatory features according to organ failure severity and in-ICU mortality. 28D Mortality. | |
| Biomarkers in supine position | ||||
| NON-COVID ARDS | Mrozek S. | 2016 | sRAGE | To characterize focal and non-focal patterns of lung CT-based imaging with biomarkers. ARDS phenotype, duration of MV, 28D, and 90D mortality |
| Rosenberg CM | 2023 | Angiopoietin-2 | To evaluate if plasma Ang-2 would be associated with the development of ARDS and poor clinical outcomes. Development of ARDS, severity of illness, and 30D mortality. | |
| Bendib I | 2021 | BAL fluid to serum ratio of IL8, BAL fluid to serum ratio of IL1, IL6, IP-10/CXCL, and IL10. TNFa, IFNg, ICAM-1, GM-CSF, VEGF, Angiopoetin1/2, RAGE, SP-D, HLA-DR CD8+ lymphocytes, and PD-1. | To evaluate the interrelation of ARDS/sepsis biomarkers in the alveolar and blood compartments and explore their association with clinical outcomes. Hospital mortality. | |
| Dong X | 2020 | Plasma IGFBP7, vWF, t-PA | To identify causal protein biomarkers for ARDS 28-D mortality using muti-stage Mendelian randomization. | |
| Headley AS | 1997 | TNF-α, IL1B, IL6, IL8, | To evaluate the relationships among clinical variables and biological markers of SIRS and patient outcome. Mortality. | |
| Yao-Ling-Lee | 2021 | IL6, IL8, IL1B, IL10, TNF-α, CRP | To determine whether biomarkers and endotoxins on the first day of ICU are associated with hospital mortality in severe pneumonia. Mortality | |
| Ware | 2013 | SP-D, RAGE, IL-8, CC-16, IL-6, ANG 2, MMP 1/3/9, PCPIII, BNP | To test if a biomarker panel would be useful for biologic confirmation of the clinical diagnosis of ARDS. The development of ARDS and the severity of the illness. | |
| C-ARDS | Bain W | 2021 | IL-6, IL-8, IL-10 | To compare key demographic and physiologic parameters, biomarkers, and clinical outcomes of COVID ARDS and non-COVID ARDS. 60D mortality, days of MV, MV parameters. |
| Grasselli G | 2020 | D-dimer | To examine the functional and morphological features of COVID ARDS and to compare them with non-COVID ARDS. Mortality. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadaro, S.; Jimenez-Santana, J.D.; La Rosa, R.; Spinazzola, G.; Argente Navarro, P.; Volta, C.A.; Scaramuzzo, G. Prone Positioning and Molecular Biomarkers in COVID and Non-COVID ARDS: A Narrative Review. J. Clin. Med. 2024, 13, 317. https://doi.org/10.3390/jcm13020317
Spadaro S, Jimenez-Santana JD, La Rosa R, Spinazzola G, Argente Navarro P, Volta CA, Scaramuzzo G. Prone Positioning and Molecular Biomarkers in COVID and Non-COVID ARDS: A Narrative Review. Journal of Clinical Medicine. 2024; 13(2):317. https://doi.org/10.3390/jcm13020317
Chicago/Turabian StyleSpadaro, Savino, Jose Daniel Jimenez-Santana, Riccardo La Rosa, Giorgia Spinazzola, Pilar Argente Navarro, Carlo Alberto Volta, and Gaetano Scaramuzzo. 2024. "Prone Positioning and Molecular Biomarkers in COVID and Non-COVID ARDS: A Narrative Review" Journal of Clinical Medicine 13, no. 2: 317. https://doi.org/10.3390/jcm13020317







