Transcription Profile Analysis of Chlorophyll Biosynthesis in Leaves of Wild-Type and Chlorophyll b-Deficient Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
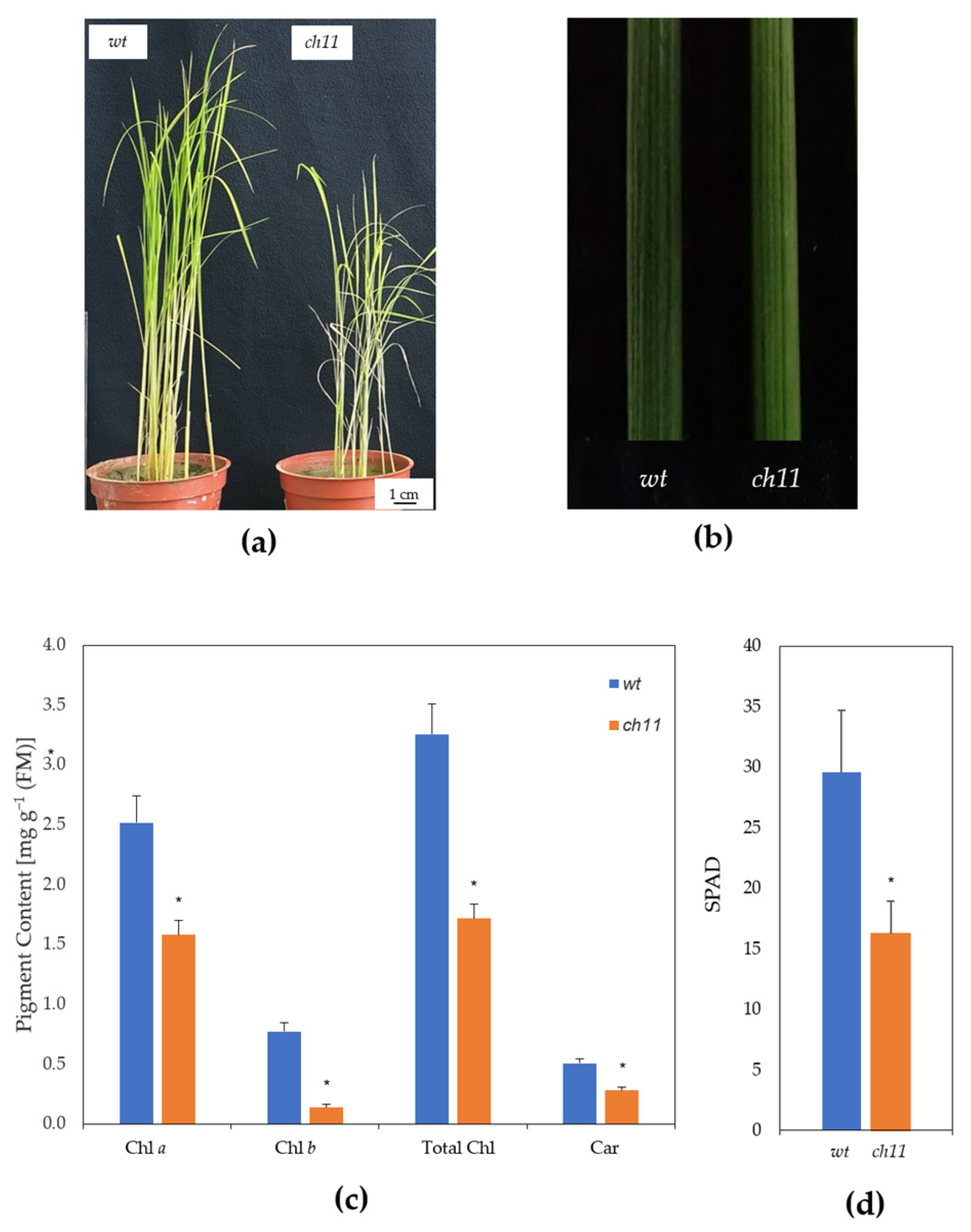
2.2. Assessment of Pigment Contents
2.3. Ultrastructure Microscope
2.4. cDNA Libraries Construction and Transcriptome Sequencing
2.5. Transcriptome Analysis
2.6. Quantitative RT-qPCR
2.7. Statistical Analysis
3. Results
3.1. Characterization of ch11 Rice
3.2. Genome Mapping and Gene Expression Analysis
3.3. Detection and Annotation of Differentially Expressed Genes
3.4. Role of Chlorophyll Metabolism Genes in Leaf Coloration
3.5. Role of Transcription Factors in Leaf Coloration
3.6. RT-qPCR Validation of Differentially Expressed Chlorophyll Biosynthesis Genes
4. Discussion
4.1. Pigment Contents and Chloroplast Development
4.2. Chloroplast-Related Differentially Expressed Genes and Photosynthetic Capacity
4.3. RNA-Seq Analysis and Chlorophyll-Related Differentially Expressed Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fageria, N.K. Yield physiology of rice. J. Plant Nutr. 2007, 30, 843–879. [Google Scholar] [CrossRef]
- Xiong, Y.; Xie, Y.; Song, Q.; Zeng, W. The relationship between meteorological factors and rice yield in Liuzhi special zone. Guizhou Agric. Sci. 2009, 10, 79–81. [Google Scholar]
- Liu, S.L.; Pu, C.; Ren, Y.X.; Zhao, X.L.; Zhao, X.; Chen, F.; Xiao, X.P.; Zhang, H.L. Yield variation of double-rice in response to climate change in Southern China. Eur. J. Agron. 2016, 81, 161–168. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J.C.; Cai, G.Z. Advances in the research of elevation on rice yield and quality. Chin. Agric. Sci. Bull. 2013, 29, 1–4. [Google Scholar]
- Man, Y.; Wang, B.; Wang, J.; Slaný, M.; Yan, H.; Li, P.; El-Naggar, A.; Shaheen, S.M.; Rinklebe, J.; Feng, X. Use of biochar to reduce mercury accumulation in Oryza sativa L: A trial for sustainable management of historically polluted farmlands. Environ. Int. 2021, 153, 106527. [Google Scholar] [CrossRef]
- Kruger, E.L.; Volin, J.C. Reexamining the empirical relation between plant growth and leaf photosynthesis. Funct. Plant Biol. 2006, 33, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flood, P.J.; Kruijer, W.; Schnabel, S.K.; van der Schoor, R.; Jalink, H.; Snel, J.F.H.; Harbinson, J.; Aarts, M.G.M. Phenomics for photosynthesis, growth and reflectance in arabidopsis thaliana reveals circadian and long-term fluctuations in heritability. Plant Methods 2016, 12, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, G.; Li, X.; Huang, J.; Zheng, J. Heredity. physiology and mapping of a chlorophyll content gene of rice (Oryza sativa L.). J. Plant Physiol. 2008, 165, 324–330. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Rühle, W.; Reiländer, H.; Otto, K.D.; Wild, A. Chlorophyll-protein-complexes of thylakoids of wild type and chlorophyll b mutants of Arabidopsis thaliana. Photosynth. Res. 1983, 4, 301–305. [Google Scholar] [CrossRef]
- Yang, C.M.; Osterman, J.C.; Markwell, J. Temperature sensitivity as a general phenomenon in a collection of chlorophyll-deficient mutants of sweetclover (Melilotus alba). Biochem. Genet. 1990, 28, 31–40. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Han, S.; Zhang, X.; Yu, D. Identification and gene mapping of a soybean chlorophyll-deficient mutant. Plant Breed. 2011, 130, 133–138. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Naqvi, K.R.; Melø, T.B.; Bangar Raju, B. Assaying the chromophore composition of photosynthetic systems by spectral reconstruction: Application to the light-harvesting complex (lhc ii) and the total pigment content of higher plants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 2229–2234. [Google Scholar] [CrossRef]
- Sager, J.C.; McFarlane, J.C. Radiation-Plant Growth Chamber Handbook; Iowa State University of Science and Technology: Ames, IA, USA, 1997; pp. 1–29. [Google Scholar]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ohtsuka, T.; Tanaka, A. Conversion of chlorophyll b to chlorophyll a via 7-hydroxymethyl chlorophyll. J. Biol. Chem. 1996, 271, 1475–1479. [Google Scholar] [CrossRef] [Green Version]
- Gupta, J. Climate Change and Water Law. In Impact of Climate Change on Water and Health; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Avenson, T.J.; Cruz, J.A.; Kanazawa, A.; Kramer, D.M. Regulating the proton budget of higher plant photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9709–9713. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.L.; Sheehy, J.E. Supercharging rice photosynthesis to increase yield. New Phytol. 2006, 171, 688–693. [Google Scholar] [CrossRef]
- Huang, J.; Qin, F.; Zang, G.; Kang, Z.; Zou, H.; Hu, F.; Yue, C.; Li, X.; Wang, G. Mutation of OSDET1 increases chlorophyll content in rice. Plant Sci. 2013, 210, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Fujita, Y. Regulation and evolution of chlorophyll metabolism. Photochem. Photobiol. Sci. 2008, 7, 1131–1149. [Google Scholar] [CrossRef]
- Lai, Y.C.; Wang, S.Y.; Gao, H.Y.; Nguyen, K.M.; Nguyen, C.H.; Shih, M.C.; Lin, K.H. Physicochemical properties of starches and expression and activity of starch biosynthesis-related genes in sweet potatoes. Food Chem. 2016, 199, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Shih, T.H.; Lin, S.H.; Huang, W.D.; Yang, C.M. Transcription analysis of chlorophyll biosynthesis in wildtype and chlorophyll b-lacking rice (Oryza sativa L.). Photosynthetica 2020, 58, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, V.; Ingenfeld, A.; Apel, K. Characterization of the snowy cotyledon 1 mutant of Arabidopsis thaliana: The impact of chloroplast elongation factor g on chloroplast development and plant vitality. Plant Mol. Biol. 2006, 60, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogson, B.J.; Ganguly, D.; Albrecht-Borth, V. Insights into chloroplast biogenesis and development. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, T.; Matsuyama, T.; Sekido, S.; Yamaguchi, I.; Yoshida, S.; Kameya, T. Chlorophyll-deficient mutants of rice demonstrated the deletion of a DNA fragment by heavy-ion irradiation. J. Radiat. Res. 2002, 43, S157–S161. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.W.; Lee, Y.J.; Koh, H.J.; Lee, B.M.; Nam, Y.W.; Paek, N.C. Isolation, characterization, and mapping of the stay green mutant in rice. Theor. Appl. Genet. 2002, 104, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Hur, J.; Ryu, C.H.; Choi, Y.; Chung, Y.Y.; Miyao, A.; Hirochika, H.; An, G. Characterization of a rice chlorophyll-deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol. 2003, 44, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, W.; Xie, Y.; Lu, W.; Zhang, R. Comparative proteomics of thylakoid membrane from a chlorophyll b-less rice mutant and its wild type. Plant Sci. 2007, 173, 397–407. [Google Scholar] [CrossRef]
- Chu, P.; Yan, G.X.; Yang, Q.; Zhai, L.N.; Zhang, C.; Zhang, F.Q.; Guan, R.Z. ITRAQ-based quantitative proteomics analysis of brassica napus leaves reveals pathways associated with chlorophyll deficiency. J. Proteom. 2015, 113, 244–259. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Y.Y.; Zhai, H.; He, S.Z.; Zhao, N.; LIU, Q.Q.C. Transcriptome profiling reveals insights into the molecular mechanism of drought tolerance in sweetpotato. J. Integr. Agric. 2019, 18, 9–23. [Google Scholar] [CrossRef]
- Vothknecht, U.C.; Kannangara, C.G.; Von Wettstein, D. Expression of catalytically active barley glutamyl tRNAglu reductase in escherichia coli as a fusion protein with glutathione s-transferase. Proc. Natl. Acad. Sci. USA 1996, 93, 9287–9291. [Google Scholar] [CrossRef] [Green Version]
- Harpster, M.H.; Mayfield, S.P.; Taylor, W.C. Effects of pigment-deficient mutants on the accumulation of photosynthetic proteins in maize. Plant Mol. Biol. 1984, 3, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Droppa, M.; Ghirardi, M.L.; Horvath, G.; Melis, A. Chlorophyll b deficiency in soybean mutants. II. thylakoid membrane development and differentiation. BBA Bioenerg. 1988, 932, 138–145. [Google Scholar] [CrossRef]
- Zhao, H.B.; Guo, H.J.; Zhao, L.S.; Gu, J.Y.; Zhao, S.R.; Li, J.H.; Liu, L.X. Agronomic traits and photosynthetic characteristics of chlorophyll-deficient wheat mutant induced by spaceflight environment. Acta Agron. Sin. 2011, 37, 119–126. [Google Scholar]
- Somerville, C.R. Analysis of photosynthesis with mutants of higher plants and algae. Annu. Rev. Plant Physiol. 1986, 37, 467–506. [Google Scholar] [CrossRef]
- Terao, T.; Yamashita, A.; Katoh, S. Chlorophyll b-deficient mutants of rice: II. antenna chlorophyll a/b-proteins of photosystem I and II. Plant Cell Physiol. 1985, 26, 1369–1377. [Google Scholar]
- Terao, T.; Yamashita, A.; Katoh, S. Chlorophyll b-deficient mutants of rice. 1. Absorption and fluorescence spectra and chlorophyll a/b ratios. Plant Cell Physiol. 1985, 26, 1361–1367. [Google Scholar]
- Terao, T.; Sonoike, K.; Yamazaki, J.; Kamimura, Y.; Katoh, S. Stoichiometries of photosystem I and photosystem II in rice mutants differently deficient in chlorophyll b. Plant Cell Physiol. 1996, 37, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.M.; Chang, K.W.; Yin, M.H.; Huang, H.M. Methods for the determination of the chlorophylls and their derivatives. Taiwania 1998, 43, 116–122. [Google Scholar]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Audic, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bujaldon, S.; Kodama, N.; Rappaport, F.; Subramanyam, R.; de Vitry, C.; Takahashi, Y.; Wollman, F.A. Functional accumulation of antenna proteins in chlorophyll b-less mutants of Chlamydomonas reinhardtii. Mol. Plant 2017, 10, 115–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Sun, L.; Wu, Q.; Men, X.; Yao, L.; Xing, S. Transcriptome profile analysis reveals the ontogenesis of rooted chichi in Ginkgo biloba L. Gene 2018, 669, 8–14. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Xu, B.; Li, Y.; Ma, Y.; Wang, G. Phenotype and transcriptome analysis reveals chloroplast development and pigment biosynthesis together influenced the leaf color formation in mutants of Anthurium andraeanum ‘sonate’. Front. Plant Sci. 2015, 6, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, J. Changes in the photosynthetic characteristics and photosystem stoichiometries in wild-type and Chl b-deficient mutant rice seedlings under various irradiances. Photosynthetica 2010, 48, 521–529. [Google Scholar] [CrossRef]
- Rüdiger, W. Chlorophyll metabolism: From outer space down to the molecular level. Phytochemistry 1997, 46, 1151–1167. [Google Scholar] [CrossRef]
- Liu, W.; Fu, Y.; Hu, G.; Si, H.; Zhu, L.; Wu, C.; Sun, Z. Identification and fine mapping of a thermo-sensitive chlorophyll deficient mutant in rice (Oryza sativa L.). Planta 2007, 226, 785–795. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Li, M.; Su, L.; Lian, S.; Zhang, B.; Li, X.; Ge, K.; Li, L. AhGLK1 Affects chlorophyll biosynthesis and photosynthesis in peanut leaves during recovery from drought. Sci. Rep. 2018, 8, 2250. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Y.; Xia, X.W.; Fang, W.; Fu, Y.; An, M.M.; Zhou, M.B. Identification of genes involved in spontaneous leaf color variation in Pseudosasa japonica. Genet. Mol. Res. 2015, 14, 11827–11840. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.M.; Zhang, X.; Wang, J.L.; Wan, J.M. Leaf chloroplast ultrastructure and photosynthetic properties of a chlorophyll-deficient mutant of rice. Photosynthetica 2014, 52, 217–222. [Google Scholar] [CrossRef]
- Seo, T.S.; Bai, X.; Ruparel, H.; Li, Z.; Turro, N.J.; Ju, J. Photocleavable fluorescent nucleotides for dna sequencing on a chip constructed by site-specific coupling chemistry. Proc. Natl. Acad. Sci. USA 2004, 101, 5488–5493. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Wang, P.; Wang, S.; Ma, L.; Li, L.; Yang, R.; Ma, Y.; Wang, Q. Comprehensive transcriptome analysis discovers novel candidate genes related to leaf color in a lagerstroemia indica yellow leaf mutant. Genes Genom. 2015, 37, 851–863. [Google Scholar] [CrossRef]
- Schmitz, A.J.; Glynn, J.M.; Olson, B.J.; Stokes, K.D.; Osteryoung, K.W. Arabidopsis FtsZ2-1 and FtsZ2-2 are functionally redundant, but FtsZ-based plastid division is not essential for chloroplast partitioning or plant growth and development. Mol. Plant 2009, 2, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Swid, N.; Nevo, R.; Kiss, V.; Kapon, R.; Dagan, S.; Snir, O.; Adam, Z.; Falconet, D.; Reich, Z.; Charuvi, D. Differential impacts of FtsZ proteins on plastid division in the shoot apex of Arabidopsis. Dev. Biol. 2018, 441, 83–94. [Google Scholar] [CrossRef]
- Goral, T.K.; Johnson, M.P.; Duffy, C.D.; Brain, A.P.R.; Ruban, A.V.; Mullineaux, C.W. Light-harvesting antenna composition controls the macrostructure and dynamics of thylakoid membranes in Arabidopsis. Plant J. 2012, 69, 289–301. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, T.; Feng, B.; Zhang, C.; Peng, S.; Zhang, X.; Fu, G.; Tao, L. Non-photochemical quenching plays a key role in light acclimation of rice plants differing in leaf color. Front. Plant Sci. 2017, 7, 1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.X.; Hall, M.; Funk, C.; Schröder, W.P. Photosystem ii, a growing complex: Updates on newly discovered components and low molecular mass proteins. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarnath, K.; Bennett, D.I.G.; Schneider, A.R.; Fleming, G.R. Multiscale model of light harvesting by photosystem II in plants. Proc. Natl. Acad. Sci. USA 2016, 113, 1156–1161. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J. Protein phosphorylation in green plant chloroplasts. Annu. Rev. Plant Biol. 1991, 42, 281–311. [Google Scholar] [CrossRef]
- Allen, J.F. How does protein phosphorylation regulate photosynthesis? Trends Biochem. Sci. 1992, 17, 12–17. [Google Scholar] [CrossRef]
- Standfuss, J.; Terwisscha van Scheltinga, A.C.; Lamborghini, M.; Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 2005, 24, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.M.; Chen, H.Y. Grana stacking is normal in a chlorophyll-deficient LT8 mutant of rice. Bot. Bull. Acad. Sin. 1996, 37, 31–34. [Google Scholar]
- Jenny, A.; Mark, W.; Robin, G.W.; Caroline, A.H.; Alexander, V.R.; Peter, H.; Stefan, J. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II–effects on photosynthesis, grana stacking and fitness. Plant J. 2003, 35, 350–361. [Google Scholar] [CrossRef]
- Kim, E.H.; Li, X.P.; Razeghifard, R.; Anderson, J.M.; Niyogi, K.K.; Pogson, B.J.; Chow, W.S. The multiple roles of light-harvesting chlorophyll a/b-protein complexes define structure and optimize function of Arabidopsis chloroplasts: A study using two chlorophyll b-less mutants. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Tyutereva, E.; Ivanova, A.; Voitsekhovskaja, O. On the role of chlorophyll b in ontogenetic adaptations of plants. Biol. Bull. Rev. 2014, 4, 507–514. [Google Scholar] [CrossRef]
- Barber, J. Influence of surface charges on thylakoid structure and function. Annu. Rev. Plant Physiol. 1982, 33, 261–295. [Google Scholar] [CrossRef]
- Mulo, P. Chloroplast-targeted ferredoxin-nadp+ oxidoreductase (fnr): Structure, function and location. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Fromme, P.; Melkozernov, A.; Jordan, P.; Krauss, N. Sstructure and function of photosystem I: Interaction with its soluble electron carriers and external antenna systems. FEBS Lett. 2003, 555, 40–44. [Google Scholar] [CrossRef]
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 2006, 57, 521–565. [Google Scholar] [CrossRef] [Green Version]
- Voitsekhovskaja, O.V.; Tyutereva, E.V. Chlorophyll b in angiosperms: Functions in photosynthesis, signaling and ontogenetic regulation. J. Plant Physiol. 2015, 189, 51–64. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, S.; Zhang, F.; Zhao, A.; Zhang, W.; Zhang, M.; Liu, L. The Arabidopsis glutamyl-tRNA reductase (GLUTR) forms a ternary complex with flu and GLUTR-binding protein. Sci. Rep. 2016, 6, 19756. [Google Scholar] [CrossRef]
- Sheng, Z.; Lv, Y.; Li, W.; Luo, R.; Wei, X.; Xie, L.; Jiao, G.; Shao, G.; Wang, J.; Tang, S.; et al. Yellow-leaf 1 encodes a magnesium-protoporphyrin ix monomethyl ester cyclase, involved in chlorophyll biosynthesis in rice (Oryza sativa L.). PLoS ONE 2017, 12, e0177989. [Google Scholar] [CrossRef] [Green Version]
- Nomata, J.; Swem, L.R.; Bauer, C.E.; Fujita, Y. Overexpression and characterization of dark-operative protochlorophyllide reductase from Rhodobacter capsulatus. Biochim. Biophys. Acta Bioenerg. 2005, 1708, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y. Protochlorophyllide reduction: A key step in the greening of plants. Plant Cell Physiol. 1996, 37, 411–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apel, K. Chlorophyll biosynthesis—Metabolism and strategies of higher plants to avoid photooxidative stress. In Regulation of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2001; pp. 235–252. [Google Scholar]
- Schoefs, B. The protochlorophyllide–chlorophyllide cycle. Photosynth. Res. 2001, 70, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, Y.; Ito, H.; Tanaka, A. Conversion of chlorophyll b to chlorophyll a precedes magnesium dechelation for protection against necrosis in Arabidopsis. Plant J. 2012, 72, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tanaka, R. The biochemistry, physiology, and evolution of the chlorophyll cycle. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]






| Function | Gene ID | TF Family | Log2 Fold Change | Expression in ch11 Compared to wt | Annotation |
|---|---|---|---|---|---|
| Chlorophyll biosynthesis | Osa_4328118 | GLuRS | 1.4 | Up-regulated | glutamyl-tRNA synthetase |
| Osa_4337415 | POR | −1.7 | Down-regulated | protochlorophyllide reductase A | |
| Osa_4348519 | NCCR | 1.7 | Up-regulated | Red chlorophyll catabolite reductase | |
| Photosynthesis | Osa_107276047 | PsbR | −1.7 | Down-regulated | photosystem II 10 kDa protein |
| Osa_4342395 | PsbR | −4.5 | Down-regulated | photosystem II 10 kDa protein | |
| Osa_4334338 | PetH | −1.1 | Down-regulated | ferredoxin-NADP+ reductase | |
| Antenna protein | Osa_4324705 | Lhcb1 | −1.7 | Down-regulated | Photosynthesis-antenna protein |
| Chloroplast division | Osa_4333567 | FtsZ1 | −2.1 | Down-regulated | Cell division protein FtsZ homolog 2-1 |
| Transcription Factor Family | Total No. Genes | No. Genes Up-Regulated | No. Genes Down-Regulated |
|---|---|---|---|
| ABI3VP1 | 1 | 0 | 1 |
| Afin-like | 1 | 1 | 0 |
| AP2-EREBP | 13 | 1 | 12 |
| ARF | 5 | 0 | 5 |
| ARR-B | 4 | 0 | 4 |
| BBR/BPC | 2 | 2 | 0 |
| bHLH | 34 | 5 | 29 |
| bZIP | 4 | 0 | 4 |
| C2C2-Dof | 6 | 0 | 6 |
| C2C2-GATA | 1 | 0 | 1 |
| C2C2-YABBY | 4 | 0 | 4 |
| C2H2 | 6 | 0 | 6 |
| C3H | 1 | 0 | 1 |
| CSD | 1 | 0 | 1 |
| E2F-DP | 2 | 0 | 2 |
| FAR1 | 14 | 12 | 2 |
| G2-like | 8 | 0 | 8 |
| GRAS | 8 | 1 | 7 |
| GRF | 4 | 1 | 3 |
| HB | 1 | 0 | 1 |
| HSF | 1 | 0 | 1 |
| LIM | 1 | 0 | 1 |
| LOB | 1 | 0 | 1 |
| MADS | 12 | 2 | 10 |
| mTERF | 5 | 3 | 2 |
| MYB | 42 | 4 | 38 |
| MYB-related | 32 | 4 | 28 |
| NAC | 28 | 1 | 27 |
| OFP | 1 | 0 | 1 |
| PBF-2-like | 1 | 1 | 0 |
| SBP | 1 | 0 | 1 |
| Sigma70-like | 2 | 2 | 0 |
| SRS | 2 | 0 | 2 |
| TCP | 2 | 2 | 0 |
| Tify | 6 | 0 | 6 |
| Trihelix | 2 | 0 | 2 |
| TUB | 1 | 0 | 1 |
| WRKY | 26 | 4 | 22 |
| zf-HD | 2 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.K.; Shih, T.-H.; Lin, S.-H.; Lin, J.-W.; Nguyen, H.C.; Yang, Z.-W.; Yang, C.-M. Transcription Profile Analysis of Chlorophyll Biosynthesis in Leaves of Wild-Type and Chlorophyll b-Deficient Rice (Oryza sativa L.). Agriculture 2021, 11, 401. https://doi.org/10.3390/agriculture11050401
Nguyen MK, Shih T-H, Lin S-H, Lin J-W, Nguyen HC, Yang Z-W, Yang C-M. Transcription Profile Analysis of Chlorophyll Biosynthesis in Leaves of Wild-Type and Chlorophyll b-Deficient Rice (Oryza sativa L.). Agriculture. 2021; 11(5):401. https://doi.org/10.3390/agriculture11050401
Chicago/Turabian StyleNguyen, Minh Khiem, Tin-Han Shih, Szu-Hsien Lin, Jun-Wei Lin, Hoang Chinh Nguyen, Zhi-Wei Yang, and Chi-Ming Yang. 2021. "Transcription Profile Analysis of Chlorophyll Biosynthesis in Leaves of Wild-Type and Chlorophyll b-Deficient Rice (Oryza sativa L.)" Agriculture 11, no. 5: 401. https://doi.org/10.3390/agriculture11050401
APA StyleNguyen, M. K., Shih, T. -H., Lin, S. -H., Lin, J. -W., Nguyen, H. C., Yang, Z. -W., & Yang, C. -M. (2021). Transcription Profile Analysis of Chlorophyll Biosynthesis in Leaves of Wild-Type and Chlorophyll b-Deficient Rice (Oryza sativa L.). Agriculture, 11(5), 401. https://doi.org/10.3390/agriculture11050401






