Biobeds, a Microbial-Based Remediation System for the Effective Treatment of Pesticide Residues in Agriculture
Abstract
:1. Introduction
2. Release of Pesticide Residues into the Environment
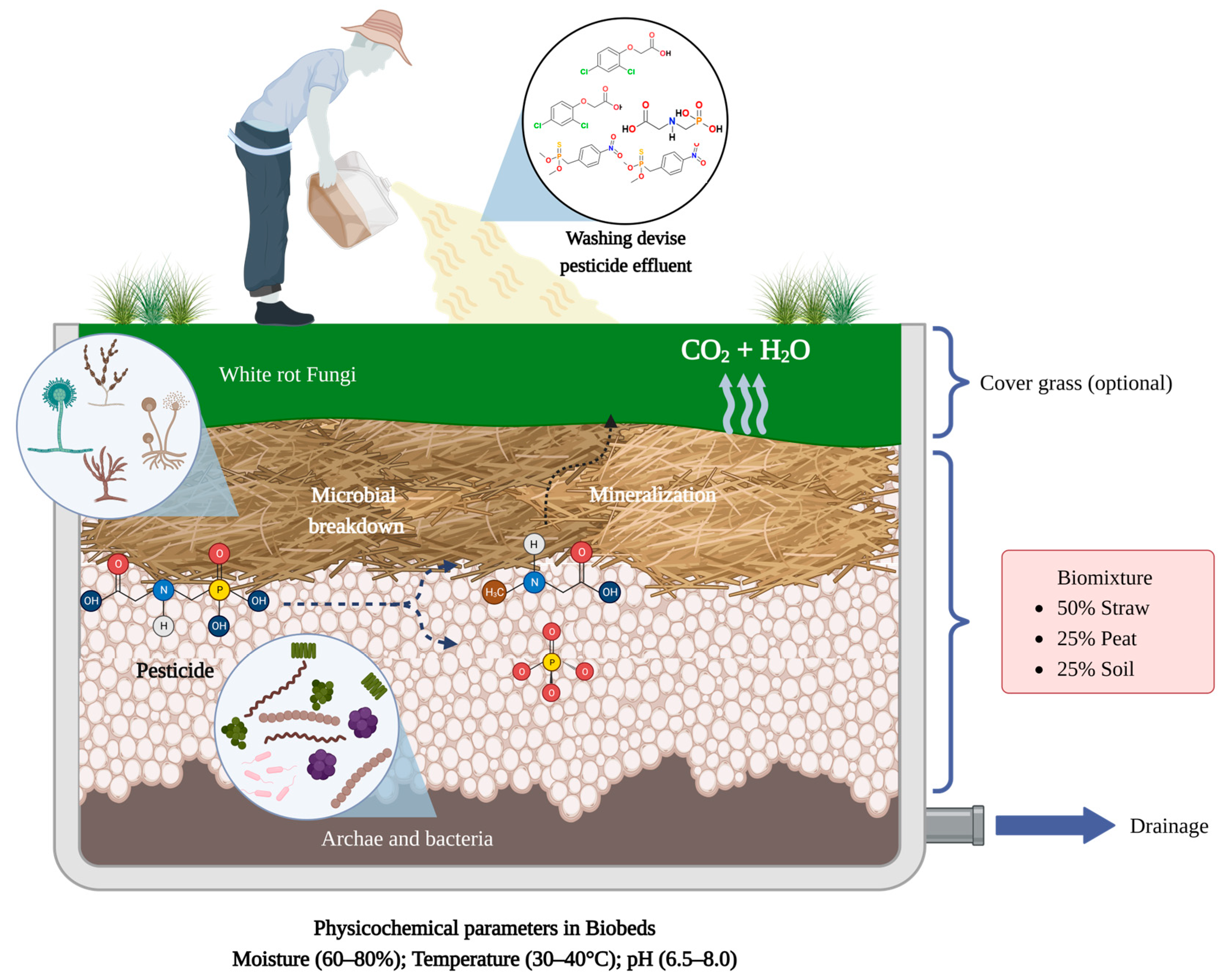
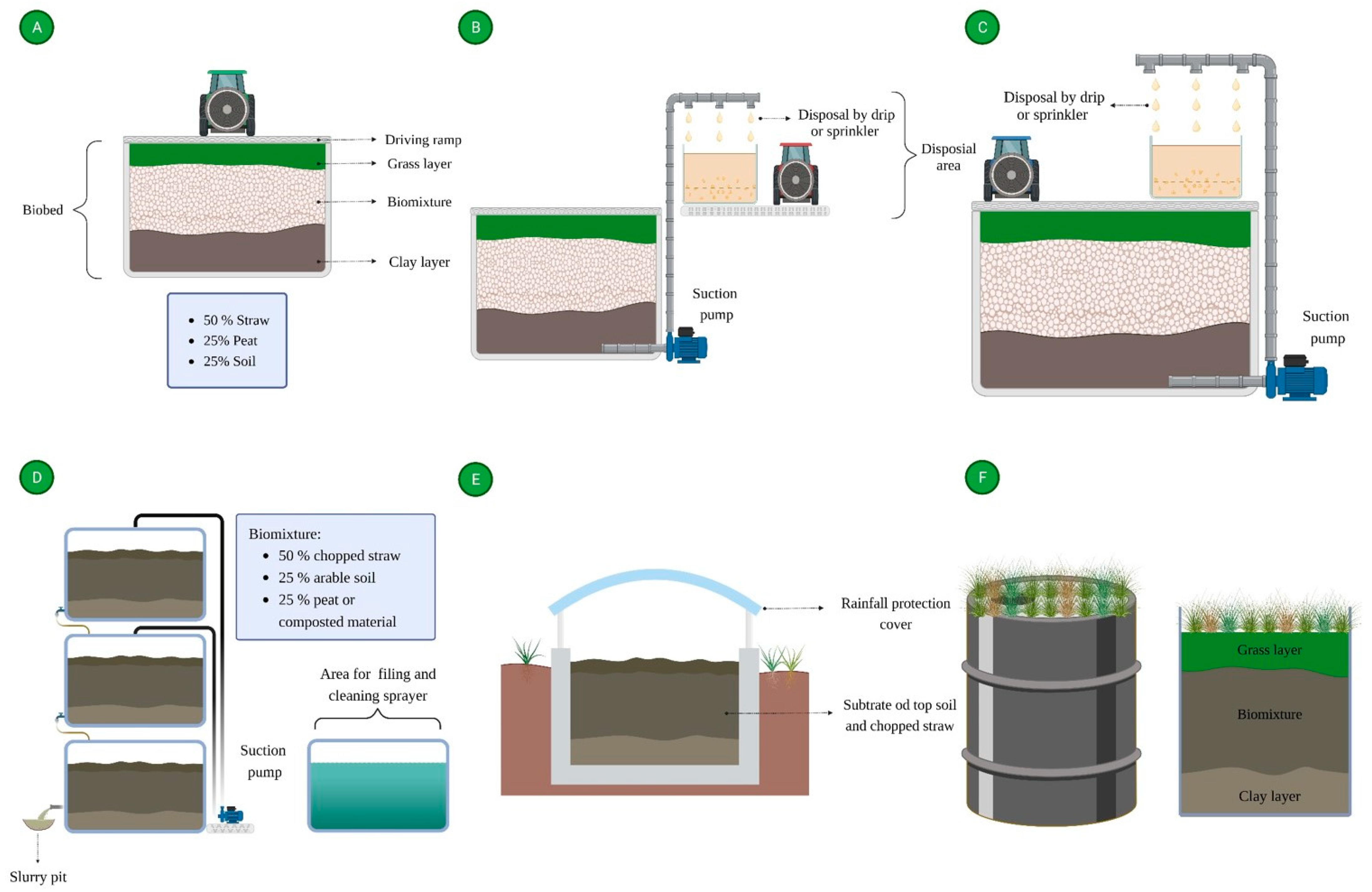
3. What Are Biobeds?
4. Key Factors in Biobeds’ Effectiveness
4.1. Biomixture
4.2. Microorganisms
4.3. Physicochemical Parameters
4.4. Analysis of the Biobeds’ Treated Effluents
5. Recent Studies of Pesticide Biodegradation in Biobed Systems
5.1. Fungicides
| Chemical Family | Fungicide | Biomixture (%) | Microorganisms | Concentration (mg·kg−1) | Degradation (%) | Degradation Time (Days) | Analytical Determination | Reference |
|---|---|---|---|---|---|---|---|---|
| Acylalanine | 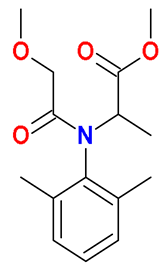 Metalaxyl | Wheat straw, spent mushroom substrate, and soil (25:50:25) | Soil’s indigenous microbial community | 9.5 † | 50 | 3 | HPLC-DAD | [42] |
| Vine branches, garden compost, and soil (40:40:20) | Soil’s indigenous microbial community | 131.6 | 47 | 150 | HPLC-UV | [74] | ||
| Wheat straw, spent mushroom substrate, and soil (25:50:25) | Soil’s indigenous microbial community | 28 | 80 | 100 | HPLC | [39] | ||
| Coconut fiber, garden compost, and soil (45:13:42) | Trametes versicolor | 30 | 97 | 16 | UHPLC-MS/MS | [117] | ||
| Vine shoots, vermicompost, and soil (25:50:25) | Soil’s indigenous microbial community | 50 | 48 | 15 | HPLC-DAD | [74] | ||
| Coconut fiber, garden compost, soil (50:25:25), and antibiotic oxytetracycline (10 mg·kg−1) | Proteobacteria, Firmicutes, Actinobacteria, and Acidobacteria | 30 | 100 | 16 | UHPLC-MS/MS | [79] | ||
| Benzimidazoles |  Carbendazim | Millet stubble and soil (50:50) | Soil’s indigenous microbial community | 1.14 | 99 | 60 | HPLC-MS | [41] |
| Coconut fiber, garden compost, and soil (45:13:42) | Trametes versicolor | 40 | 97 | 16 | UHPLC-MS/MS | [117] | ||
| Coconut fiber, garden compost, soil (50:25:25), and antibiotic oxytetracycline (10 mg·kg−1) | Proteobacteria, Firmicutes, Actinobacteria, and Acidobacteria | 40 | 100 | 16 | UHPLC-MS/MS | [79] | ||
 Thiabendazole | Wheat straw, spent mushroom substrate, and soil (25:50:25) | Soil’s indigenous microbial community | 2.3 † | 50 | 45 | HPLC-DAD | [42] | |
| Carboxamides | 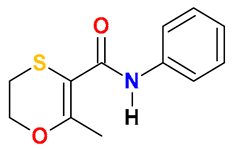 Carboxin | Wheat straw, spent mushroom substrate, and soil (25:50:25) | Soil’s indigenous microbial community | 5.14 † | 50 | 3.7 | HPLC-DAD | [42] |
| 52 | 100 | 20 | HPLC | [39] | ||||
 Fluxapyroxad | Wheat straw, spent mushroom substrate, and soil (25:50:25) | Soil’s indigenous microbial community | 8.7 † | 50 | 143 | HPLC-DAD | [42] | |
| 34 | 30 | 100 | HPLC | [39] | ||||
| Chloronitriles |  Chlorothalonil | Wheat straw, spent mushroom substrate, and soil (25:50:25) | Soil’s indigenous microbial community | 8.1 † | 50 | 3 | HPLC-DAD | [42] |
| Imidazoles | 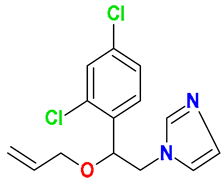 Imazalil | Wheat straw, spent mushroom substrate, and soil (25:50:25) | Soil’s indigenous microbial community | 75 † | 50 | 29 | HPLC-DAD | [42] |
| Methoxt-acrylates |  Azoxystrobin | Rice straw, compost, and soil (40:20:20) | Soil’s indigenous microbial community | 100 † | 82 | 45 | HPLC-MS | [75] |
| Corn cobs, compost, and soil (40:20:20) | 69 | |||||||
| Rice straw, compost, and soil (40:20:20) | Soil’s indigenous microbial community | 30 † | 94.8 | 28 | HPLC | [114] | ||
| Rice straw, peat, and soil (40:40:20) | 98.5 | |||||||
| Corn cobs, compost, and soil (40:20:20) | 98.4 | |||||||
| Corn cobs, peat, and soil (40:20:20) | 95.3 | |||||||
| Phenylpyrroles |  Fludioxonil | Wheat straw, spent mushroom substrate, and soil (25:50:25) | Soil’s indigenous microbial community | 3.2 † | 50 | 79 | HPLC-DAD | [42] |
| Wheat straw, spent mushroom substrate, and soil (25:50:25) | Uncharacterized bacterial consortium | 10 | 50 | 42.4 | HPLC | [39] | ||
| 20 | ||||||||
| 150 | ||||||||
| Triazoles | 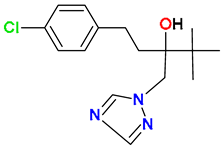 Tebuconazole | Wheat straw, peat, and soil (50:25:25) | Soil’s indigenous microbial community | 0.0945 | 100 | 168 | UHPLC-MS/MS | [118] |
| Vine shoots, vermicompost, and soil (25:50:25) | Soil’s indigenous microbial community | 50 | 13 | 15 | HPLC-DAD | [43] |
5.2. Herbicides
| Chemical Family | Herbicide | Biomixture (%) | Microorganisms | Concentration (mg·kg−1) | Degradation (%) | Degradation Time (Days) | Analytical Determination | Reference |
|---|---|---|---|---|---|---|---|---|
| Organophosphates (glycines) |  Glyphosate | Millet stubble and soil (50:50) | Soil’s indigenous microbial community | 9.6 | 99 | 60 | HPLC-MS | [38] |
| Corn husk and soil (50:50) | White-rot fungi | 0.8 | 99 | 20 | GC-ECD-NPD | [46] | ||
| Alfalfa straw and soil (50:50) + Eisenia fetida | Soil’s indigenous microbial community | 1000 | 90 | 90 | HPLC-UV | [121] | ||
| Alfalfa straw, river waste, and soil (50:25:25) + Eisenia fetida | ||||||||
| Wheat stubble and soil (50:50) + Eisenia fetida | 100 | |||||||
| Wheat straw and soil (50:50) | A total of 21 species of bacteria, Pseudomonas nitroreducens, Rhodospirillales order, and Pseudomonas sp. | 50 † | 90 | 15 | GC-ECD-NPD | [119] | ||
| Alfalfa straw and soil (50:50) | Soil’s indigenous microbial community | 1000 | 85 | 63 | HPLC-UV | [120] | ||
| Alfalfa straw, river waste, and soil (50:25:25) | 100 | |||||||
| Wheat stubble and soil (50:50) | ||||||||
| Wheat stubble, river waste, and soil (50:25:25) | ||||||||
| Corn stover and soil (50:50) | A total of 23 species of archaea, 598 species of bacteria, and 64 species of fungi | 1800 † | 100 | 41 | GC-ECD | [108] | ||
| Seaweed, compost, and soil (25:25:50) | ||||||||
| Corn stover, compost, and soil (25:25:50) | ||||||||
| Corn stover, sisal, and soil (25:25:50) | ||||||||
| Phenoxy-carboxylates | 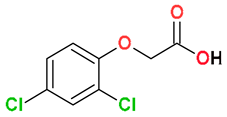 2,4-Dichlorophenoxyacetic acid (2,4-D) | Wheat stubble and soil (50:50) | Soil’s indigenous microbial community | 1200 | 100 | 30 | HPLC-UV | [122] |
| Corn husk and soil (50:50) | White-rot fungi | 22,250 | 99 | 20 | GC-ECD | [46] | ||
| Corn stover and soil (50:50) | A total of 23 species of archaea, 598 species of bacteria, and 64 species of fungi | 5400 † | 88 | 41 | GC-ECD | [108] | ||
| Seaweed, compost, and soil (25:25:50) | ||||||||
| Corn stover, compost, and soil (25:25:50) | ||||||||
| Corn stover, sisal, and soil (25:25:50) | ||||||||
| Triazines | 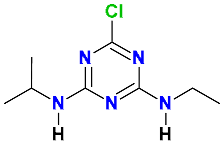 Atrazine | Millet stubble and soil (50:50) | Soil’s indigenous microbial community | 0.68 | 99 | 20 | HPLC-MS | [41] |
| Rice straw, compost, and soil (25:25:50) | Atrazine degrading microbial consortium Bacillus megaterium | 50 † | 98 | 60 | HPLC-MS/MS | [76] | ||
| Rice straw, compost + rice husk ash, and soil (50:25:25) | 94 | |||||||
| Rice straw, compost + straw biochar, and soil (50:25:25) | 98 | |||||||
| Corn husk and soil (50:50) | White-rot fungi | 1116 | 99 | 20 | GC-ECD-NPD | [46] | ||
| Coconut fiber, garden compost, and soil (45:13:42) | Trametes versicolor | 40 | 68.4 | 16 | UHPLC-MS/MS | [117] | ||
| Corn stover and soil (50:50) | A total of 23 species of archaea, 598 species of bacteria, and 64 species of fungi | 12,500 | 91 | 41 | GC-ECD | [108] | ||
| Seaweed, compost, and soil (25:25:50) | ||||||||
| Corn stover, compost, and soil (25:25:50) | ||||||||
| Corn stover, sisal, and soil (25:25:50) | ||||||||
| Coconut fiber, garden compost, and soil (bioaugmented) (45:13:42) | Proteobacteria, Firmicutes, and Actinobacteria | 40 | 70 | 16 | UHPLC-MS/MS | [79] | ||
| Coconut fiber, garden compost, soil, and antibiotic oxytetracycline (45:13:42) | ||||||||
| Coconut fiber, garden compost, and soil (bioaugmented + oxytetracycline) (45:13:42) | 72 | |||||||
| Coconut fiber, garden compost, and soil (45:13:42) | 68 | |||||||
| Wheat straw, peat, and andisol topsoil (50:25:25) | Actinobacteria, bacteria and fungi | 50 | 93 | 30 | HPLC | [123] | ||
 Prometryn | Millet stubble and soil (50:50) | Soil’s indigenous microbial community | 4.1 | 99 | 60 | HPLC | [41] | |
| Ureas | 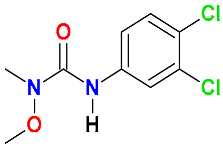 Linuron | Coconut fiber, compost, and soil (50:25:25) | Soil’s indigenous microbial community | 64 | 100 | 14 | HPLC-MS | [73] |
5.3. Insecticides
| Chemical Family | Insecticide | Biomixture (%) | Microorganisms | Concentration (mg·kg−1) | Degradation (%) | Degradation Time (Days) | Analytical Determination | Reference |
|---|---|---|---|---|---|---|---|---|
| Carbamates | 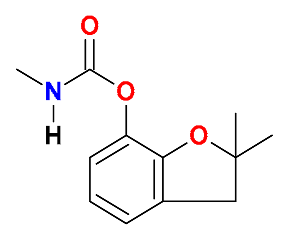 Carbofuran | Corn husk and soil (50:50) | White-rot fungi | 511 | 99 | 41 | GC-MS | [46] |
| Coconut fiber, garden compost, and soil (45:13:42) | Trametes versicolor | 30 | 99 | 16 | UHPLC-MS/MS | [117] | ||
| Coconut fiber, garden compost, and soil + oxytetracycline (45:13:42) | ||||||||
| Coconut fiber, garden compost, and soil + bacterial bioaugmentation (45:13:42) | ||||||||
| Coconut fiber, garden compost, and soil + oxytetracycline and bacterial bioaugmentation (45:13:42) | ||||||||
| Coconut fiber, garden compost, and soil (45:13:42) | Proteobacteria, firmicutes, actinobacteria, and acidobacteria | 30 | 100 | 16 | UHPLC | [79] | ||
| Coconut fiber, garden compost, and soil + oxytetracycline (45:13:42) | ||||||||
| Coconut fiber, garden compost, and soil bioaugmentation (45:13:42) | Proteobacteria (pseudomonas and sphingobium) | |||||||
| Coconut fiber, garden compost, and soil + oxytetracycline and bacterial bioaugmentation (45:13:42) | ||||||||
| Corn stover and soil (50:50) | A total of 23 species of archaea, 598 species of bacteria, and 64 species of fungi | 1145 | 98 | 20 | GC-ECD-NPD | [108] | ||
| Seaweed, compost, and soil (25:25:50) | ||||||||
| Corn stover, compost and soil (25:25:50) | ||||||||
| Corn stover, sisal and soil (25:25:50) | ||||||||
| Neonicotinoids | 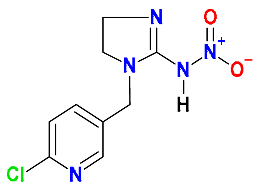 Imidacloprid | Millet stubble and soil (50:50) | Soil’s indigenous microbial community | 9 | 40 | 60 | HPLC-MS | [41] |
| Rice straw, compost, and soil (40:40:20) | Soil’s indigenous microbial community | 100 † | 100 | 45 | HPLC-MS/MS | [76] | ||
| Corn cob, compost, and soil (40:40:20) | ||||||||
| Rice straw, compost and soil (40:40:20) | Soil’s indigenous microbial community | 20 | 70 | 28 | HPLC | [114] | ||
| Rice straw, peat, and soil (40:40:20) | 74 | |||||||
| Corn cobs, peat, and soil (40:40:20) | 84 | |||||||
| Corn cobs, compost, and soil (40:40:20) | 90 | |||||||
| Vine shoots, vermicompost, and soil (25:50:25) | Soil’s indigenous microbial community | 50 | 22 | 15 | HPLC-DAD | [43] | ||
| Organophosphates |  Chlorpyrifos | Cereal bran, peat, and soil (50:25:25 MB1) | Abortiporus biennis | 60 | 92 | 50 | GC-ECD | [125] |
| Cereal bran, peat, and soil (50:25:25 MB2) | 64 | 21 | GC-MS | |||||
| Vine branches, compost, and soil (40:40:20) | Soil’s indigenous microbial community | 26.3 | 32 | 126 | HPLC-UV | [74] | ||
| Wheat straw, peat, and soil (50:25:25) | Streptomyces spp. | 50 † | 6 | 28 | HPLC | [124] | ||
| Wheat straw, peat, and soil (50:25:25) | Actinobacteria, bacteria, and fungi | 50 | 100 | 30 | HPLC | [123] | ||
 Diazinon | Corn husk and soil (50:50) | White-rot fungi | 766 | 99 | 35 | GC-MS | [46] | |
| Wheat straw, peat, and soil (50:25:25) | Streptomyces spp. | 50 † | 100 | 240 | HPLC | [124] | ||
| Corn stover and soil (50:50) | A total of 23 species of archaea, 598 species of bacteria, and 64 species of fungi | 1718 † | 99 | 41 | GC-ECD-NPD | [108] | ||
| Seaweed, compost, and soil (25:25:50) | 99 | |||||||
| Corn stover, compost, and soil (25:25:50) | 99 | |||||||
| Corn stover, sisal, and soil (25:25:50) | 98 | |||||||
 Phosmet | Corn straw, peat, and soil (50:25:25 SB) | Soil’s indigenous microbial community | 35 | 91 | 90 | UHPLC-MS/MS | [79] | |
| Corn straw, pine litter, and soil (50:25:25 PB) | 96 | |||||||
| Corn straw, vermicompost, and soil (50:25:25 VB) | 99 | |||||||
| Organophosphate mixture | Coconut fiber, compost, and farm soil (50:25:25) | Soil’s indigenous microbial community | 125 | 68.5 | 128 | UHPL-MS | [72] | |
| Phenylpyrazole |  Fipronil | Rice straw, compost + rice husk ash, and soil (50:25:25) | Soil’s indigenous microbial community | 50 † | 80 | 60 | HPLC-MS/MS | [76] |
| Rice straw, compost + wheat straw biochar, and soil (50:25:25) | 84 | |||||||
| Compost, wheat straw biochar, and soil (50:25:25) | 86 | |||||||
| Pyrethroid | 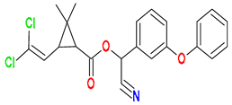 Cypermethrin | Clay and Acacia concinna 10% (90:10) | Bordetella petrii I GV 34, Bordetella petrii II GV 36, and Achromobacter xyloxidans GV 47 | 50 † | 95 | 32 | GC-MS | [126] |
| Clay and sawdust 5% (95:5) | 97 |
5.4. Characteristics and Risk Profiles of the Pesticides Treated in Biobeds
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawlak, K.; Kołodziejczak, M. The role of agriculture in ensuring food security in developing countries: Considerations in the context of the problem of sustainable food production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Pereira, P.; Barceló, D.; Panagos, P. Soil and water threats in a changing environment. Environ. Res. 2020, 186, 109501. [Google Scholar] [CrossRef] [PubMed]
- Maja, M.M.; Ayano, S.F. The impact of population growth on natural resources and farmers’ capacity to adapt to climate change in low-income countries. Earth Syst. Environ. 2021, 5, 271–283. [Google Scholar] [CrossRef]
- Molajou, A.; Afshar, A.; Khosravi, M.; Soleimanian, E.; Vahabzadeh, M.; Variani, H.A. A new paradigm of water, food, and energy nexus. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Gomes, H.D.O.; Menezes, J.M.C.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P.; do Nascimento, R.F. A socio-environmental perspective on pesticide use and food production. Ecotoxicol. Environ. Saf. 2020, 197, 110627. [Google Scholar] [CrossRef]
- Mandal, A.; Sarkar, B.; Mandal, S.; Vithanage, M.; Patra, A.K.; Manna, M.C. Impact of agrochemicals on soil health. In Agrochemicals Detection, Treatment and Remediation, Pesticides and Chemical Fertilizers; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 161–187. [Google Scholar] [CrossRef]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, history, and classification. In Natural Remedies for Pest, Disease and Weed Control; Chukwuebuka Egbuna, C., Sawicka, B., Eds.; Academic Press: Oxford, UK, 2020; pp. 29–42. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Maksymiv, I. Pesticides: Benefits and hazards. J. Vasyl Stefanyk Precarpath. Nat. Univ. 2015, 2, 70–76. [Google Scholar] [CrossRef]
- Castrejón-Godínez, M.L.; Rodríguez, A.; Sánchez-Salinas, E.; Mussali-Galante, P.; Tovar-Sánchez, E.; Ortiz-Hernández, M.L. Soils Contaminated with Persistent Organic Pollutants (POPs): Current Situations, Management, and Bioremediation Techniques: A Mexican Case Study. In Pesticides Bioremediation; Siddiqui, S., Meghvansi, M.K., Chaudhary, K.K., Eds.; Springer: Cham, Switzerland, 2022; pp. 413–453. [Google Scholar] [CrossRef]
- Brühl, C.A.; Zaller, J.G. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci. 2019, 7, 177. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation, Pesticides and Chemical Fertilizers; Agrochemicals detection, treatment and remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Dhananjayan, V.; Jayanthi, P.; Jayakumar, S.; Ravichandran, B. Agrochemicals Impact on Ecosystem and Bio-monitoring. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020; pp. 349–388. [Google Scholar] [CrossRef]
- Phat, C.; Ty, B.; Kuok, F.; Andrews, E.M.; Kurniawan, W.; Hinode, H. Residual Pesticides. In Water and Life in Tonle Sap Lake; Yoshimura, C., Khanal, R., Sovannara, U., Eds.; Springer: Singapore, 2022; pp. 387–397. [Google Scholar] [CrossRef]
- Shalaby, S.; Abdou, G. The influence of soil microorganisms and bio-or-organic fertilizers on dissipation of some pesticides in soil and potato tubers. J. Plant Prot. Res. 2010, 50, 86–92. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Dollimore, L.; Schimpf, W. Obsolete pesticide stocks–the past 25 years, lessons learned and observations for the future. Outlooks Pest Manag. 2013, 24, 251–256. [Google Scholar] [CrossRef]
- Vijgen, J.; Weber, R.; Lichtensteiger, W.; Schlumpf, M. The legacy of pesticides and POPs stockpiles—A threat to health and the environment. Environ. Sci. Pollut. Res. 2018, 25, 31793–31798. [Google Scholar] [CrossRef] [Green Version]
- Alshemmari, H. Inventories and assessment of POPs in the State of Kuwait as a basis for Stockholm Convention implementation. Emerg. Contam. 2021, 7, 88–98. [Google Scholar] [CrossRef]
- Mit, N.; Cherednichenko, O.; Mussayeva, A.; Khamdiyeva, O.; Amirgalieva, A.; Begmanova, M.; Tolebaeva, A.; Koishekenova, G.; Zaypanova, S.; Pilyugina, A.; et al. Ecological risk assessment and long-term environmental pollution caused by obsolete undisposed organochlorine pesticides. J. Environ. Sci. Health B 2021, 56, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Chitara, M.K.; Singh, R.P.; Gupta, P.K.; Mishra, D.; Jatav, S.S.; Sharma, S.; Jatav, H.S. The risk associated with crop ecosystem management and pesticides pollution. In Ecosystem Services: Types, Management and Benefits; Jatav, H.S., Rajput, V.D., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2022; pp. 151–163. [Google Scholar]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, M.K. Soil pollution and human health. In Plant Responses to Soil Pollution; Singh, P., Singh, S.K., Prasad, S.M., Eds.; Springer: Singapore, 2020; pp. 205–220. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Narayanan, M.; Kandasamy, S.; He, Z.; Kumarasamy, S. Ecological impacts of pesticides on soil and water ecosystems and its natural degradation process. In Pesticides in the Natural Environment; Singh, P., Singh, S., Sillanpää, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–49. [Google Scholar] [CrossRef]
- Sarker, S.; Akbor, M.A.; Nahar, A.; Hasan, M.; Islam, A.R.M.T.; Siddique, M.A.B. Level of pesticides contamination in the major river systems: A review on South Asian countries perspective. Heliyon 2021, 7, e07270. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, J.; Xu, Y.; Liu, H.; Yang, G.; He, Y.; Xu, J.; Lu, Z. Suspecting screening “known unknown” pesticides and transformation products in soil at pesticide manufacturing sites. Sci. Total Environ. 2022, 808, 152074. [Google Scholar] [CrossRef]
- Bagheri, A.; Emami, N.; Damalas, C.A. Farmers’ behavior towards safe pesticide handling: An analysis with the theory of planned behavior. Sci. Total Environ. 2021, 751, 141709. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, S.N.; Rahman, M.A.; Juraimi, A.S.; Uddin, M.K.; Brown, C.L.; Arshad, A. Chronic effects of organic pesticides on the aquatic environment and human health: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100740. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarmi, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water—A review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef] [PubMed]
- Boudh, S.; Singh, J.S. Pesticide contamination: Environmental problems and remediation strategies. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R., Chowdhary, P., Eds.; Springer: Singapore, 2019; pp. 245–269. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, distribution pathways and effects on human health–a review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sial, M.U.; Bashir, M.A.; Bilal, M.; Raza, Q.U.A.; Ali Raza, H.M.; Rehim, A.; Geng, Y. Pesticides xenobiotics in soil ecosystem and their remediation approaches. Sustainability 2022, 14, 3353. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Raffa, C.M.; Chiampo, F. Bioremediation of agricultural soils polluted with pesticides: A review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef]
- Karimi, H.; Mahdavi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rajput, V.D.; Minkina, T.; Astatkie, T. Insights on the bioremediation technologies for pesticide-contaminated soils. Environ. Geochem. Health 2022, 44, 1329–1354. [Google Scholar] [CrossRef]
- Karas, P.; Metsoviti, A.; Zisis, V.; Ehaliotis, C.; Omirou, M.; Papadopoulou, E.S.; Menkissoglou-Spiroudi, U.; Manta, S.; Komiotis, D.; Karpouzas, D.G. Dissipation, metabolism and sorption of pesticides used in fruit-packaging plants: Towards an optimized depuration of their pesticide-contaminated agro-industrial effluents. Sci. Total Environ. 2015, 530, 129–139. [Google Scholar] [CrossRef]
- Papazlatani, C.V.; Karas, P.A.; Tucat, G.; Karpouzas, D.G. Expanding the use of biobeds: Degradation and adsorption of pesticides contained in effluents from seed-coating, bulb disinfestation and fruit-packaging activities. J. Environ. Manag. 2019, 248, 109221. [Google Scholar] [CrossRef]
- Rezende, S.; Cesio, M.V.; Archondo, L.; Russi, C.; Martínez, P.; Rivero, A.; Besil, N. Pilot study of biobeds application for the remediation of citrus agro-industrial wastewaters. Int. J. Environ. Anal. Chem. 2021, 1–17. [Google Scholar] [CrossRef]
- Lescano, M.; Fussoni, N.; Vidal, E.; Zalazar, C. Biodegradation of pesticide-contaminated wastewaters from a formulation plant employing a pilot scale biobed. Sci. Total Environ. 2022, 807, 150758. [Google Scholar] [CrossRef] [PubMed]
- Papazlatani, C.V.; Karas, P.A.; Lampronikou, E.; Karpouzas, D.G. Using biobeds for the treatment of fungicide-contaminated effluents from various agro-food processing industries: Microbiome responses and mobile genetic element dynamics. Sci. Total Environ. 2022, 823, 153744. [Google Scholar] [CrossRef]
- Romero, E.; Delgado-Moreno, L.; Nogales, R. Pesticide dissipation and enzyme activities in ungrassed and grassed biomixtures, composed of winery wastes, used in biobed bioremediation systems. Water Air Soil Pollut. 2019, 230, 33. [Google Scholar] [CrossRef] [Green Version]
- Carniel, L.S.C.; Niemeyer, J.C.; de Oliveira Filho, L.C.I.; Alexandre, D.; Gebler, L.; Klauberg-Filho, O. Are there any risks of the disposal of pesticide effluents in soils? Biobed system meets ecotoxicology ensuring safety to soil fauna. Ecotoxicology 2020, 29, 1409–1421. [Google Scholar] [CrossRef]
- Pinto, A.P.; Lopes, M.E.; Dordio, A.; Castanheiro, J.E.F. Bioaugmentation an effective strategy to improve the performance of biobeds: A review. In Agrochemicals Detection, Treatment and Remediation; Prassad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–240. [Google Scholar] [CrossRef]
- Córdova-Méndez, E.A.; Góngora-Echeverría, V.R.; González-Sánchez, A.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Pesticide treatment in biobed systems at microcosms level under critical moisture and temperature range using an Orthic Solonchaks soil from southeastern Mexico amended with corn husk as support. Sci. Total Environ. 2021, 772, 145038. [Google Scholar] [CrossRef] [PubMed]
- Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, environmental pollution, and health. In Environmental Health Risk-Hazardous Factors to Living Species; Larramendy, M.L., Soloneski, S., Eds.; Intech Open: London, UK, 2016; pp. 3–27. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.H.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Dhananjayan, V.; Jayakumar, S.; Ravichandran, B. Conventional methods of pesticide application in agricultural field and fate of the pesticides in the environment and human health. In Controlled Release of Pesticides for Sustainable Agriculture; Rakhimol, K.R., Sabu, T., Tatiana, V., Jayachandran, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–37. [Google Scholar] [CrossRef]
- Le Cor, F.; Slaby, S.; Dufour, V.; Iuretig, A.; Feidt, C.; Dauchy, X.; Banas, D. Occurrence of pesticides and their transformation products in headwater streams: Contamination status and effect of ponds on contaminant concentrations. Sci. Total Environ. 2021, 788, 147715. [Google Scholar] [CrossRef] [PubMed]
- Brhich, A.; Ait Sidi Brahim, M.; Merzouki, H.; Chatoui, R.; Merzouki, M. Fate and impact of pesticides: Environmental and human health issues. In Nutrition and Human Health; Chatoui, H., Merzouki, M., Moummou, H., Tilaoui, M., Saadaoui, N., Brhich, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 41–53. [Google Scholar] [CrossRef]
- Dias, L.D.A.; Gebler, L.; Niemeyer, J.C.; Itako, A.T. Destination of pesticide residues on biobeds: State of the art and future perspectives in Latin America. Chemosphere 2020, 248, 126038. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Bazhari, S.; Nogales, R.; Romero, E. Innovative application of biobed bioremediation systems to remove emerging contaminants: Adsorption, degradation and bioaccesibility. Sci. Total Environ. 2019, 651, 990–997. [Google Scholar] [CrossRef]
- Torstensson, L.; Castillo, M. Use of biobeds in Sweden to minimize environmental spillages from agricultural spraying equipment. Pestic. Outlook 1997, 8, 24–27. [Google Scholar]
- Torstensson, L. Experiences of biobeds in practical use in Sweden. Pestic. Outlook 2000, 11, 206–211. [Google Scholar] [CrossRef]
- Castillo, M.D.P.; Torstensson, L.; Stenström, J. Biobeds for environmental protection from pesticide use—A review. J. Agric. Food Chem. 2008, 56, 6206–6219. [Google Scholar] [CrossRef]
- Biobeds.org. The International Biobed Site. 2022. Available online: https://bricksite.com/biobed (accessed on 1 February 2023).
- Henriksen, V.V.; Helweg, A.; Spliid, N.H.; Felding, G.; Stenvang, L. Capacity of model biobeds to retain and degrade mecoprop and isoproturon. Pest Manag. Sci. 2003, 59, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Fogg, P.; Boxall, A.B.; Walker, A.; Jukes, A. Leaching of pesticides from biobeds: Effect of biobed depth and water loading. J. Agric. Food Chem. 2004, 52, 6217–6227. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.D.P.; Torstensson, L. Biobeds-Biotechnology for environmental protection from pesticide pollution. In Methods and Techniques for Cleaning-Up Contaminated Sites; Annable, M.D., Teodorescu, M., Hlavinek, P., Diels, L., Eds.; Springer: Cham, Switzerland, 2008; pp. 145–151. [Google Scholar]
- Cooper, R.J.; Fitt, P.; Hiscock, K.M.; Lovett, A.A.; Gumm, L.; Dugdale, S.J.; Rambohul, J.; Williamson, A.; Noble, L.; Beamish, J.; et al. Assessing the effectiveness of a three-stage on-farm biobed in treating pesticide contaminated wastewater. J. Environ. Manag. 2016, 181, 874–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez, J.M.; Bigliardo, A.L.; Raimondo, E.E.; Briceño, G.E.; Polti, M.A.; Benimeli, C.S. Lindane dissipation in a biomixture: Effect of soil properties and bioaugmentation. Ecotoxicol. Environ. Saf. 2018, 156, 97–105. [Google Scholar] [CrossRef]
- Castillo, M.D.P.; Torstensson, L. Effect of biobed composition, moisture, and temperature on the degradation of pesticides. J. Agric. Food Chem. 2007, 55, 5725–5733. [Google Scholar] [CrossRef]
- De Wilde, T.; Spanoghe, P.; Debaer, C.; Ryckeboer, J.; Springael, D.; Jaeken, P. Overview of on-farm bioremediation systems to reduce the occurrence of point source contamination. Pest Manag. Sci. 2007, 63, 111–128. [Google Scholar] [CrossRef]
- Karanasios, E.; Karpouzas, D.G.; Tsiropoulos, N.G. Key parameters and practices controlling pesticide degradation efficiency of biobed substrates. J. Environ. Sci. Health B 2012, 47, 589–598. [Google Scholar] [CrossRef]
- Urrutia, C.; Rubilar, O.; Tortella, G.R.; Diez, M.C. Degradation of pesticide mixture on modified matrix of a biopurification system with alternatives lignocellulosic wastes. Chemosphere 2013, 92, 1361–1366. [Google Scholar] [CrossRef]
- Karanasios, E.; Tsiropoulos, N.G.; Karpouzas, D.G.; Menkissoglu-Spiroudi, U. Novel biomixtures based on local Mediterranean lignocellulosic materials: Evaluation for use in biobed systems. Chemosphere 2010, 80, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Diez, M.C.; Tortella, G.R.; Briceño, G.; Castillo, M.D.P.; Díaz, J.; Palma, G.; Altamirano, C.; Calderón, C.; Rubilar, O. Influence of novel lignocellulosic residues in a biobed biopurification system on the degradation of pesticides applied in repeatedly high doses. Electron. J. Biotechnol. 2013, 16, 1–11. [Google Scholar] [CrossRef]
- Góngora-Echeverría, V.R.; Martin-Laurent, F.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Agricultural effluent treatment in biobed systems using novel substrates from southeastern Mexico: The relationship with physicochemical parameters of biomixtures. Environ. Sci. Pollut. Res. 2017, 24, 9741–9753. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liang, J.; Pizzul, L.; Feng, X.M.; Zhang, K.; del Pilar Castillo, M. Evaluation of spent mushroom substrate as substitute of peat in chinese biobeds. Int. Biodeterior. Biodegrad. 2015, 98, 107–112. [Google Scholar] [CrossRef]
- Yarpuz-Bozdogan, N.; Bozdogan, A.M.; Daglioglu, N.; Sagliker, H. Determination of the effect of agricultural wastes alternative to peat in biobed. Fresenius Environ. Bull. 2020, 29, 9906–9913. [Google Scholar]
- Masís-Mora, M.; Lizano-Fallas, V.; Tortella, G.; Beita-Sandí, W.; Rodríguez-Rodríguez, C.E. Removal of triazines, triazoles and organophophates in biomixtures and application of a biopurification system for the treatment of laboratory wastewaters. Chemosphere 2019, 233, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Sánchez, A.; Soto-Garita, C.; Masís-Mora, M.; Cambronero-Heinrichs, J.C.; Rodríguez-Rodríguez, C.E. Impaired pesticide removal and detoxification by biomixtures during the simulated pesticide application cycle of a tropical agricultural system. Ecotoxicol. Environ. Saf. 2020, 195, 110460. [Google Scholar] [CrossRef]
- Vischetti, C.; Monaci, E.; Casucci, C.; De Bernardi, A.; Cardinali, A. Adsorption and degradation of three pesticides in a vineyard soil and in an organic biomix. Environments 2020, 7, 113. [Google Scholar] [CrossRef]
- Kumari, A.; Kumari, U.; Gupta, S.; Singh, N. Azoxystrobin and imidacloprid degradation in biobed setup under laboratory conditions. J. Environ. Anal. Chem. 2021, 103, 2292–2299. [Google Scholar] [CrossRef]
- Kumari, U.; Banerjee, T.; Narayanan, N.; Yadav, S.; Singh, N. Ash and biochar mixed biomixtures to degrade co-applied atrazine and fipronil in bio-augmented biobeds. J. Environ. Anal. Chem. 2022. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Nogales, R.; Romero, E. Biodegradation of high doses of commercial pesticide products in pilot-scale biobeds using olive-oil agroindustry wastes. J. Environ. Manag. 2017, 204, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Moreno, L.; Nogales, R.; Romero, E. Wastes from the olive oil production in sustainable bioremediation systems to prevent pesticides water contamination. Int. J. Environ. Sci. Technol. 2017, 14, 2471–2484. [Google Scholar] [CrossRef]
- Dias, L.D.A.; Itako, A.T.; Gebler, L.; Tolentino Júnior, J.B.; Pizzutti, I.R.; Fontana, M.E.; Janish, B.D.; Niemeyer, J.C. Pine litter and vermicompost as alternative substrates for biobeds: Efficiency in pesticide degradation. Water Air Soil Pollut. 2021, 232, 283. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Hellín, P.; Flores, P.; Vela, N.; Navarro, S. Use of different organic wastes as strategy to mitigate the leaching potential of phenylurea herbicides through the soil. Environ. Sci. Pollut. Res. 2015, 22, 4336–4349. [Google Scholar] [CrossRef] [PubMed]
- Kravvariti, K.; Tsiropoulos, N.G.; Karpouzas, D.G. Degradation and adsorption of terbuthylazine and chlorpyrifos in biobed biomixtures from composted cotton crop residues. Pest Manag. Sci. 2010, 66, 1122–1128. [Google Scholar] [CrossRef]
- Lemerhyeratte, A.; Zougagh, M.; El Mouden, O.I.; Salghi, R.; Bazzi, L.; Hormatallah, A.; Zine, S. Biobed system to reduce four pesticide organophosphorus point contamination at farm level. Orient. J. Chem. 2010, 26, 15–22. [Google Scholar]
- Ruiz-Hidalgo, K.; Chin-Pampillo, J.S.; Masís-Mora, M.; Carazo-Rojas, E.; Rodríguez-Rodríguez, C.E. Optimization of a fungally bioaugmented biomixture for carbofuran removal in on-farm biopurification systems. Water Air Soil Pollut. 2016, 227, 3. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, V.; Masís-Mora, M.; Carazo-Rojas, E.; Mora-López, M.; Rodríguez-Rodríguez, C.E. Impact of oxytetracycline and bacterial bioaugmentation on the efficiency and microbial community structure of a pesticide-degrading biomixture. Environ. Sci. Pollut. Res. 2018, 25, 11787–11799. [Google Scholar] [CrossRef]
- Cessna, A.J.; Knight, J.D.; Ngombe, D.; Wolf, T.M. Effect of temperature on the dissipation of seven herbicides in a biobed matrix. Can. J. Soil Sci. 2017, 97, 717–731. [Google Scholar] [CrossRef] [Green Version]
- El Bakouri, H.; Morillo, J.; Usero, J.; Vanderlinden, E.; Vidal, H. Effectiveness of acid-treated agricultural stones used in biopurification systems to avoid pesticide contamination of water resources caused by direct losses: Part I. Equilibrium experiments and kinetics. Bioresour. Technol. 2010, 101, 5084–5091. [Google Scholar] [CrossRef]
- Karanasios, E.; Tsiropoulos, N.G.; Karpouzas, D.G.; Ehaliotis, C. Degradation and adsorption of pesticides in compost-based biomixtures as potential substrates for biobeds in Southern Europe. J. Agric. Food Chem. 2010, 58, 9147–9156. [Google Scholar] [CrossRef]
- Rojas, R.; Morillo, J.; Usero, J.; Vanderlinden, E.; El Bakouri, H. Adsorption study of low-cost and locally available organic substances and a soil to remove pesticides from aqueous solutions. J. Hydrol. 2015, 520, 461–472. [Google Scholar] [CrossRef]
- Briceño, G.; Tortella, G.; Rubilar, O.; Palma, G.; Diez, M.C. Advances in Chile for the treatment of pesticide residues: Biobeds technology. In Bioremediation in Latin America: Current Research and Perspectives; Alvarez, A., Polti, M., Eds.; Springer: Cham, Switzerland, 2014; pp. 53–68. [Google Scholar] [CrossRef]
- Kumari, U.; Banerjee, T.; Singh, N. Evaluating ash and biochar mixed biomixtures for atrazine and fipronil degradation. Environ. Technol. Innov. 2021, 23, 101745. [Google Scholar] [CrossRef]
- Karas, P.A.; Makri, S.; Papadopoulou, E.S.; Ehaliotis, C.; Menkissoglu-Spiroudi, U.; Karpouzas, D.G. The potential of organic substrates based on mushroom substrate and straw to dissipate fungicides contained in effluents from the fruit-packaging industry–Is there a role for Pleurotus ostreatus? Ecotoxicol. Environ. Saf. 2016, 124, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Adak, T.; Mahapatra, B.; Swain, H.; Patil, N.B.; Gowda, G.B.; Annamalai, M.; Pokhare, S.S.; Meena, K.S.; Rath, P.C.; Jena, M. Indigenous biobed to limit point source pollution of imidacloprid in tropical countries. J. Environ. Manag. 2020, 272, 111084. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.; Rath, S.K.; Mohapatra, P.K. Bioremediation of insecticides by white-rot fungi and its environmental relevance. In Mycoremediation and Environmental Sustainability; Prasad, R., Ed.; Springer: Cham, Switzerland, 2018; pp. 181–212. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa. Fungal Enzymes for Bioremediation of Contaminated Soil. In Recent Advancement in White Biotechnology through Fungi; Yadav, A., Singh, S., Mishra, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 189–215. [Google Scholar] [CrossRef]
- Singh, R.K.; Tripathi, R.; Ranjan, A.; Srivastava, A.K. Fungi as potential candidates for bioremediation. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–191. [Google Scholar] [CrossRef]
- Kathiravan, A.; Gnanadoss, J.J. White-rot fungi-mediated bioremediation as a sustainable method for xenobiotic degradation. Environ. Exp. Biol. 2021, 19, 103–119. [Google Scholar] [CrossRef]
- Tomer, A.; Singh, R.; Singh, S.K.; Dwivedi, S.A.; Reddy, C.U.; Keloth, M.R.A.; Rachel, R. Role of fungi in bioremediation and environmental sustainability. In Mycoremediation and Environmental Sustainability; Prasad, R., Nayak, S.C., Kharwar, R.N., Dubey, N.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 187–200. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, A.; Sharma, B. Role of fungal enzymes for bioremediation of hazardous chemicals. In Recent Advancement in White Biotechnology through Fungi; Yadav, A., Singh, S., Mishra, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 237–256. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wei, J.; Yang, B.; Zhang, M.; Zhuo, R. Bioremediation of organic pollutants by white rot fungal cytochrome P450: The role and mechanism of CYP450 in biodegradation. Chemosphere 2022, 301, 134776. [Google Scholar] [CrossRef]
- Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Dantán-González, E.; Castrejón-Godínez, M.L. Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. In Biodegradation-Life of Science; Chamy, R., Rosenkranz, F., Eds.; IntechOpen: London, UK, 2013; pp. 251–287. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; López-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, P.; Malyan, S.K.; Sharma, J.; Kumar, V. A comprehensive review on enzymatic degradation of the organophosphate pesticide malathion in the environment. J. Environ. Sci. Health C 2019, 37, 288–329. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Das, J. Role of microbial enzymes for biodegradation and bioremediation of environmental pollutants: Challenges and future prospects. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 325–346. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef]
- Bergsveinson, J.; Perry, B.J.; Sheedy, C.; Braul, L.; Reedyk, S.; Gossen, B.D.; Yost, C.K. Identifying the core bacterial and fungal communities within four agricultural biobeds used for the treatment of pesticide rinsates. J. Appl. Microbiol. 2018, 125, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Echeverría, V.R.; Quintal-Franco, C.; Arena-Ortiz, M.L.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Identification of microbial species present in a pesticide dissipation process in biobed systems using typical substrates from southeastern Mexico as a biomixture at a laboratory scale. Sci. Total Environ. 2018, 628, 528–538. [Google Scholar] [CrossRef]
- Russell, J.N.; Perry, B.J.; Bergsveinson, J.; Freeman, C.N.; Sheedy, C.; Nilsson, D.; Braul, L.; Yost, C.K. Metagenomic and metatranscriptomic analysis reveals enrichment for xenobiotic-degrading bacterial specialists and xenobiotic-degrading genes in a Canadian prairie two-cell biobed system. Environ. Microbiol. Rep. 2021, 13, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Magan, N.; Gouma, S.; Fragoeiro, S.; Shuaib, M.E.; Bastos, A.C. Bacterial and fungal bioremediation strategies. In Microbial Biodegradation and Bioremediation; Das, S., Dash, H.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 193–212. [Google Scholar] [CrossRef]
- Fernández-Alberti, S.; Rubilar, O.; Tortella, G.R.; Diez, M.C. Chlorpyrifos degradation in a biomix: Effect of pre-incubation and water holding capacity. J. Soil Sci. Plant Nutr. 2012, 12, 785–799. [Google Scholar] [CrossRef] [Green Version]
- Tortella, G.R.; Rubilar, O.; Castillo, M.; Cea, M.; Mella-Herrera, R.; Diez, M.C. Chlorpyrifos degradation in a biomixture of biobed at different maturity stages. Chemosphere 2012, 88, 224–228. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, N.; Ramakrishnan, B. Parameters affecting azoxystrobin and imidacloprid degradation in biobed substrates in the North Indian tropical environment. J. Environ. Sci. Health B 2019, 54, 843–857. [Google Scholar] [CrossRef]
- Basford, B. BIOBEDS. Outlooks Pest Manag. 2010, 21, 78–80. [Google Scholar] [CrossRef]
- Spliid, N.H.; Helweg, A.; Heinrichson, K. Leaching and degradation of 21 pesticides in a full-scale model biobed. Chemosphere 2006, 65, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gutiérrez, V.; Masís-Mora, M.; Carazo-Rojas, E.; Mora-López, M.; Rodríguez-Rodríguez, C.E. Fungal and bacterial co-bioaugmentation of a pesticide-degrading biomixture: Pesticide removal and community structure variations during different treatments. Water Air Soil Pollut. 2019, 230, 247. [Google Scholar] [CrossRef]
- Veiga-Bernardelli, P.; Nagel-Hassemer, M.; Gebler, L. Introduction of advanced oxidative pre-treatment in the biobed reactor system in the final disposal process of pesticide effluents. Chem. Eng. Trans. 2021, 86, 685–690. [Google Scholar] [CrossRef]
- Lescano, M.R.; Masin, C.E.; Rodríguez, A.R.; Godoy, J.L.; Zalazar, C.S. Earthworms to improve glyphosate degradation in biobeds. Environ. Sci. Pollut. Res. 2020, 27, 27023–27031. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Echeverría, V.; García-Escalante, R.; Rojas-Herrera, R.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Pesticide bioremediation in liquid media using a microbial consortium and bacteria-pure strains isolated from a biomixture used in agricultural areas. Ecotoxicol. Environ. Saf. 2020, 200, 110734. [Google Scholar] [CrossRef] [PubMed]
- Lescano, M.R.; Pizzul, L.; Castillo, M.D.P.; Zalazar, C.S. Glyphosate and aminomethylphosphonic acid degradation in biomixtures based on alfalfa straw, wheat stubble and river waste. J. Environ. Manag. 2018, 228, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.E.; Morel, M.R.; Clebot, A.C.; Zalazar, C.S.; Ballari, M.M. Effectiveness of a simple biomixture for the adsorption and elimination of 2,4-dichlorophenoxyacetic acid (2,4-D) herbicide and its metabolite, 2,4-dichlorophenol (2,4-DCP), for a biobed system. J. Environ. Chem. Eng. 2022, 10, 106877. [Google Scholar] [CrossRef]
- Diez, M.C.; Leiva, B.; Gallardo, F. Novel insights in biopurification system for dissipation of a pesticide mixture in repeated applications. Environ. Sci. Pollut. Res. 2018, 25, 21440–21450. [Google Scholar] [CrossRef]
- Rivero, A.; Gérez, N.; Jesús, F.; Niell, S.; Pia-Cerdeiras, M.; Heinzen, H.; Cesio, M.V. Unambiguous evaluation of chlorpyrifos and TCP bioremediation in laboratory and field experiments. Int. J. Environ. Anal. Chem. 2022, 102, 6845–6857. [Google Scholar] [CrossRef]
- Briceño, G.; Vergara, K.; Schalchli, H.; Palma, G.; Tortella, G.; Fuentes, M.S.; Diez, M.C. Organophosphorus pesticide mixture removal from environmental matrices by a soil S treptomyces mixed culture. Environ. Sci. Pollut. Res. 2018, 25, 21296–21307. [Google Scholar] [CrossRef]
- Odukkathil, G.; Vasudevan, N. Bacteria amended clay biochar composite biobed system to treat agriculture runoff. J. Environ. Manag. 2020, 269, 110694. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.I. Groundwater ubiquity score: A simple method for assessing pesticide leachability. Environ. Toxicol. Chem. Int. J. 1989, 8, 339–357. [Google Scholar] [CrossRef]
- Inter-Organization Programme for the Sound Management of Chemicals; World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020; 92p. Available online: https://www.who.int/publications/i/item/9789240005662 (accessed on 15 June 2023).
- Hertfordshire University. PPDB: Pesticide Properties DataBase; University of Hertfordshire: Hatfield, UK, 2017; Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/ (accessed on 12 June 2023).
| Characteristics | Risk Profile | ||||||
|---|---|---|---|---|---|---|---|
| Pesticide | Water Solubility 1 | Persistence in Soil 2 | Leachability 3 | Bioaccumulative Potential 4 | Acute Toxicity 5 | Ecotoxicity 6 | Human Health 7 |
| Fungicides | |||||||
| Metalaxyl | High | Moderately persistent | Moderate | Low | Moderately hazardous | Moderate acute toxicity in birds, fish, and Daphnia magna Moderate chronic toxicity in Daphnia magna and earthworms | Moderate acute toxicity in mammals Moderate chronic toxicity in mammals |
| Carboxin | Moderate | Not persistent | Moderate | Low | Slightly hazardous | Moderate acute and chronic toxicity in birds, fish, Daphnia magna, and earthworms | Moderate chronic toxicity in mammals and possible reproduction/development effects |
| Imazalil | Moderate | Moderately persistent | Low | Low | Moderately hazardous | High chronic toxicity in fish | Endocrine disrupter and reproduction/development effects |
| Azoxystrobin | Low | Moderately persistent | High | Low | Unlikely to present acute hazard | Moderate acute toxicity in fish, Daphnia magna, bees, and earthworms Moderate chronic toxicity in fish, Daphnia magna, and earthworms | Moderate chronic toxicity in mammals and possible reproduction/development effects |
| Carbendazim | Low | Moderately persistent | Moderate | Low | Unlikely to present acute hazard | High chronic toxicity in fish, Daphnia magna, and earthworms | Endocrine disrupter and reproduction/development effects |
| Chlorothalonil | Low | Not persistent | Low | Threshold for concern | Unlikely to present acute hazard | High acute and chronic toxicity in fish and Daphnia magna | Carcinogen, endocrine disrupter, and reproduction/development effect |
| Fludioxonil | Low | Persistent | Low | Threshold for concern | Unlikely to present acute hazard | High acute and chronic toxicity in fish and Daphnia magna | Possible carcinogen and possible reproduction/development effects |
| Fluxapyroxad | Low | Persistent | Moderate | Low | Slightly hazardous | High chronic toxicity in fish | Possible carcinogen and possible reproduction/development effects |
| Tebuconazole | Low | Moderately persistent | Moderate | Low | Moderately hazardous | High chronic toxicity in birds and fish | Endocrine disrupter and reproduction/development effects |
| Thiabendazole | Low | Very persistent | Moderate | Low | Slightly hazardous | High chronic toxicity in fish | Moderate chronic toxicity in mammals, possible carcinogen, and possible reproduction/development effects |
| Herbicides | |||||||
| 2,4 D | High | Not persistent | High | Low | Moderately hazardous | Moderate acute toxicity in birds, fish, bees, and earthworms Moderate chronic toxicity in birds, fish, and earthworms | Endocrine disrupter, reproduction/development effects, and neurotoxicant. |
| Glyphosate | High | Not persistent | Low | Low | Slightly hazardous | Moderate chronic toxicity in birds, fish, and earthworms | Possible carcinogen, possible endocrine disrupter, and possible reproduction/development effects |
| Linuron | Moderate | Moderately persistent | Moderate | Low | Slightly hazardous | Moderate acute toxicity in birds, fishes, Daphnia magna, bees, and earthworms Moderatechronic toxicity in birds, fish, Daphnia magna, and earthworms | High chronic toxicity in mammals and high reproduction/development effects |
| Atrazine | Low | Moderately persistent | Moderate | Low | Slightly hazardous | Moderate acute toxicity in fish, Daphnia magna, and earthworms Moderate chronic toxicity in fish and Daphnia magna | Endocrine disrupter |
| Prometryn | Low | Moderately persistent | Low | Low | Slightly hazardous | High chronic toxicity in fish | Endocrine disrupter |
| Insecticides | |||||||
| Imidacloprid | High | Persistent | High | Low | Slightly hazardous | High acute toxicity in birds and bees High chronic toxicity in birds | Reproduction/development effects |
| Carbofuran | Moderate | Not persistent | Moderate | Low | Highly hazardous | High acute toxicity in birds, Daphnia magna, and bees High chronic toxicity in fish and Daphnia magna | High acute toxicity in mammals, endocrine disrupter, and reproduction/development effects |
| Diazinon | Moderate | Not persistent | Low | Threshold for concern | Slightly hazardous | High acute toxicity in birds, Daphnia magna, and bees High chronic toxicity in Daphnia magna | Endocrine disrupter, acetyl cholinesterase inhibitor, and neurotoxicant |
| Chlorpyrifos | Low | Very persistent | Low | Threshold for concern | Slightly hazardous | High acute toxicity in birds, fish, Daphnia magna, and bees High chronic toxicity in birds, fish, Daphnia magna, and earthworms | High acute and chronic toxicity in mammals, endocrine disrupter, reproduction/development effects, acetyl cholinesterase inhibitor, and neurotoxicant |
| Cypermethrin | Low | Not persistent | Low | Threshold for concern | Slightly hazardous | High acute toxicity in fish, Daphnia magna, and bees High chronic toxicity in Daphnia magna | High acute toxicity in mammals and endocrine disrupter |
| Fipronil | Low | Persistent | Moderate | Threshold for concern | Slightly hazardous | High acute toxicity in birds and bees High chronic toxicity in birds and fish | High acute and chronic toxicity in mammals and neurotoxicant |
| Phosmet | Low | Not persistent | Low | Low | Slightly hazardous | High acute toxicity in Daphnia magna and bees High chronic toxicity in fish and Daphnia magna | Reproduction/development effects, acetyl cholinesterase inhibitor, and neurotoxicant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussali-Galante, P.; Castrejón-Godínez, M.L.; Díaz-Soto, J.A.; Vargas-Orozco, Á.P.; Quiroz-Medina, H.M.; Tovar-Sánchez, E.; Rodríguez, A. Biobeds, a Microbial-Based Remediation System for the Effective Treatment of Pesticide Residues in Agriculture. Agriculture 2023, 13, 1289. https://doi.org/10.3390/agriculture13071289
Mussali-Galante P, Castrejón-Godínez ML, Díaz-Soto JA, Vargas-Orozco ÁP, Quiroz-Medina HM, Tovar-Sánchez E, Rodríguez A. Biobeds, a Microbial-Based Remediation System for the Effective Treatment of Pesticide Residues in Agriculture. Agriculture. 2023; 13(7):1289. https://doi.org/10.3390/agriculture13071289
Chicago/Turabian StyleMussali-Galante, Patricia, María Luisa Castrejón-Godínez, José Antonio Díaz-Soto, Ángela Patricia Vargas-Orozco, Héctor Miguel Quiroz-Medina, Efraín Tovar-Sánchez, and Alexis Rodríguez. 2023. "Biobeds, a Microbial-Based Remediation System for the Effective Treatment of Pesticide Residues in Agriculture" Agriculture 13, no. 7: 1289. https://doi.org/10.3390/agriculture13071289










