Eph/ephrin Signaling and Biology of Mesenchymal Stromal/Stem Cells
Abstract
:1. Mesenchymal Stromal/Stem Cells (MSCs)
2. Eph/ephrin Molecules
3. Eph and MSCs
3.1. Expression of Eph/ephrins on MSCs
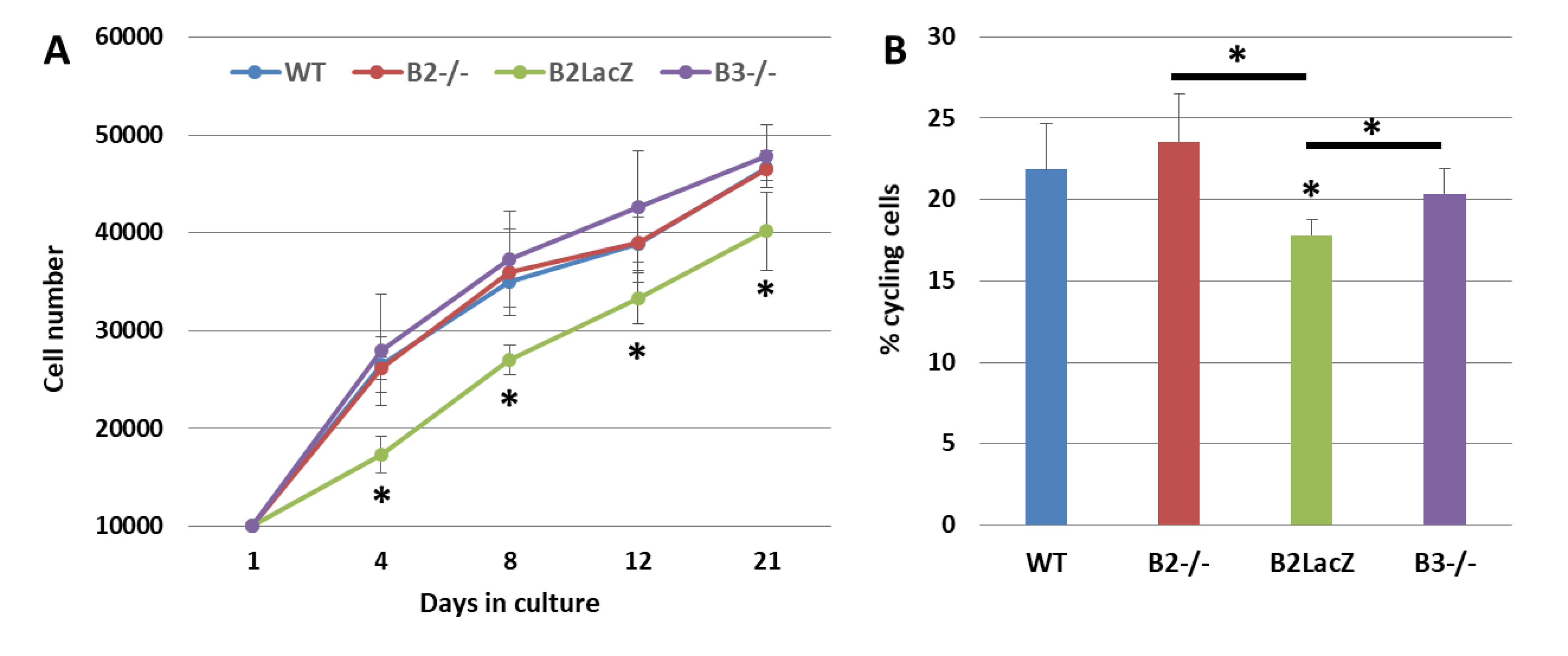
3.2. Effects of Eph/ephrins on the Survival, Proliferation, and Differentiation of MSCs
3.3. Eph/ephrin-Mediated Effects of MSCs on Progenitor Cells
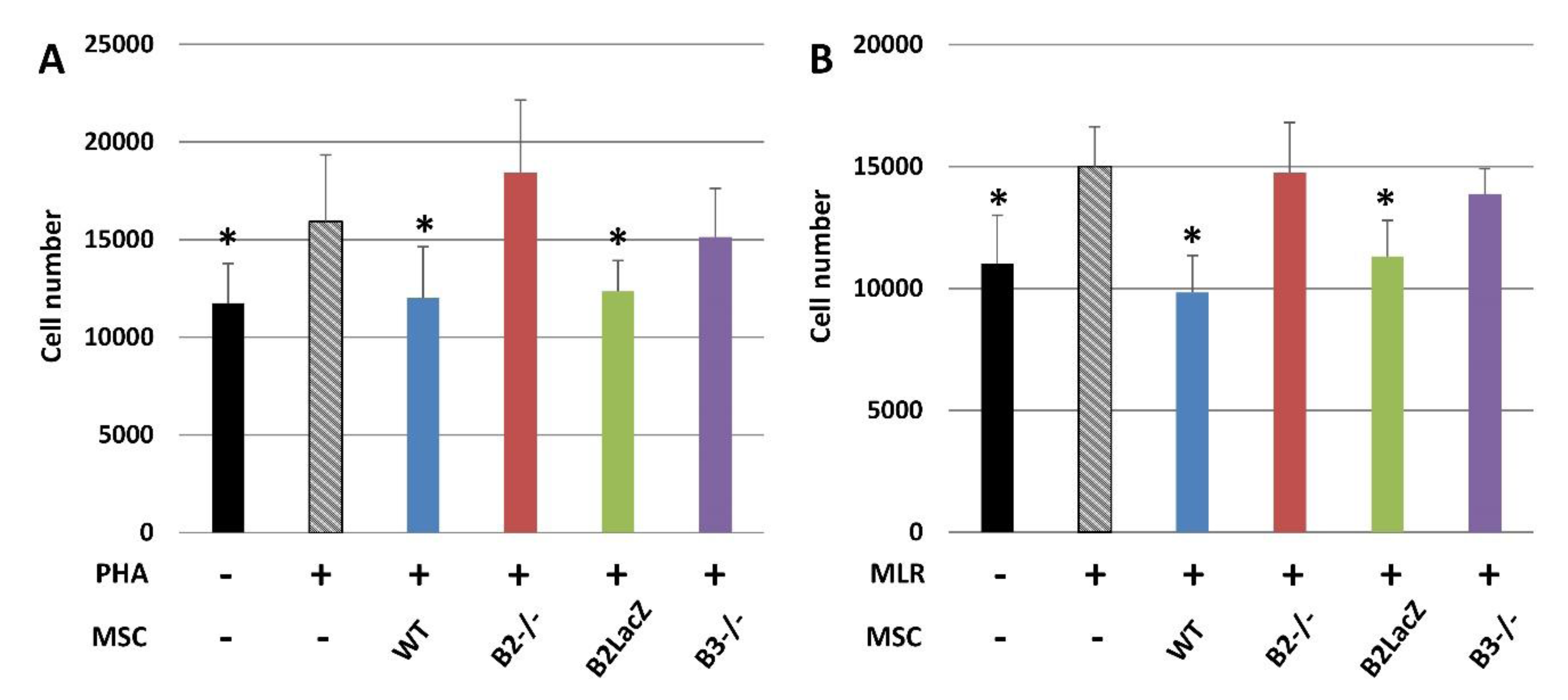
3.4. The Role of Eph and ephrins in the Immunomodulatory Properties of MSCs
4. Conclusions and Further Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation List
| MSCs | mesenchymal stromal/stem cells |
| CFU-Fb | colony forming unit-fibroblastic-like cells |
| BM | bone marrow |
| IFN-γ | interferon gamma |
| PDGFR | platelet-derived growth factor receptor |
| NGFR | nerve growth factor receptor |
| IL | interleukin |
| CCR | C chemokine receptor |
| CXCR | C-X-C chemokine receptor |
| TLR | Toll-like receptor |
| TNF-α | tumor necrosis factor alpha |
| DC | dendritic cells |
| TGFb | transforming growth factor beta |
| HGF | hepatocyte growth factor |
| PGE2 | prostaglandin E2 |
| IDO | indoleamine 2,3-dioxygenase |
| BMP | bone morphogenetic proteins |
| PSGL-1 | P-selectin glycoprotein ligand |
| sLeX | sialyl Lewis X |
| GPI | glycosyl-phosphatidyl-inositol |
| BM-MSC | MSCs derived from the stromal fraction of bone marrow |
| Ad-MSC | MSCs derived from the adipose tissue |
| WT | wild-type |
| HPSCs | hematopoietic progenitor stem cells |
| HSCs | hematopoietic stem cells |
| BFU-E | erythroid burst-forming units |
| PHA | phytohemagglutinin |
| MLR | mixed lymphocyte reaction |
| iNOS | inducible nitric oxide synthase |
References
- Galipeau, J.; Sensebé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Najar, M.; Bouhtit, F.; Melki, R.; Afif, H.; Hamal, A.; Fahmi, H.; Merimi, M.; Lagneaux, L. Mesenchymal Stromal Cell-Based Therapy: New Perspectives and Challenges. J. Clin. Med. 2019, 8, 626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; I Piatetzky-Shapiro, I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Pittenger, M.F. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lin, C.S.; Xin, Z.C.; Dai, J.; Lue, T.F. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol. Histopathol. 2013, 28, 1109–1116. [Google Scholar]
- Baddoo, M.; Hill, K.; Wilkinson, R.; Gaupp, D.; Hughes, C.; Kopen, G.C.; Phinney, D.G. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J. Cell. Biochem. 2003, 89, 1235–1249. [Google Scholar] [CrossRef]
- Meirelles, L.D.S.; Nardi, N.B. Murine marrow-derived mesenchymal stem cell: Isolation, in vitroexpansion, and characterization. Br. J. Haematol. 2003, 123, 702–711. [Google Scholar] [CrossRef]
- Peister, A.; Mellad, J.A.; Larson, B.L.; Hall, B.M.; Gibson, L.F.; Prockop, D.J. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 2004, 103, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.K.; Guo, Z.K.; Xiao, X.R.; Liu, B.; Liu, X.D.; Tang, P.H.; Mao, N. Isolation of Mouse Marrow Mesenchymal Progenitors by a Novel and Reliable Method. Stem Cells 2003, 21, 527–535. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhu, R.J.; Li, H.L.; Li, J.; Han, Q.; Zhao, R.C. Mesenchymal stem cells and immune disorders: From basic science to clinical transition. Front. Med. 2019, 13, 138–151. [Google Scholar] [CrossRef]
- De Castro, L.L.; Lopes-Pacheco, M.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J. Mol. Med. 2019, 97, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, G.Q.; Liu, M.; Zhou, T.T.; Zhou, H.B. Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. J. Biosci. Bioeng. 2016, 121, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef]
- Slukvin, I.I.; Kumar, A. The mesenchymoangioblast, mesodermal precursor for mesenchymal and endothelial cells. Cell. Mol. Life Sci. 2018, 75, 3507–3520. [Google Scholar] [CrossRef]
- Isern, J.; García-García, A.; Martín, A.M.; Arranz, L.; Martín-Pérez, D.; Torroja, C.; Sanchez-Cabo, F.; Méndez-Ferrer, S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 2014, 3, e03696. [Google Scholar] [CrossRef]
- Shi, S.; Gronthos, S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.K.; Chen, C.C.; Luppen, C.A.; Kim, J.B.; DeBoer, A.T.; Wei, K.; Helms, J.A.; Kuo, C.J.; Kraft, D.L.; Weissman, I.L. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 2009, 457, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017, 20, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Kallens, P.; Terraza, C.; Oyarce, K.; Gajardo, T.; Campos-Mora, M.; Barroilhet, M.T.; Alvarez, C.; Fuentes, R.; Figueroa, F.; Khoury, M.; et al. Mesenchymal stem cells and their immunosuppressive role in transplantation tolerance. Ann. N. Y. Acad. Sci. 2018, 1417, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057623. [Google Scholar] [CrossRef] [PubMed]
- Poltavtseva, R.A.; Poltavtsev, A.V.; Lutsenko, G.V.; Svirshchevskaya, E.V. Myths, reality and future of mesenchymal stem cell therapy. Cell Tissue Res. 2019, 375, 563–574. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [Green Version]
- Sackstein, R.; Merzaban, J.S.; Cain, D.W.; Dagia, N.M.; Spencer, J.A.; Lin, C.P.; Wohlgemuth, R. Ex Vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008, 14, 181–187. [Google Scholar] [CrossRef]
- García-Ceca, J.; Alfaro, D.; Montero-Herradón, S.; Tobajas, E.; Muñoz, J.J.; Zapata, A.G. Eph/Ephrins-Mediated Thymocyte–Thymic Epithelial Cell Interactions Control Numerous Processes of Thymus Biology. Front. Immunol. 2015, 6, 333. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.; Campbell, J.; Nobes, C.D. Ephs and ephrins. Curr. Biol. 2017, 27, R90–R95. [Google Scholar] [CrossRef] [Green Version]
- Barquilla, A.; Pasquale, E.B. Eph receptors and ephrins: Therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 465–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuzi, N.; Gullick, W. eph, the largest known family of putative growth factor receptors. Br. J. Cancer 1994, 69, 417–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulthard, M.G.; Duffy, S.; Down, M.; Evans, B.; Power, M.; Smith, F.; Stylianou, C.; Kleikamp, S.; Oates, A.; Lackmann, M.; et al. The role of the Eph-ephrin signalling system in the regulation of developmental patterning. Int. J. Dev. Biol. 2002, 46, 375–384. [Google Scholar]
- Sasaki, E.; Hikono, H.; Kaku, Y.; Kuwana, T.; Naito, M.; Sakurai, M. ephA9, a novel avian receptor tyrosine kinase gene. Gene 2003, 316, 103–110. [Google Scholar] [CrossRef]
- Kania, A.; Klein, R. Mechanisms of ephrin–Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef]
- Alfaro, D.; Garcia-Ceca, J.; Montero-Herradón, S.; Zapata, A.G. Lymphoid Seeding in the Thymus: A New Function for EphB2 and EphB3. Immunogenet Open Access 2016, 1, 3. [Google Scholar]
- Arthur, A.; Zannettino, A.; Panagopoulos, R.; Koblar, S.A.; Sims, N.A.; Stylianou, C.; Matsuo, K.; Gronthos, S. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone 2011, 48, 533–542. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, S.J.; Oh, S.Y.; Lee, H.J.; Ryu, J.M.; Han, H.J. Oleic acid enhances the motility of umbilical cord blood derived mesenchymal stem cells through EphB2-dependent F-actin formation. Biochim. Biophys. Acta BBA Mol. Cell Res. 2015, 1853, 1905–1917. [Google Scholar] [CrossRef] [Green Version]
- Kwak, H.; Salvucci, O.; Weigert, R.; Martinez-Torrecuadrada, J.L.; Henkemeyer, M.; Poulos, M.G.; Butler, J.M.; Tosato, G. Sinusoidal ephrin receptor EPHB4 controls hematopoietic progenitor cell mobilization from bone marrow. J. Clin. Investig. 2016, 126, 4554–4568. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Arthur, A.; Panagopoulos, R.; Paton, S.; Hayball, J.D.; Zannettino, A.C.; Purton, L.E.; Matsuo, K.; Gronthos, S. EphB4 Expressing Stromal Cells Exhibit an Enhanced Capacity for Hematopoietic Stem Cell Maintenance. Stem Cells 2015, 33, 2838–2849. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.R.; Kim, J.; Wergedal, J.; Chen, S.T.; Mohan, S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Mol. Cell. Biol. 2010, 30, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Azab, F.; Azab, A.K.; Maiso, P.; Calimeri, T.; Flores, L.; Liu, Y.; Quang, P.; Roccaro, A.M.; Sacco, A.; Ngo, H.T.; et al. Eph-B2/ephrin-B2 interaction plays a major role in the adhesion and proliferation of Waldenstrom’s macroglobulinemia. Clin. Cancer Res. 2012, 18, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro, D.; Zapata, A.G. Eph/Ephrin-mediated stimulation of human bone marrow mesenchymal stromal cells correlates with changes in cell adherence and increased cell death. Stem Cell Res. Ther. 2018, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Alfonso, Z.; Zuk, P.A.; Elbarbary, A.; Zhu, M.; Ashjian, P.; Benhaim, P.; Hedrick, M.H.; Fraser, J.K. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 2003, 89, 267–270. [Google Scholar] [CrossRef]
- Ahmadian Kia, N.; Bahrami, A.R.; Ebrahimi, M.; Matin, M.M.; Neshati, Z.; Almohaddesin, M.R.; Aghdami, N.; Bidkhori, H.R. Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J. Mol. Neurosci. 2011, 44, 178–185. [Google Scholar] [CrossRef]
- Freywald, A.; Sharfe, N.; Rashotte, C.; Grünberger, T.; Roifman, C.M. The EphB6 Receptor Inhibits JNK Activation in T Lymphocytes and Modulates T Cell Receptor-mediated Responses. J. Biol. Chem. 2003, 278, 10150–10156. [Google Scholar] [CrossRef] [Green Version]
- Freywald, A.; Sharfe, N.; Miller, C.D.; Rashotte, C.; Roifman, C.M. EphA receptors inhibit anti-CD3-induced apoptosis in thymocytes. J. Immunol. 2006, 176, 4066–4074. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Mao, J.; Wu, Y.; Luo, H.; Wu, J. Ephrin-B1 Is Critical in T-cell Development. J. Biol. Chem. 2006, 281, 10222–10229. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.Y.; Wan, X.C.; Wu, Y.L.; Wu, J.P. Cross-linking of EphB6 resulting in signal transduction and apoptosis in Jurkat cells. J. Immunol. 2001, 167, 1362–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro, D.; García-Ceca, J.J.; Cejalvo, T.; Jimenez, E.; Jenkinson, E.J.; Anderson, G.; Muñoz, J.J.; Zapata, A. EphrinB1-EphB signaling regulates thymocyte-epithelium interactions involved in functional T cell development. Eur. J. Immunol. 2007, 37, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996, 12, 463–519. [Google Scholar] [CrossRef] [Green Version]
- Vail, M.E.; Murone, C.; Tan, A.; Hii, L.; Abebe, D.; Janes, P.W.; Lee, F.T.; Baer, M.; Palath, V.; Bebbington, C.; et al. Targeting EphA3 Inhibits Cancer Growth by Disrupting the Tumor Stromal Microenvironment. Cancer Res. 2014, 74, 4470–4481. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, D.; Munoz, J.J.; Garcia-Ceca, J.; Cejalvo, T.; Jimenez, E.; Zapata, A.G. The Eph/ephrinB signal balance determines the pattern of T-cell maturation in the thymus. Immunol. Cell Biol. 2011, 89, 844–852. [Google Scholar] [CrossRef]
- Chukkapalli, S.; Amessou, M.; Dilly, A.K.; Dekhil, H.; Zhao, J.; Liu, Q.; Bejna, A.; Thomas, R.D.; Bandyopadhyay, S.; Bismar, T.A.; et al. Role of the EphB2 receptor in autophagy, apoptosis and invasion in human breast cancer cells. Exp. Cell Res. 2014, 320, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Li, X.Y.; Cui, P.; Gao, F.; Zhu, K.; Li, L.Z.; Sun, X.H.; Wang, Z.F. Involvement of mGluR I in EphB/ephrinB reverse signaling activation induced retinal ganglion cell apoptosis in a rat chronic hypertension model. Brain Res. 2018, 1683, 27–35. [Google Scholar] [CrossRef]
- Arthur, A.; Koblar, S.; Shi, S.; Gronthos, S. Eph/ephrinB Mediate Dental Pulp Stem Cell Mobilization and Function. J. Dent. Res. 2009, 88, 829–834. [Google Scholar] [CrossRef]
- Arthur, A.; Nguyen, T.M.; Paton, S.; Zannettino, A.C.; Gronthos, S. Loss of EfnB1 in the osteogenic lineage compromises their capacity to support hematopoietic stem/progenitor cell maintenance. Exp. Hematol. 2019, 69, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhang, Z.H.; Sun, N.; Dong, S.W.; Xu, J.Z.; Dai, F. EphB4 Promotes Osteogenesis of CTLA4-Modified Bone Marrow-Derived Mesenchymal Stem Cells Through Cross Talk with Wnt Pathway in Xenotransplantation. Tissue Eng. Part A 2015, 21, 2404–2416. [Google Scholar] [CrossRef]
- Inada, T.; Iwama, A.; Sakano, S.; Ohno, M.; Sawada, K.I.; Suda, T. Selective Expression of the Receptor Tyrosine Kinase, HTK, on Human Erythroid Progenitor Cells. Blood 1997, 89, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.B.; Dimos, J.T.; Schaniel, C.; Hackney, J.A.; Moore, K.A.; Lemischka, I.R. A Stem Cell Molecular Signature. Science 2002, 298, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Yanai, N.; Obinata, M. Stromal cells modulate ephrinB2 expression and transmigration of hematopoietic cells. Exp. Hematol. 2006, 34, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Steidl, U.; Bork, S.; Schaub, S.; Selbach, O.; Seres, J.; Aivado, M.; Schroeder, T.; Rohr, U.P.; Fenk, R.; Kliszewski, S.; et al. Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood 2004, 104, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.M.; Arthur, A.; Zannettino, A.C.; Gronthos, S. EphA5 and EphA7 forward signaling enhances human hematopoietic stem and progenitor cell maintenance, migration, and adhesion via Rac1 activation. Exp. Hematol. 2017, 48, 72–78. [Google Scholar] [CrossRef]
- Ting, M.J.; Day, B.W.; Spanevello, M.D.; Boyd, A.W. Activation of ephrin A proteins influences hematopoietic stem cell adhesion and trafficking patterns. Exp. Hematol. 2010, 38, 1087–1098. [Google Scholar] [CrossRef]
- Lazarova, P.; Wu, Q.; Kvalheim, G.; Suo, Z.; Haakenstad, K.W.; Metodiev, K.; Nesland, J.M. Growth factor receptors in hematopoietic stem cells: EPH family expression in CD34+ and CD133+ cell populations from mobilized peripheral blood. Int. J. Immunopathol. Pharmacol. 2006, 19, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Miura, N.; Bonelli, A.; Mole, P.; Carlesso, N.; Olson, D.P.; Scadden, D.T. Receptor tyrosine kinase, EphB4 (HTK), accelerates differentiation of select human hematopoietic cells. Blood 2002, 99, 2740–2747. [Google Scholar] [CrossRef] [Green Version]
- Suenobu, S.; Takakura, N.; Inada, T.; Yamada, Y.; Yuasa, H.; Zhang, X.Q.; Sakano, S.; Oike, Y.; Suda, T. A role of EphB4 receptor and its ligand, ephrin-B2, in erythropoiesis. Biochem. Biophys. Res. Commun. 2002, 293, 1124–1131. [Google Scholar] [CrossRef]
- Foo, S.S.; Turner, C.J.; Adams, S.; Compagni, A.; Aubyn, D.; Kogata, N.; Lindblom, P.; Shani, M.; Zicha, D.; Adams, R.H. Ephrin-B2 Controls Cell Motility and Adhesion during Blood-Vessel-Wall Assembly. Cell 2006, 124, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, D.; García-Ceca, J.; Farias-De-Oliveira, D.A.; Terra-Granado, E.; Montero-Herradón, S.; Cotta-De-Almeida, V.; Savino, W.; Zapata, A. EphB2 and EphB3 play an important role in the lymphoid seeding of murine adult thymus. J. Leukoc. Biol. 2015, 98, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.; Lauranzano, E.; Soldani, C.; Ploia, C.; Angioni, R.; D’Amico, G.; Sarukhan, A.; Mazzon, C.; Viola, A. Identification of a novel agrin-dependent pathway in cell signaling and adhesion within the erythroid niche. Cell Death Differ. 2016, 23, 1322–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohban, R.; Pieber, T.R. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017, 2017, 5173732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.M.; Arthur, A.; Gronthos, S. Eph/Ephrin-mediated Mesenchymal Stem Cell Regulation of T-cell Activation and Function. J. Clin. Cell. Immunol. 2016. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro, D.; Rodríguez-Sosa, M.R.; Zapata, A.G. Eph/ephrin Signaling and Biology of Mesenchymal Stromal/Stem Cells. J. Clin. Med. 2020, 9, 310. https://doi.org/10.3390/jcm9020310
Alfaro D, Rodríguez-Sosa MR, Zapata AG. Eph/ephrin Signaling and Biology of Mesenchymal Stromal/Stem Cells. Journal of Clinical Medicine. 2020; 9(2):310. https://doi.org/10.3390/jcm9020310
Chicago/Turabian StyleAlfaro, David, Mariano R. Rodríguez-Sosa, and Agustín G. Zapata. 2020. "Eph/ephrin Signaling and Biology of Mesenchymal Stromal/Stem Cells" Journal of Clinical Medicine 9, no. 2: 310. https://doi.org/10.3390/jcm9020310




