Impact of Lentinus sajor-caju on Lignocellulosic Biomass, In Vitro Rumen Digestibility and Antioxidant Properties of Astragalus membranaceus var. mongholicus Stems under Solid-State Fermentation Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Preparation
2.2. Experimental Set-Up
2.3. Structural Characterization of AMM Stems Using Scanning Electron Microscopy
2.4. Chemical Analysis
2.5. In Vitro Rumen Fermentation
2.6. Antioxidant Activity
2.7. Metabolomic Profiling Analysis
2.8. Statistical Analyses
3. Results and Discussion
3.1. Morphological Observations and Structural Characterization
3.2. Lignocellulose Composition
3.3. In Vitro Rumen Digestibility
3.4. The Antioxidant Activity of AMM Stem Extracts
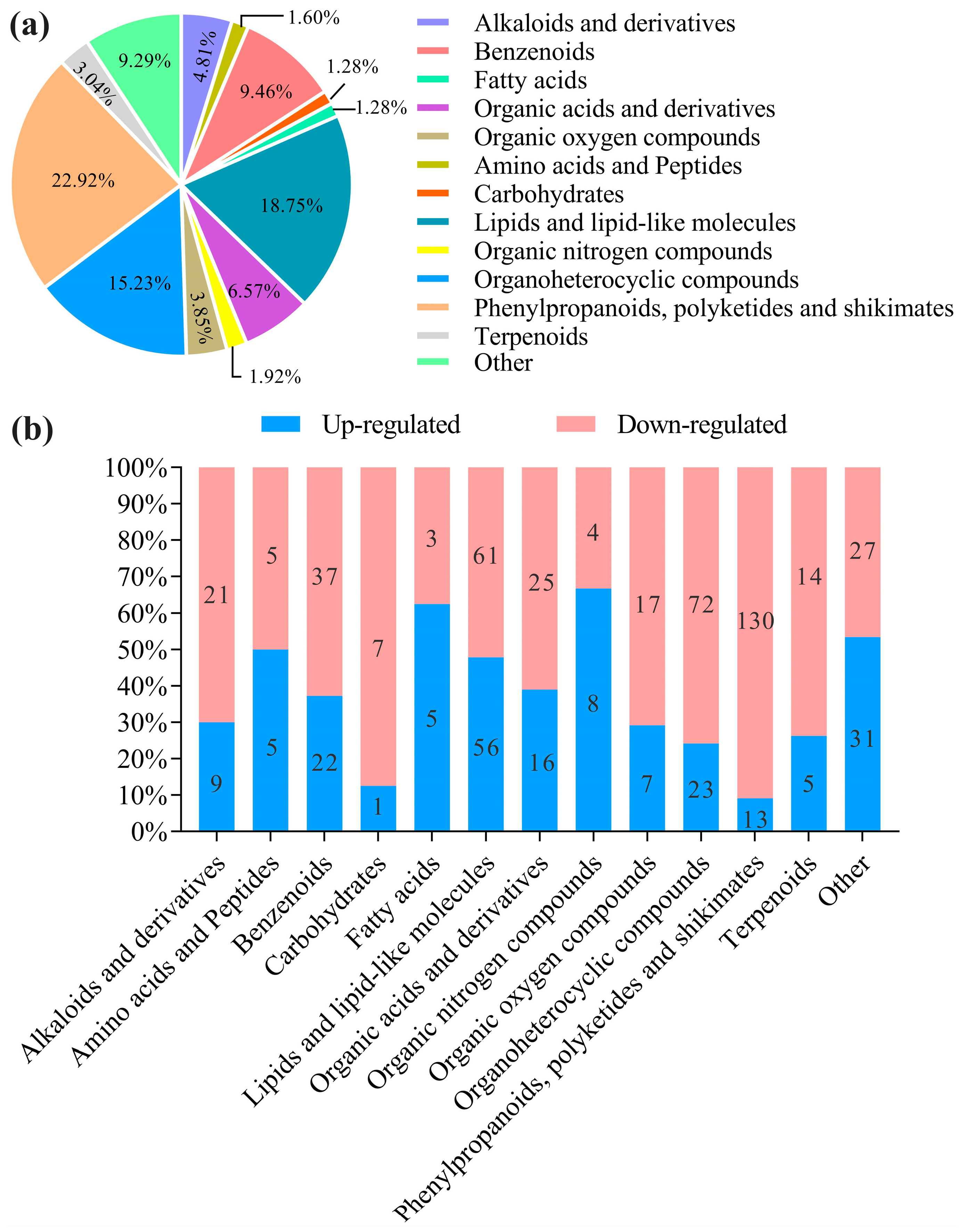
3.5. Metabolite Differences and Classification
3.5.1. Analysis of Differential Metabolite
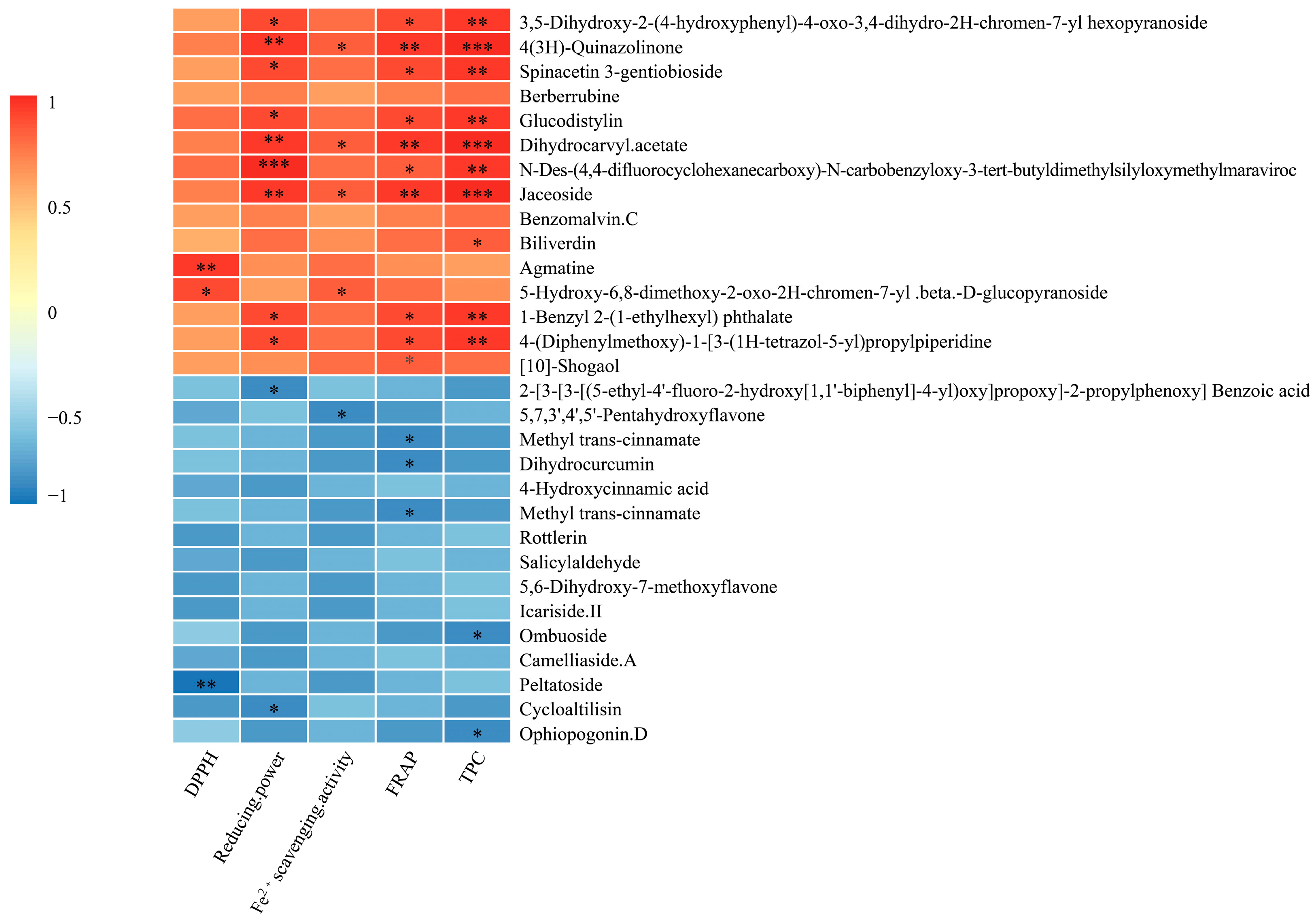
3.5.2. Antioxidant Composition in AMM Stems Fermented and Unfermented
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, R.Q.; Yin, M.Z.; Yang, M.; Chu, S.S.; Han, X.J.; Wang, M.J.; Peng, H.S. Developmental anatomy of anomalous structure and classification of commercial specifications and grades of the Astragalus membranaceus var. mongholicus. Microsc. Res. Tech. 2018, 81, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Lyu, X.; Chen, X.M.; Guo, S. Nutritional characterization and untargeted metabolomics of oyster mushroom produced using Astragalus membranaceus var. mongolicus stems and leaves as substrates. Front. Plant Sci. 2022, 13, 802801. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Findlay, J.A. Constituents of Astragalus membranaceus. J. Nat. Prod. 1991, 54, 810–815. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Noh, H.J.; Choi, J.; Lee, K.H.; Lee, M.H.; Lee, J.H.; Hong, Y.; Lee, S.E.; Kim, S.Y.; Kim, G.S. Anti-inflammatory cycloartane-type saponins of Astragalus membranaceus. Molecules 2013, 18, 3725–3732. [Google Scholar] [CrossRef]
- Li, S.G.; Zhang, Y.Q. Characterization and renal protective effect of a polysaccharide from Astragalus membranaceus. Carbohydr. Polym. 2009, 78, 343–348. [Google Scholar] [CrossRef]
- Cui, R.T.; He, J.C.; Wang, B.; Zhang, F.K.; Chen, G.Y.; Yin, S.S.; Shen, H. Suppressive effect of Astragalus membranaceus bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother. Pharmacol. 2003, 51, 75–80. [Google Scholar] [CrossRef]
- Zhang, G. Investigation of active ingredients from roots and stem leaves of Astragalus mongholicus Bunge. Tianjin Pharm. 2010, 5, 5–7. [Google Scholar]
- Luo, Y.L.; Su, L.; Su, R.; Wang, B.H.; Liu, C.; Wang, Z.G.; Zhao, L.H.; Jin, Y. Effects of Astragalus membranaceus supplementation on oxidative stability of Cashmere goat. Food Sci. Nutr. 2020, 8, 5550–5556. [Google Scholar] [CrossRef]
- Moussaoui, Y.; Ferhi, F.; Elaloui, E.; Ben Salem, R.B.; Belgacem, M.N. Utilisation of Astragalus armatus roots in papermaking. BioResources 2011, 6, 4969–4978. [Google Scholar] [CrossRef]
- Rauf, A.; Shafeeq, A.; Shahzad, K. Delignification of corn straw using the ionic liquid triethylammonium hydrogen sulfate. Chem. Eng. Technol. 2022, 45, 1106–1113. [Google Scholar] [CrossRef]
- Sharma, R.K.; Arora, D.S. Bioprocessing of wheat and paddy straw for their nutritional up-gradation. Bioprocess. Biosyst. Eng. 2014, 37, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Atiwesh, G.; Parrish, C.C.; Banoub, J.; Le, T.T. Lignin degradation by microorganisms: A review. Biotechnol. Prog. 2022, 38, e3226. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Gou, C.L.; Chen, L.M.; Liao, Y.C.; Zhang, H.; Luo, L.L.; Ji, J.H.; Qi, Y. Solid-state fermentation with white rot fungi (Pleurotus species) improves the chemical composition of highland barley straw as a ruminant feed and enhances in vitro rumen digestibility. J. Fungi 2023, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Goering, H.K.; Soest, P.J.V. Forage Fiber Analyses: Apparatus, Reagents, Procedures, and Some Applications. In Agricultural Handbook; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1970; Volume 379, pp. 1–20. [Google Scholar]
- Van Soest, P.J.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Arora, D.S.; Chandra, P. Assay of antioxidant potential of two Aspergillus isolates by different methods under various physio-chemical conditions. Braz. J. Microbiol. 2010, 41, 765–777. [Google Scholar] [CrossRef]
- Chang, L.W.; Yen, W.J.; Huang, S.C.; Duh, P.D. Antioxidant activity of sesame coat. Food Chem. 2002, 78, 347–354. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, Y.; Jia, Y.N.; Xue, Z.H.; Chen, Z.Q.; Zhang, M.; Pharkphoom, P.; Yang, S.Y.; Chen, H.X. Chrysophyllum cainito. L alleviates diabetic and complications by playing antioxidant, antiglycation, hypoglycemic roles and the chemical profile analysis. J. Ethnopharmacol. 2021, 281, 114569. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, P.P.; Zhu, Z.D.; Chen, C.; Xu, X.Q. Production of phenolic compounds and antioxidant activity via bioconversion of wheat straw by Inonotus obliquus under submerged fermentation with the aid of a surfactant. J. Sci. Food Agric. 2021, 101, 1021–1029. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrate and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Shinya, T.; Iwata, E.; Nakahama, K.; Fukuda, Y.; Hayashi, K.; Nanto, K.; Rosa, A.C.; Kawaoka, A. Transcriptional profiles of hybrid Eucalyptus genotypes with contrasting lignin content reveal that monolignol biosynthesis-related genes regulate wood composition. Front. Plant Sci. 2016, 7, 443. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, Y.; Hu, H.; Xie, F.; Wei, X.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Datsomor, O.; Gou-Qi, Z.; Miao, L. Effect of ligninolytic axenic and coculture white-rot fungi on rice straw chemical composition and in vitro fermentation characteristics. Sci. Rep. 2022, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Wang, F.; Fang, Y.; Zhou, D.W.; Wang, S.P.; Wu, D.Q.; Wang, L.X.; Zhong, R.Z. High-potency white-rot fungal strains and duration of fermentation to optimize corn straw as ruminant feed. Bioresour. Technol. 2020, 312, 123512. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Luo, Y.; Luo, L.L.; Zhang, H.; Liao, Y.C.; Gou, C.L. Enhancement of the nutritional value of fermented corn stover as ruminant feed using the fungi Pleurotus Spp. Sci. Rep. 2021, 11, 11961. [Google Scholar] [CrossRef]
- Ding, C.H.; Wang, X.; Li, M.X. Evaluation of six white-rot fungal pretreatments on corn stover for the production of cellulolytic and ligninolytic enzymes, reducing sugars, and ethanol. Appl. Microbiol. Biotechnol. 2019, 103, 5641–5652. [Google Scholar] [CrossRef]
- Reddy, G.V. Bioconversion of Banana Waste into Protein by Two Pleurotus Species (P. ostreatus and P. sajor-caju). Biotechnological Approach. Ph.D. Thesis, Sardar Patel University, Gujarat, India, 2001. [Google Scholar]
- Ghimire, S. Volatile Fatty Acid Production in Ruminants. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, 2015. [Google Scholar]
- Datsomor, O.; Yan, Q.; Wang, K.; Mohamed, S.; Opoku-Mensah, L.; Zhao, G.Q.; Miao, L. Effect of ammoniated and/or basidiomycete white-rot fungi treatment on rice straw proximate composition, cell wall component, and in vitro rumen fermentation characteristics. Fermentation 2022, 8, 228. [Google Scholar] [CrossRef]
- Sufyan, A.; Khan, N.A.; AbuGhazaleh, A.; Ahmad, N.; Tang, S.; Tan, Z. Novel techniques for the mass production of nutritionally improved, fungus-treated lignocellulosic biomass for ruminant nutrition. J. Sci. Food. Agri. 2024, 4, 2215–2224. [Google Scholar] [CrossRef]
- Chakraborty, A.; Majumdar, S.; Bhowal, J. Phytochemical screening and antioxidant and antimicrobial activities of crude extracts of different filamentous fungi. Arch. Microbiol. 2021, 203, 6091–6108. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality—Invited review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Huang, M.Y.; Lin, K.H.; Lu, C.C.; Chen, L.R.; Hsiung, T.C.; Chang, W.T. The intensity of blue light-emitting diodes influences the antioxidant properties and sugar content of oyster mushrooms (Lentinus sajor-caju). Sci. Hortic. Amst. 2017, 218, 8–13. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.S.; Aboshanab, K.M.; Yassien, M.A. Antiviral, cytotoxic, and antioxidant activities of three edible Agaricomycetes mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef]
- Su, W.Y.; Gao, S.Y.; Zhan, S.J.; Wu, Q.; Chen, G.M.; Han, J.Z.; Lv, X.C.; Rao, P.F.; Ni, L. Evaluation of volatile profile and in vitro antioxidant activity of fermented green tea infusion with Pleurotus sajor-caju (Oyster Mushroom). Front. Nutr. 2022, 9, 865991. [Google Scholar] [CrossRef]
- Mishra, K.K.; Pal, R.S.; Arunkumar, R.; Chandrashekara, C.; Jain, S.K.; Bhatt, J.C. Antioxidant properties of different edible mushroom species and increased bioconversion efficiency of Pleurotus eryngii using locally available casing materials. Food Chem. 2013, 138, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liao, Y.; Gou, C.; Zhang, H.; Chen, L.; Bao, Y. Effect of Lentinus sajor-caju on the chemical composition and antioxidant activity of highland barley straw under solid-state fermentation. Front. Microbiol. 2024, 15, 1365254. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.; Qin, Y.; Shi, H. Influence of microbiota and metabolites on the quality of tobacco during fermentation. BMC Microb. 2020, 20, 356. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M. Biosynthesis of Diverse Class Flavonoids via Shikimate and Phenylpropanoid Pathway. In Bioactive Compounds-Biosynthesis, Characterization and Applications; Zepka, L.Q., Nascimento, T.C.d., Lopes, E.J., Eds.; IntechOpen: London, UK, 2021; p. 75392. [Google Scholar]
- Zhu, W.; Wang, W.F.; Xu, W.C.; Wu, S.; Chen, W.J.; Huang, Y.Y.; Wang, S.P. Influence of thermophilic microorganism on non-volatile metabolites during high-temperature pile-fermentation of Chinese dark tea based on metabolomic analysis. Food Sci. Biotechnol. 2022, 31, 827–841. [Google Scholar] [CrossRef]
- Gan, J.; Feng, Y.; He, Z.; Li, X.; Zhang, H. Correlations between antioxidant activity and alkaloids and phenols of maca (Lepidium meyenii). J. Food Qual. 2017, 2017, 3185945. [Google Scholar] [CrossRef]
- Shao, L.; Zhao, S.; Yang, S.; Zhou, X.; Li, Y.; Li, C.; Chen, D.; Li, Z.; Ouyang, G.; Wang, Z. Design, synthesis, antibacterial evaluation, three-dimensional quantitative structure–activity relationship, and mechanism of novel quinazolinone derivatives. J. Agr. Food Chem. 2023, 9, 3939–3949. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechno. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Ding, Y.M.; Guo, T.L.; Li, Z.W.; Zhang, B.; Kühn, F.E.; Liu, C.; Zhang, J.; Xu, D.Z.; Lei, M.; Zhang, T.; et al. Transition-metal-free synthesis of functionalized quinolines by direct conversion of β-O-4 model compounds. Angew. Chem. 2022, 134, 202206284. [Google Scholar] [CrossRef]
- Mäki, M.; Mali, T.; Hellén, H.; Heinonsalo, J.; Lundell, T.; Bäck, J. Deadwood substrate and species-species interactions determine the release of volatile organic compounds by wood-decaying fungi. Fungal Ecol. 2021, 54, 101106. [Google Scholar] [CrossRef]
- Nageen, B.; Rasul, A.; Hussain, G.; Shah, M.A.; Anwar, H.; Hussain, S.M.; Uddin, M.S.; Sarfraz, I.; Riaz, A.; Selamoglu, Z. Jaceosidin: A natural flavone with versatile pharmacological and biological activities. Curr. Pharm. Des. 2021, 4, 456–466. [Google Scholar] [CrossRef] [PubMed]
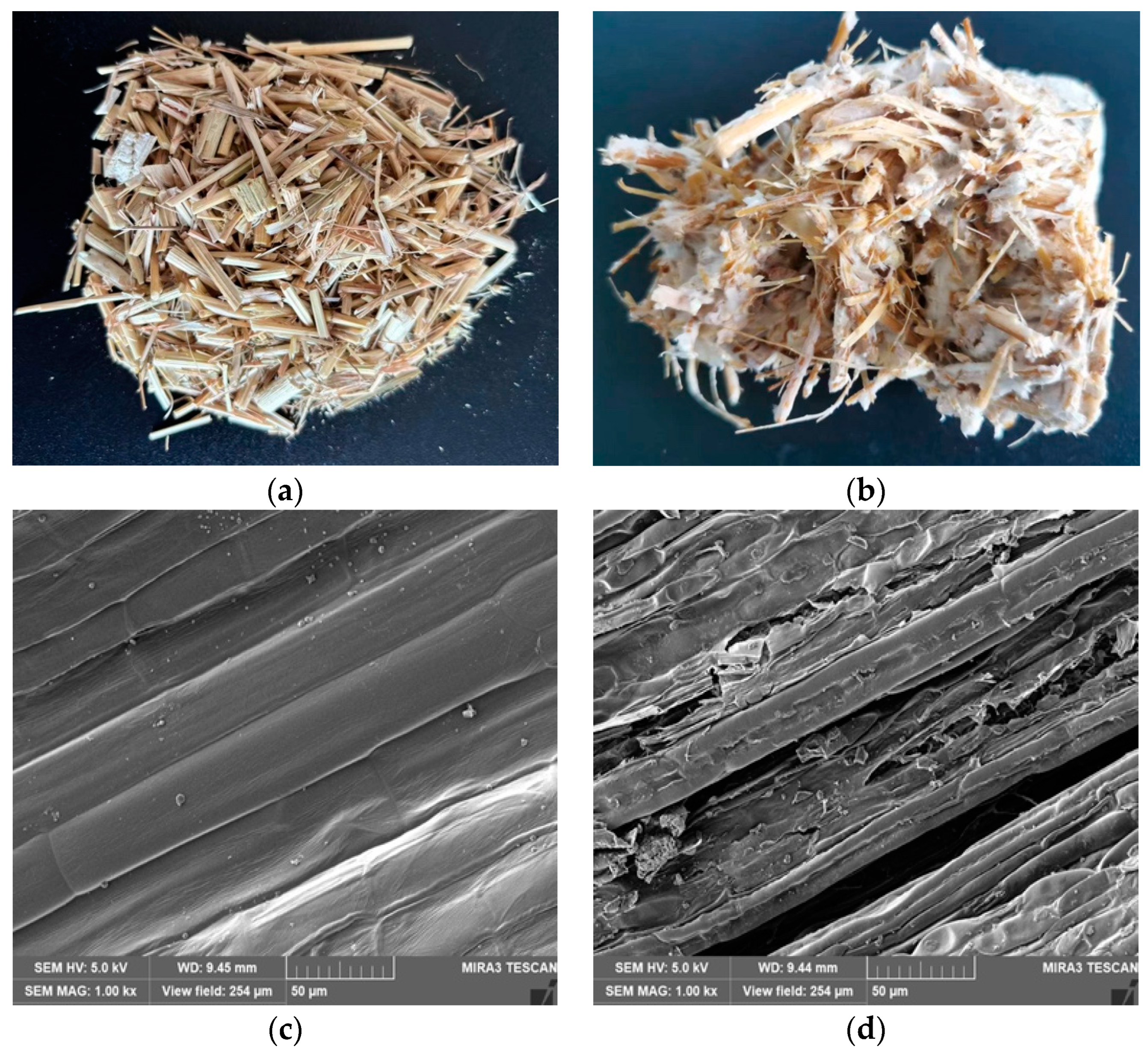

| Item | NDF | ADF | Cellulose | Hemicellulose | ADL |
|---|---|---|---|---|---|
| CK | 72.77 ± 0.41 A | 41.34 ± 0.66 A | 21.52 ± 0.80 A | 31.43 ± 0.54 A | 16.63 ± 0.23 A |
| LP | 60.85 ± 0.79 B | 36.32 ± 0.68 B | 20.60 ± 0.82 A | 24.2 ± 0.53 B | 11.46 ± 0.19 B |
| SEM | 0.63 | 0.67 | 0.08 | 0.53 | 0.21 |
| p-value | <0.001 | <0.001 | 0.81 | <0.001 | <0.001 |
| Variables | CK | LP | SEM | p-Value |
|---|---|---|---|---|
| Chemical composition digestibility | ||||
| DMD (%DM) | 60.39 ± 0.58 B | 67.95 ± 0.38 A | 0.49 | <0.001 |
| NDFD (%DM) | 48.17 ± 0.39 B | 53.42 ± 0.58 A | 0.46 | <0.001 |
| ADFD (%DM) | 46.15 ± 0.66 B | 51.68 ± 0.88 A | 0.79 | <0.001 |
| CLD (%DM) | 8.22 ± 0.36 B | 11.84 ± 0.62 A | 1.00 | <0.001 |
| HLD (%DM) | 11.99 ± 0.54 B | 15.08 ± 0.53 A | 0.54 | <0.001 |
| ADLD (%DM) | 27.19 ± 0.66 B | 33.96 ± 1.24 A | 0.51 | <0.001 |
| Fermentation variables | ||||
| pH | 6.93 ± 0.11 | 6.91 ± 0.08 | 0.10 | 0.713 |
| Acetic acid (mM) | 68.36 ± 0.35 A | 64.66 ± 0.66 B | 0.46 | <0.001 |
| Propionic acid (mM) | 16.69 ± 0.30 B | 19.49 ± 0.19 A | 0.25 | <0.001 |
| Butyric acid (mM) | 13.46 ± 0.33 B | 14.31 ± 0.32 A | 0.33 | <0.001 |
| Total VFA | 85.11 ± 0.53 B | 89.21 ± 0.46 A | 0.50 | <0.001 |
| Item | TPC (mg/g) | DPPH Assay (%) | FRAP Assay (%) | Reducing Power (%) | Fe2+ Scavenging (%) |
|---|---|---|---|---|---|
| CK | 4.42 ± 0.15 B | 41.95 ± 0.60 B | 32.08 ± 1.10 B | 0.71 ± 0.05 B | 12.05 ± 0.63 B |
| LP | 8.23 ± 0.36 A | 53.79 ± 1.83 A | 47.05 ± 1.19 A | 0.92 ± 0.03 A | 20.22 ± 1.24 A |
| SEM | 0.27 | 1.36 | 1.15 | 0.04 | 0.99 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Character | ADL Loss | TPC | DPPH | FRAP | Reducing Power | Fe2+ Scavenging |
|---|---|---|---|---|---|---|
| ADL loss | ||||||
| TPC | 0.82 ** | |||||
| DPPH | 0.78 ** | 0.87 ** | ||||
| FRAP | 0.81 ** | 0.83 ** | 0.79 ** | |||
| Reducing power | 0.82 ** | 0.86 ** | 0.76 ** | 0.71 * | ||
| Fe2+ scavenging | 0.86 ** | 0.71 * | 0.80 ** | 0.79 ** | 0.73 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Q.; Luo, L.-L.; Chen, L.-M.; Liao, Y.-C.; Zhang, H.; Gou, C.-L. Impact of Lentinus sajor-caju on Lignocellulosic Biomass, In Vitro Rumen Digestibility and Antioxidant Properties of Astragalus membranaceus var. mongholicus Stems under Solid-State Fermentation Conditions. Agriculture 2024, 14, 1702. https://doi.org/10.3390/agriculture14101702
Wang Y-Q, Luo L-L, Chen L-M, Liao Y-C, Zhang H, Gou C-L. Impact of Lentinus sajor-caju on Lignocellulosic Biomass, In Vitro Rumen Digestibility and Antioxidant Properties of Astragalus membranaceus var. mongholicus Stems under Solid-State Fermentation Conditions. Agriculture. 2024; 14(10):1702. https://doi.org/10.3390/agriculture14101702
Chicago/Turabian StyleWang, Yu-Qiong, Li-Long Luo, Li-Ming Chen, Yang-Ci Liao, Hang Zhang, and Chang-Long Gou. 2024. "Impact of Lentinus sajor-caju on Lignocellulosic Biomass, In Vitro Rumen Digestibility and Antioxidant Properties of Astragalus membranaceus var. mongholicus Stems under Solid-State Fermentation Conditions" Agriculture 14, no. 10: 1702. https://doi.org/10.3390/agriculture14101702








