Ultrasonographic Monitoring of Fetal Growth and Fetal Weight Calculation in Sows during Gestation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ultrasound Measurements
2.3. Statistical Data Analysis
3. Results
3.1. Fetal Growth
3.1.1. Fetal Weight Calculation by Linear Regression Models
3.1.2. Fetal Weight Calculation by Multiple Linear Regression Models
3.1.3. Fetal Weight Calculation by Nonlinear Regression Models
3.2. Transabdominal Ultrasound Scan
4. Discussion
4.1. Fetal Growth
4.2. Fetal Weight Calculation
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benson, C.B.; Doubilet, P.M. Fetal biometry and growth. In Callen’s Ultrasonography in Obstetrics and Gynecology; Norton, M.E., Scoutt, L.M., Feldstein, V.A., Eds.; Elsevier: Philadelphia, PA, USA, 2017; Volume 6, pp. 118–131, Appendix A and B. [Google Scholar]
- Amer, H.A. Ultrasonographic assessment of early pregnancy diagnosis, fetometry and sex determination in goats. Anim. Reprod. Sci. 2010, 117, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Beccaglia, M.; Alonge, S.; Trovo’, C.; Luvoni, G.C. Determination of gestational time and prediction of parturition in dogs and cats: An update. Reprod. Domest. Anim. 2016, 51, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, V.B.; Volkmann, D.H.; Dufour, S.; Middleton, J.R. Use of ultrasonographic fetometry for the estimation of days to kidding in dairy does. Theriogenology 2018, 118, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Moreno, J.; Gonzalez-Bulnes, A.; Gomez-Brunet, A.; Toledano-Diaz, A.; Lopez-Sebastian, A. Prediction of gestational age by transrectal ultrasonographic measurements in the mouflon (Ovis gmelini musimon). J. Zoo Wildl. Med. 2005, 36, 457–462. [Google Scholar] [CrossRef]
- Jones, A.K.; Reed, S.A. Benefits of ultrasound scanning during gestation in the small ruminant. Small Rumin. Res. 2017, 149, 163–171. [Google Scholar] [CrossRef]
- Kahn, W. Ultrasonography as a diagnostic-tool in female animal reproduction. Anim. Reprod. Sci. 1992, 28, 1–10. [Google Scholar] [CrossRef]
- Kauffold, J.; Peltoniemi, O.; Wehrend, A.; Althouse, G.C. Principles and Clinical Uses of Real-Time Ultrasonography in Female Swine Reproduction. Animals 2019, 9, 950. [Google Scholar] [CrossRef] [Green Version]
- Stenhouse, C.; Tennant, P.; Duncan, W.C.; Ashworth, C.J. Doppler ultrasound can be used to monitor umbilical arterial blood flow in lightly sedated pigs at multiple gestational ages. Reprod. Fertil. Dev. 2018, 30, 1402–1411. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Sanchez-Sanchez, R.; Perez-Solana, M.L.; Torres-Rovira, L.; Ayuso, M.; Gonzalez, J. Early-postnatal changes in adiposity and lipids profile by transgenerational developmental programming in swine with obesity/leptin resistance. J. Endocrinol. 2014, 223, M17–M29. [Google Scholar] [CrossRef] [Green Version]
- Schild, S.L.A.; Foldager, L.; Rangstrup-Christensen, L.; Pedersen, L.J. Characteristics of Piglets Born by Two Highly Prolific Sow Hybrids. Front. Vet. Sci. 2020, 7, 355. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Parraguez, V.H.; Berlinguer, F.; Barbero, A.; Garcia-Contreras, C.; Lopez-Tello, J.; Pesantez-Pacheco, J.L.; Martinez-Ros, P. The impact of prenatal environment on postnatal life and performance: Future perspectives for prevention and treatment. Theriogenology 2020, 150, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Vielwerth, S.E.; Jensen, R.B.; Larsen, T.; Holst, K.K.; Molgaard, C.; Greisen, G.; Vaag, A. The effect of birthweight upon insulin resistance and associated cardiovascular risk factors in adolescence is not explained by fetal growth velocity in the third trimester as measured by repeated ultrasound fetometry. Diabetologia 2008, 51, 1483–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merz, E. Biometrie des Fetus. In Sonografische Diagnostik in Gynaekologie und Geburtshilfe: Lehrbuch und Atlas; Merz, E., Ed.; Thieme: Stutgard, Germany; New York, NY, USA, 2002; Volume 2, pp. 139–167. [Google Scholar]
- Metzler-Zebeli, B.U.; Lang, I.S.; Görs, S.; Brüssow, K.-P.; Hennig, U.; Nürnberg, G.; Rehfeldt, C.; Otten, W.; Metges, C.C. High-protein–low-carbohydrate diet during pregnancy alters maternal plasma amino acid concentration and placental amino acid extraction but not fetal plasma amino acids in pigs. Br. J. Nutr. 2012, 108, 2176–2189. [Google Scholar] [CrossRef] [Green Version]
- Vernunft, A.; Maass, M.; Brussow, K.P. Placental Characteristics of German Landrace Sows and Their Relationships to Different Fertility Parameters. Czech J. Anim. Sci. 2018, 63, 339–346. [Google Scholar]
- Vernunft, A.; Ivell, R.; Heng, K.; Anand-Ivell, R. The Male Fetal Biomarker INSL3 Reveals Substantial Hormone Exchange between Fetuses in Early Pig Gestation. PLoS ONE 2016, 11, e0152689. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 16 November 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 43, 1689. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Cecchetto, M.; Milani, C.; Vencato, J.; Sontas, H.; Mollo, A.; Contiero, B.; Romagnoli, S. Clinical use of fetal measurements to determine the whelping day in German shepherd breed bitches. Anim. Reprod. Sci. 2017, 184, 110–119. [Google Scholar] [CrossRef]
- Riding, G.A.; Lehnert, S.A.; French, A.J.; Hill, J.R. Conceptus-related measurements during the first trimester of bovine pregnancy. Vet. J. 2008, 175, 266–272. [Google Scholar] [CrossRef]
- Ali, A.; Fahmy, S. Ultrasonographic fetometry and determination of fetal sex in buffaloes (Bubalus bubalis). Anim. Reprod. Sci. 2008, 106, 90–99. [Google Scholar] [CrossRef]
- Murase, H.; Endo, Y.; Tsuchiya, T.; Kotoyori, Y.; Shikichi, M.; Ito, K.; Sato, F.; Nambo, Y. Ultrasonographic Evaluation of Equine Fetal Growth Throughout Gestation in Normal Mares Using a Convex Transducer. J. Vet. Med. Sci. 2014, 76, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W. Recent advances in sow nutrition. Rev. Bras. Zootec. 2010, 39, 303–310. [Google Scholar] [CrossRef]
- McPherson, R.L.; Ji, F.; Wu, G.; Blanton, J.R.; Kim, S.W. Growth and compositional changes of fetal tissues in pigs. J. Anim. Sci. 2004, 82, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Pontelo, T.P.; Miranda, J.R.; Felix, M.A.R.; Pereira, B.A.; da Silva, W.E.; Avelar, G.F.; Mariano, F.C.M.Q.; Guimarães, G.C.; Zangeronimo, M.G. Histological characteristics of the gonads of pig fetuses and their relationship with fetal anatomical measurements. Res. Vet. Sci. 2018, 117, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.K.; Berg, E.P.; Berg, E.L.; Vonnahme, K.A. Effect of maternal activity during gestation on maternal behavior, fetal growth, umbilical blood flow, and farrowing characteristics in pigs. J. Anim. Sci. 2013, 91, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Kahn, W. Sonographic Fetometry in the Bovine. Theriogenology 1989, 31, 1105–1121. [Google Scholar] [CrossRef]
- Ali, A.; Al-Sobayil, F.; Derar, R.; El-Tookhy, O. Ultrasonographic fetometry and prenatal fetal sex assessment in camels (Camelus dromedarius). Theriogenology 2013, 80, 609–618. [Google Scholar] [CrossRef]
- Evans, H.E.; Sack, W.O. Prenatal Development of Domestic and Laboratory Mammals: Growth Curves, External Features and Selected References. Anat. Histol. Embryol. 1973, 2, 11–45. [Google Scholar] [CrossRef]
- Frauenholz, J.; Kähn, W.L.W. Sonogrophy for Pregnancy diagnosis in Swine—Comparison between transrectal and transcutaneous procedures. Mh. Vet.-Med. 1989, 44, 425–430. [Google Scholar]
- Merz, E.; Wellek, S.; Püttmann, S.; Bahlmann, F.; Weber, G. Orbital Diameter, Interorbital and Biocular Diameters—A Growth Model for Fetal Orbital Parameters. Ultraschall Med. 1995, 16, 12–17. [Google Scholar] [CrossRef]
- Chaoui, R.; Heling, K.S.; Bollmann, R. Sonographical Fetal Cardiac Measurements Performed in the 4-Chamber-View Plane. Geburtshilfe Frauenheilkd. 1994, 54, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Lampl, M.; Kuzawa, C.W.; Jeanty, P. Growth patterns of the heart and kidney suggest inter-organ collaboration in facultative fetal growth. Am. J. Hum. Biol. 2005, 17, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Smrcek, J.M.; Berg, C.; Geipel, A.; Fimmers, R.; Diedrich, K.; Gembruch, U. Early fetal echocardiography—Heart biometry and visualization of cardiac structures between 10 and 15 weeks’ gestation. J. Ultrasound Med. 2006, 25, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Heer, I.M.; Kümper, C.; Vögtle, N.; Müller-Egloff, S.; Dugas, M.; Strauss, A. Analysis of Factors Influencing the Ultrasonic Fetal Weight Estimation. Fetal Diagn. Ther. 2008, 23, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbagha, R.E.; Minogue, J.; Tamura, R.K.; Hungerford, S.A. Estimation of birth weight by use of ultrasonographic formulas targeted to large-, appropriate-, and small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 1989, 160, 854–862. [Google Scholar] [CrossRef]
- Siemer, J.; Hilbert, A.; Wolf, T.; Hart, N.; Muller, A.; Schild, R.L. Gender-specific weight estimation of fetuses between 2,501 and 3,999 g—New regression formulae. Fetal Diagn. Ther. 2008, 24, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Barel, O.; Vaknin, Z.; Tovbin, J.; Herman, A.; Maymon, R. Assessment of the Accuracy of Multiple Sonographic Fetal Weight Estimation Formulas. J. Ultrasound Med. 2013, 32, 815–823. [Google Scholar] [CrossRef]
- Merz, E.; Lieser, H.; Schicketanz, K.H.; Harle, J. Predicting fetal weight by ultrasound. Ultraschall Med. 1988, 9, 15–24. [Google Scholar] [CrossRef]
- Shepard, M.J.; Richards, V.A.; Berkowitz, R.L.; Warsof, S.L.; Hobbins, J.C. An evaluation of 2 equations for predicting fetal weight by ultrasound. Am. J. Obstet. Gynecol. 1982, 142, 47–54. [Google Scholar] [CrossRef]
- Vintzileos, A.M.; Campbell, W.A.; Rodis, J.F.; Borskoefoed, R.; Nochimson, D.J. Fetal weight estimation formulas with head, abdominal, femur, and thigh circumference measurements. Am. J. Obstet. Gynecol. 1987, 157, 410–414. [Google Scholar] [CrossRef]
- Jordaan, H.V.F. Estimation of fetal weight by ultrasound. J. Clin. Ultrasound 1983, 11, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective-study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.K.; Wan, C.W.; Cho, K.M. Computer-Assisted Evaluation of Ultrasonic Fetal Weight Prediction Using Multiple-Regression Equations with and without the Fetal Femur Length. J. Ultrasound Med. 1985, 4, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Carpenter, R.J.; Deter, R.L.; Park, S.K. Sonographic estimation of fetal weight—The value of femur length in addition to head and abdomen measurements. Radiology 1984, 150, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Warsof, S.L.; Wolf, P.; Coulehan, J.; Queenan, J.T. Comparison of fetal weight estimation formulas with and without head measurements. Obstet. Gynecol. 1986, 67, 569–573. [Google Scholar]
- Campbell, S.; Wilkin, D. Ultrasonic measurement of fetal abdomen circumference in estimation of fetal weight. Br. J. Obstet. Gynaecol. 1975, 82, 689–697. [Google Scholar] [CrossRef]
- Higginbottom, J.; Slater, J.; Porter, G.; Whitfield, C.R. Estimation of fetal weight from ultrasonic measurement of trunk circumference. Br. J. Obstet. Gynaecol. 1975, 82, 698–701. [Google Scholar] [CrossRef]
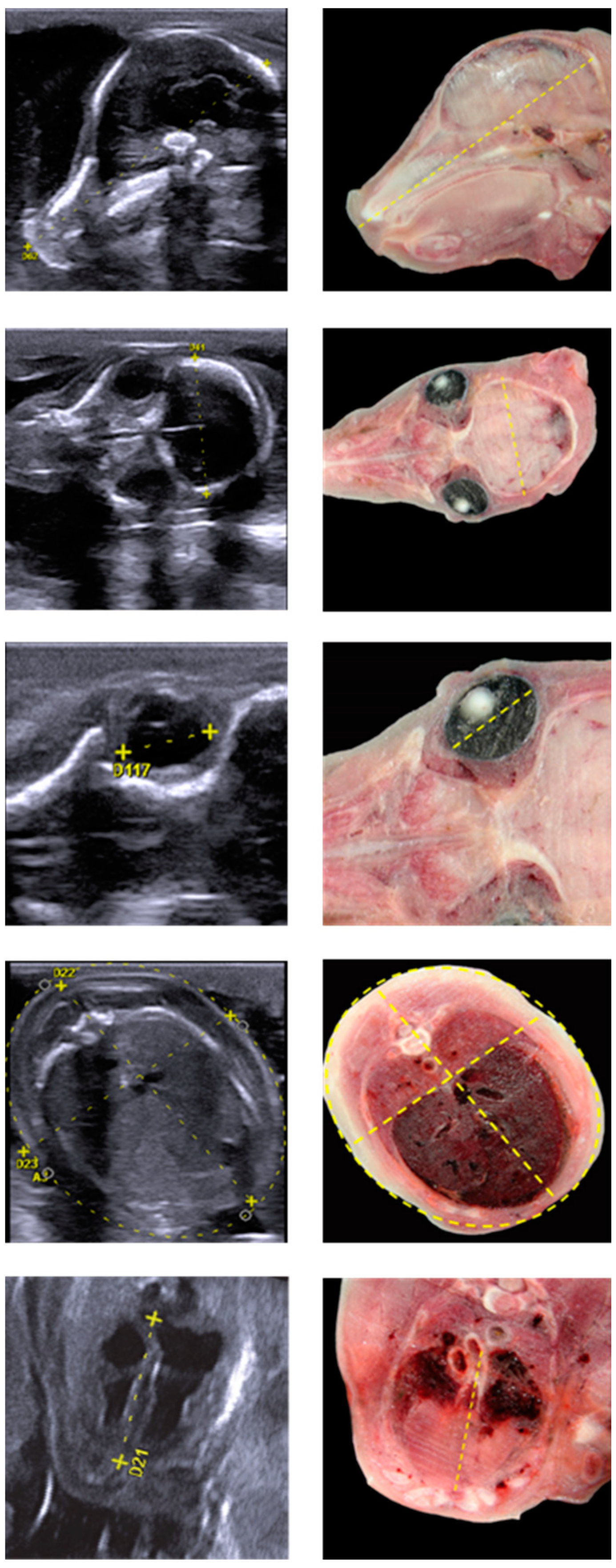

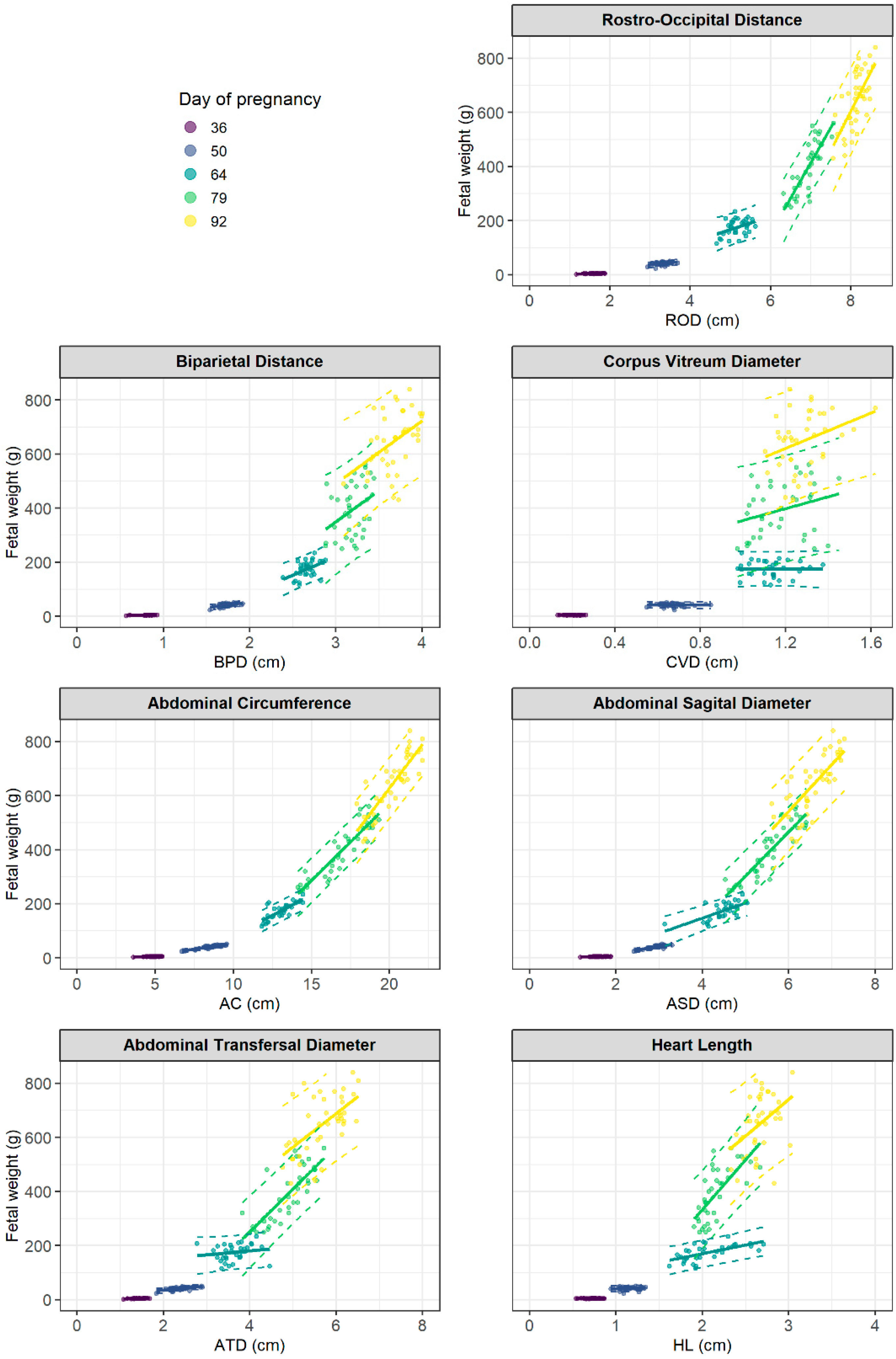

| Morphometric Parameters | Pregnancy Day | R2 | p Value | Fetal Weight Calculation |
|---|---|---|---|---|
| rostro-occipital distance | 36 | 0.37 | <0.001 | EFWPD36 = −0.28 + 2.79·RODcm |
| (ROD) | 50 | 0.20 | 0.002 | EFWPD50 = −7.15 + 14.69·RODcm |
| 64 | 0.16 | 0.023 | EFWPD64 = −74.30 + 48.28·RODcm | |
| 79 | 0.71 | <0.001 | EFWPD79 = −1408.91 + 260.54·RODcm | |
| 92 | 0.47 | <0.001 | EFWPD92 = −1725.21 + 291.47·RODcm | |
| biparietal distance | 36 | 0.30 | <0.001 | EFWPD36 = −0.34 + 5.52·BPDcm |
| (BPD) | 50 | 0.29 | <0.001 | EFWPD50 = −31.10 + 41.90·BPDcm |
| 64 | 0.24 | 0.004 | EFWPD64 = −187.98 + 136.08·BPDcm | |
| 79 | 0.13 | 0.027 | EFWPD79 = −343.57 + 231.10·BPDcm | |
| 92 | 0.21 | 0.003 | EFWPD92 = −206.23 + 232.26·BPDcm | |
| corpus vitreum diameter | 36 | <0.01 | 0.918 | EFWPD36 = 4.24 + 0.28·CVDcm |
| (CVD) | 50 | <0.01 | 0.968 | EFWPD50 = 41.94 − 0.55·CVDcm |
| 64 | <0.01 | 0.967 | EFWPD64 = 172.53 + 2.22·CVDcm | |
| 79 | 0.08 | 0.086 | EFWPD79 = 136.37 + 218.43·CVDcm | |
| 92 | 0.11 | 0.034 | EFWPD92 = 229.45 + 326.58·CVDcm | |
| abdominal circumference | 36 | 0.46 | <0.001 | EFWPD36 = −1.47 + 1.17·ACcm |
| (AC) | 50 | 0.73 | <0.001 | EFWPD50 = −30.90 + 8.36·ACcm |
| 64 | 0.63 | <0.001 | EFWPD64 = −220.28 + 30.19·ACcm | |
| 79 | 0.85 | <0.001 | EFWPD79 = −571.91 + 57.18·ACcm | |
| 92 | 0.74 | <0.001 | EFWPD92 = −898.74 + 76.35·ACcm | |
| abdominal sagittal diameter | 36 | 0.29 | <0.001 | EFWPD36 = 0.38 + 2.41·ASDcm |
| (ASD) | 50 | 0.62 | <0.001 | EFWPD50 = −34.07 + 25.38·ASDcm |
| 64 | 0.46 | <0.001 | EFWPD64 = −75.94 + 55.82·ASDcm | |
| 79 | 0.80 | <0.001 | EFWPD79 = −514.31 + 163.48·ASDcm | |
| 92 | 0.57 | <0.001 | EFWPD92 = −497.12 + 173.33·ASDcm | |
| abdominal transversal diameter | 36 | 0.34 | <0.001 | EFWPD36 = −0.12 + 3.02·ATDcm |
| (ATD) | 50 | 0.42 | <0.001 | EFWPD50 = 3.49 + 15.80·ATDcm |
| 64 | 0.03 | 0.351 | EFWPD64 = 121.46 + 14.71·ATDcm | |
| 79 | 0.61 | <0.001 | EFWPD79 = −382.70 + 158.47·ATDcm | |
| 92 | 0.37 | <0.001 | EFWPD92 = −55.26 + 123.00·ATDcm | |
| heart length | 36 | <0.01 | 0.714 | EFWPD36 = 4.03 + 0.38·HLcm |
| (HL) | 50 | 0.03 | 0.314 | EFWPD50 = 32.02 + 8.31·HLcm |
| 64 | 0.38 | <0.001 | EFWPD64 = 41.87 + 64.16·HLcm | |
| 79 | 0.51 | <0.001 | EFWPD79 = −401.49 + 367.64·HLcm | |
| 92 | 0.19 | 0.005 | EFWPD92 = −70.90 + 270.60·HLcm |
| Fixed Effects | Sum of Squares | Num DF | Den DF | F Value | p Value |
|---|---|---|---|---|---|
| abdominal circumference (AC) | 0.164 | 1 | 174.2 | 27.76 | <0.0001 |
| biparietal distance (BPD) | 0.125 | 1 | 174.6 | 21.17 | <0.0001 |
| rostro-occipital distance (ROD) | 0.082 | 1 | 174.3 | 13.91 | 0.0003 |
| abdominal sagittal diameter (ASD) | 0.033 | 1 | 174.1 | 5.54 | 0.0197 |
| heart length (HL) | 0.027 | 1 | 175.3 | 4.51 | 0.0352 |
| corpus vitreum diameter (CVD) | 0.002 | 1 | 175.5 | 0.41 | 0.5249 |
| abdominal transversal diameter (ATD) | 0.002 | 1 | 175.3 | 0.39 | 0.5339 |
| pregnancy day (PD) | 0.090 | 1 | 25.3 | 15.24 | 0.0006 |
| Pregnancy Day | Morphometric Parameters | Estimated Fetal Weight | |
|---|---|---|---|
| 36 | BPD | ROD | AC | 0.58 | EFWPD36 = −3.71 + 2.58·BPDcm + 1.19·RODcm + 0.80·ACcm |
| BPD | AC | 0.55 | EFWPD36 = −3.37 + 3.56·BPDcm + 0.95·ACcm | |
| 50 | BPD | ROD | AC | 0.75 | EFWPD50 = −56.90 + 10.00·BPDcm+ 6.00·RODcm + 7.06·ACcm |
| BPD | AC | 0.73 | EFWPD50 = −41.51 + 9.22·BPDcm + 7.74·ACcm | |
| 64 | BPD | ROD | AC | 0.76 | EFWPD64 = −538.14 + 88.19·BPDcm + 28.58·RODcm + 25.23·ACcm |
| BPD | AC | 0.71 | EFWPD64 = −414.79 + 87.51·BPDcm + 27.22·ACcm | |
| 79 | BPD | ROD | AC | 0.89 | EFWPD79 = −1161.31 + 71.36·BPDcm + 91.29·RODcm + 41.20·ACcm |
| BPD | AC | 0.86 | EFWPD79 = −814.98 + 87.88·BPDcm + 54.97·ACcm | |
| 92 | BPD | ROD | AC | 0.83 | EFWPD92 = −1837.48 + 115.79·BPDcm + 107.89·RODcm + 58.32·ACcm |
| BPD | AC | 0.79 | EFWPD92 = −1262.13 + 130.23·BPDcm + 70.66·ACcm |
| Parameter | Pregnancy Day | ||||
|---|---|---|---|---|---|
| 35 | 49 | 63 | 78 | 91 | |
| ROD | xxx | xxx | xxx | xx | xxx |
| BPD | xxx | xxx | xxx | xxx | |
| CVD | xx | xxx | xxx | xxx | xxx |
| AC | xxx | x | xx | x | |
| ASD | x | x | xx | x | |
| ATD | x | x | xx | x | |
| HL | xx | xx | x | xxx | xxx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernunft, A.; Eggert, A.; Brüssow, K.-P. Ultrasonographic Monitoring of Fetal Growth and Fetal Weight Calculation in Sows during Gestation. Agriculture 2023, 13, 16. https://doi.org/10.3390/agriculture13010016
Vernunft A, Eggert A, Brüssow K-P. Ultrasonographic Monitoring of Fetal Growth and Fetal Weight Calculation in Sows during Gestation. Agriculture. 2023; 13(1):16. https://doi.org/10.3390/agriculture13010016
Chicago/Turabian StyleVernunft, Andreas, Anja Eggert, and Klaus-Peter Brüssow. 2023. "Ultrasonographic Monitoring of Fetal Growth and Fetal Weight Calculation in Sows during Gestation" Agriculture 13, no. 1: 16. https://doi.org/10.3390/agriculture13010016






