A Low-Tech Approach to Mobilize Nutrients from Organic Residues to Produce Bioponic Stock Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Bioponic Stock Solutions
2.2. Testing the Bioponic Solution on Lettuce
2.3. Statistical Analyses
3. Results
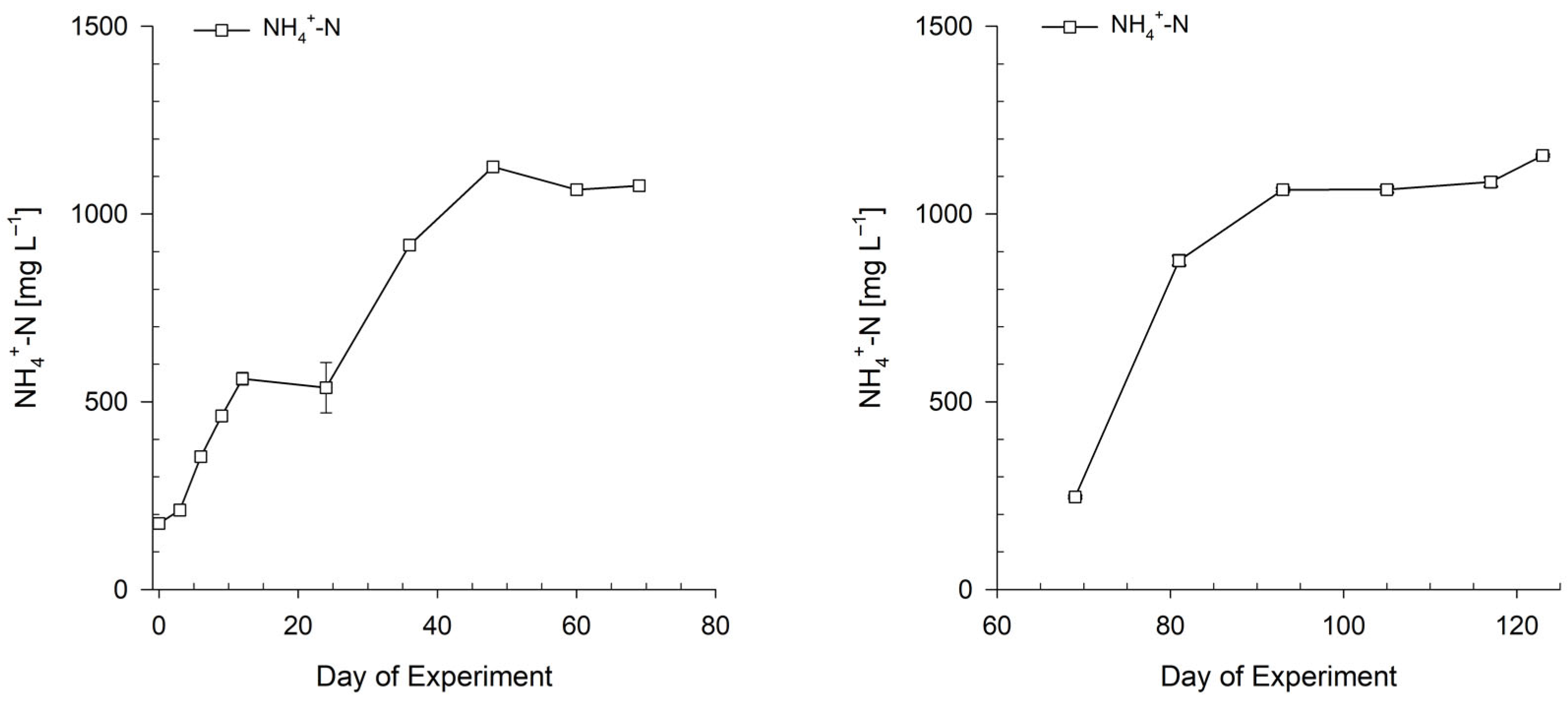
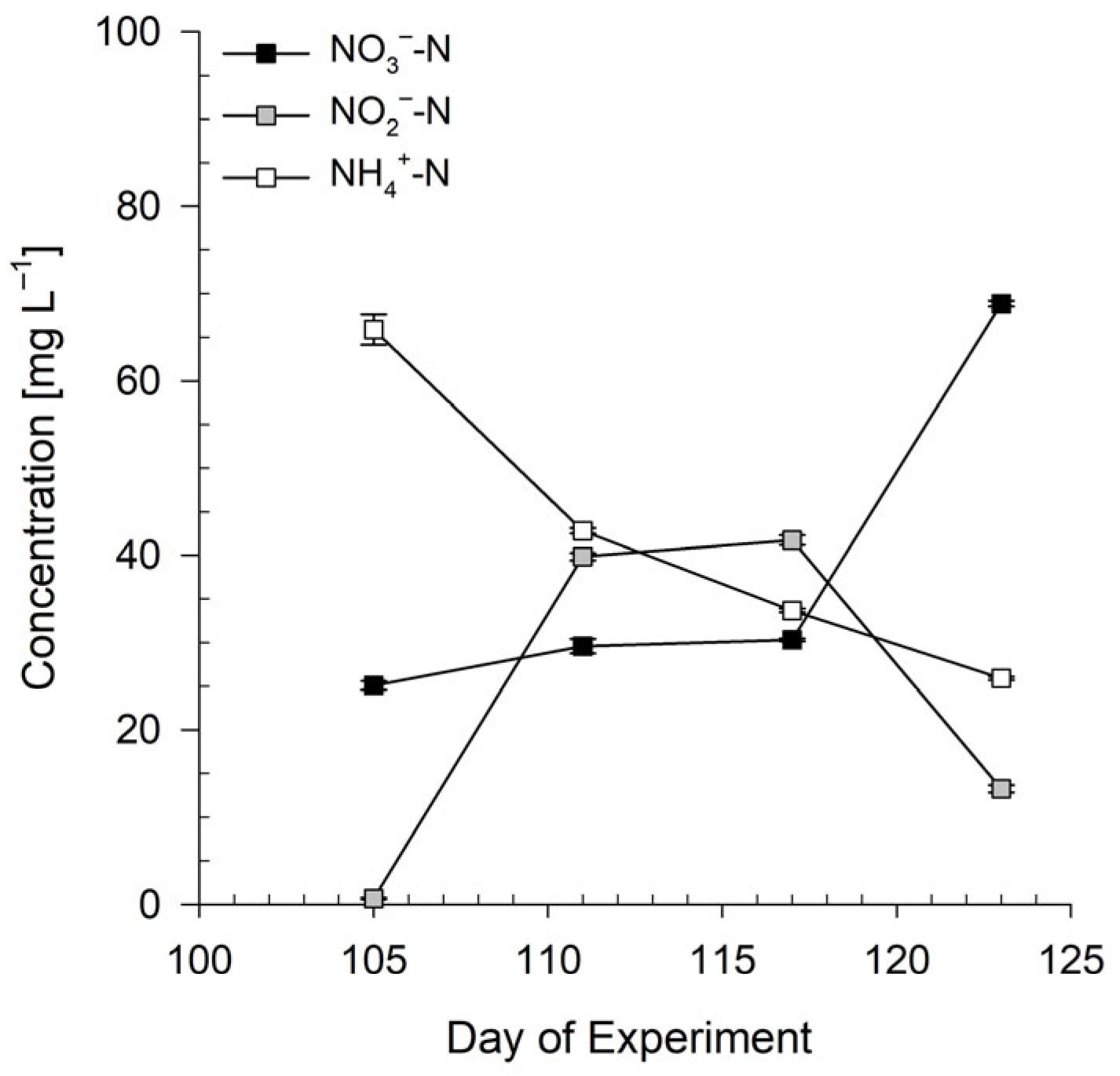
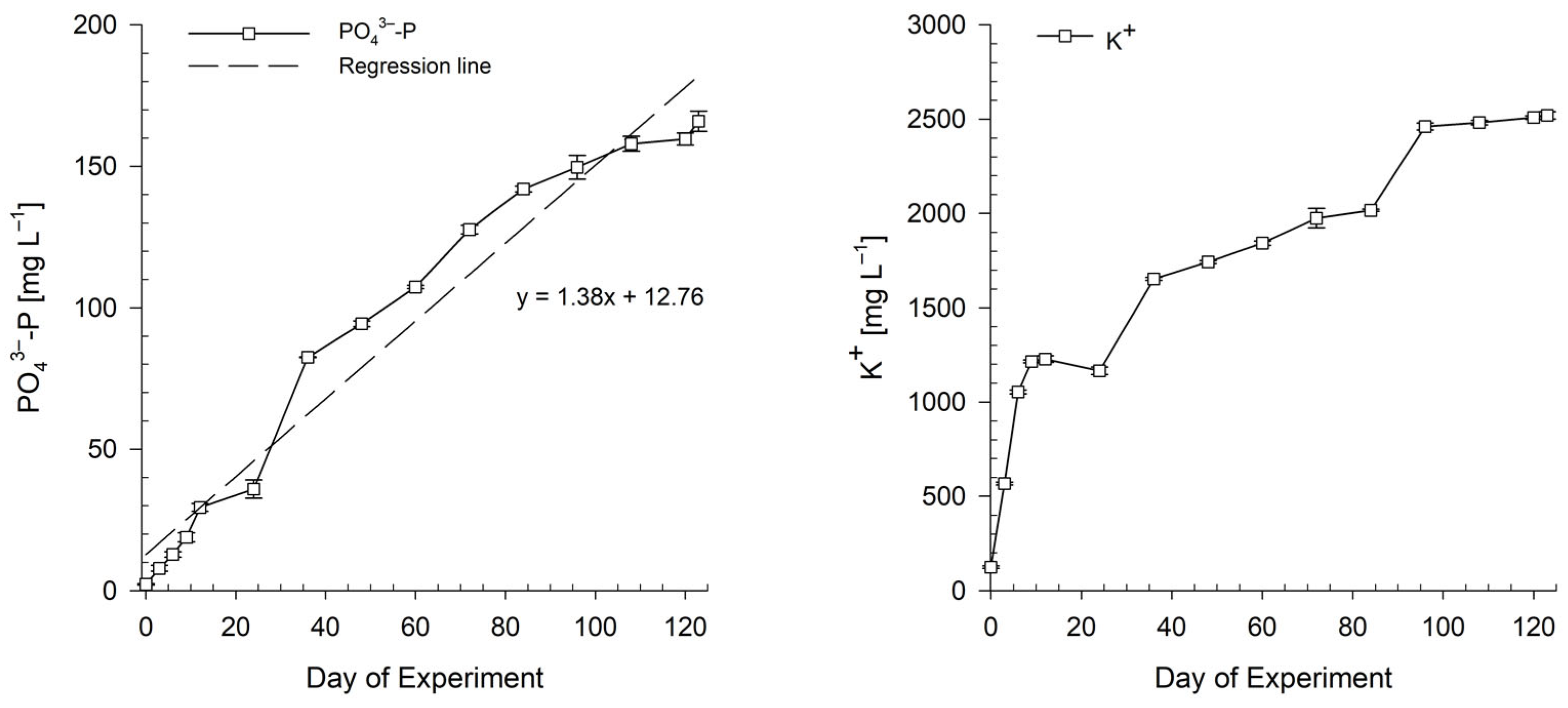
3.1. Preparation of Bioponic Stock Solutions
3.1.1. Mixing Ratio of Bioponic Stock Solutions
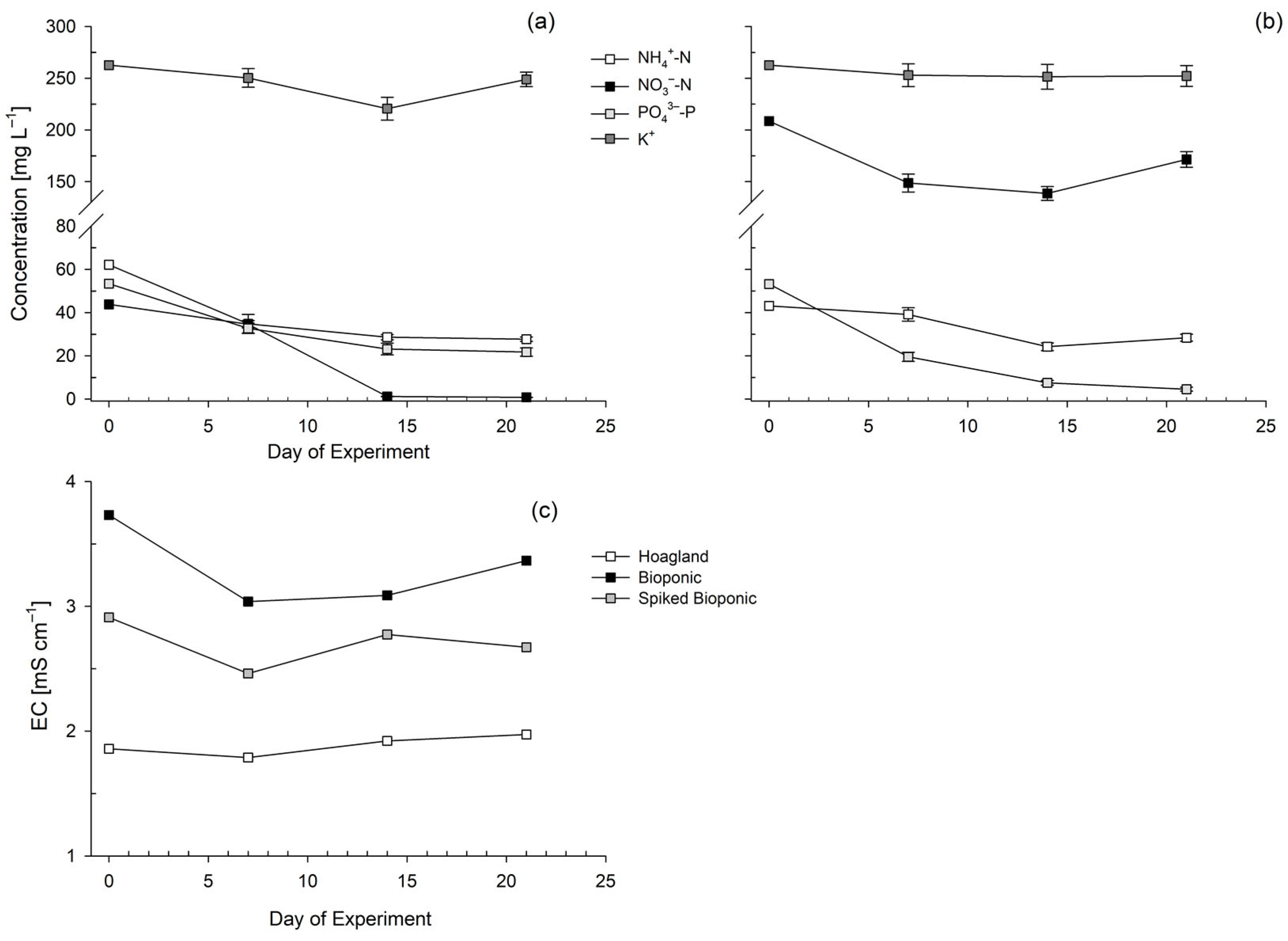
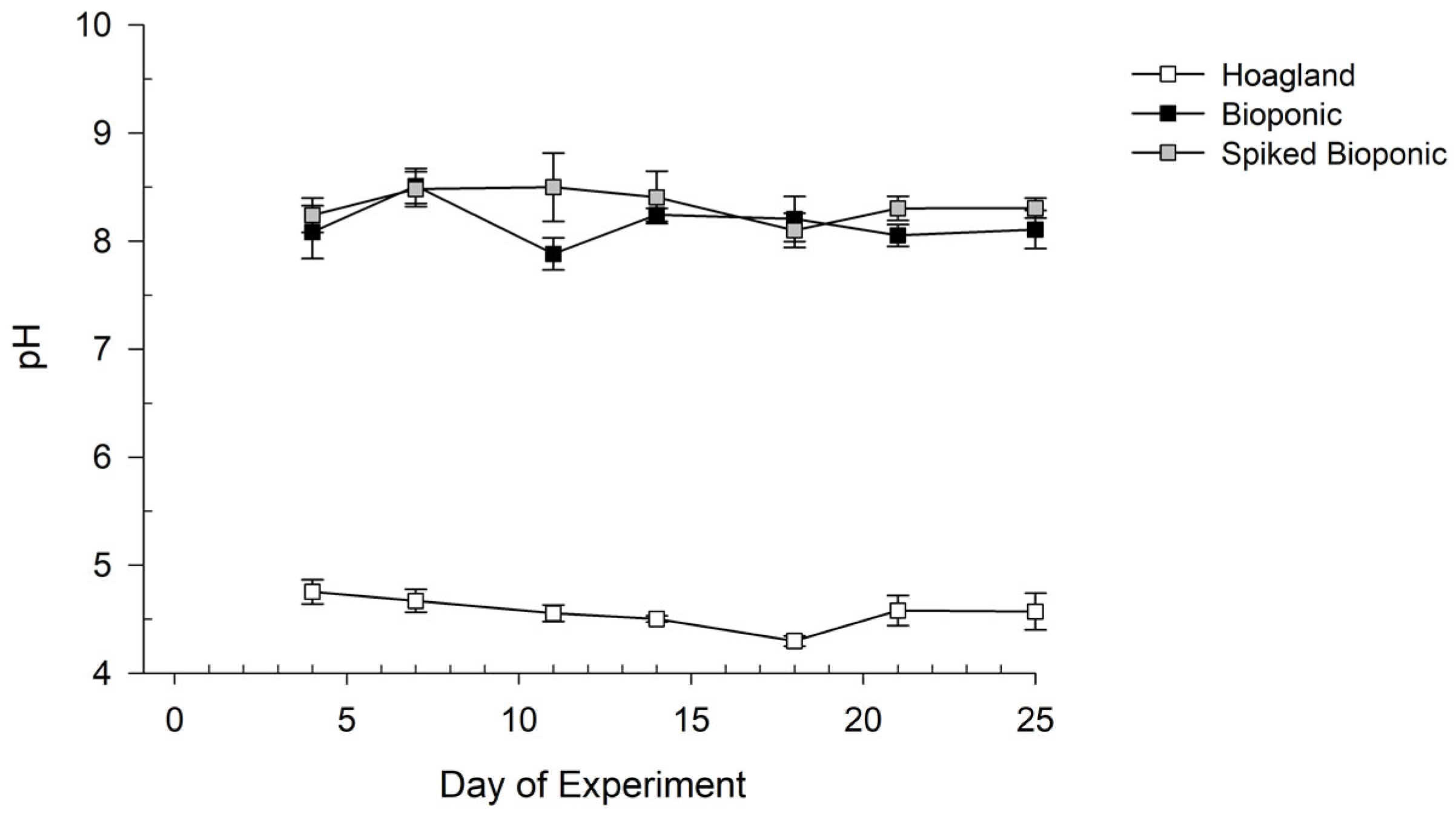
3.1.2. Nutrient Solution Stability
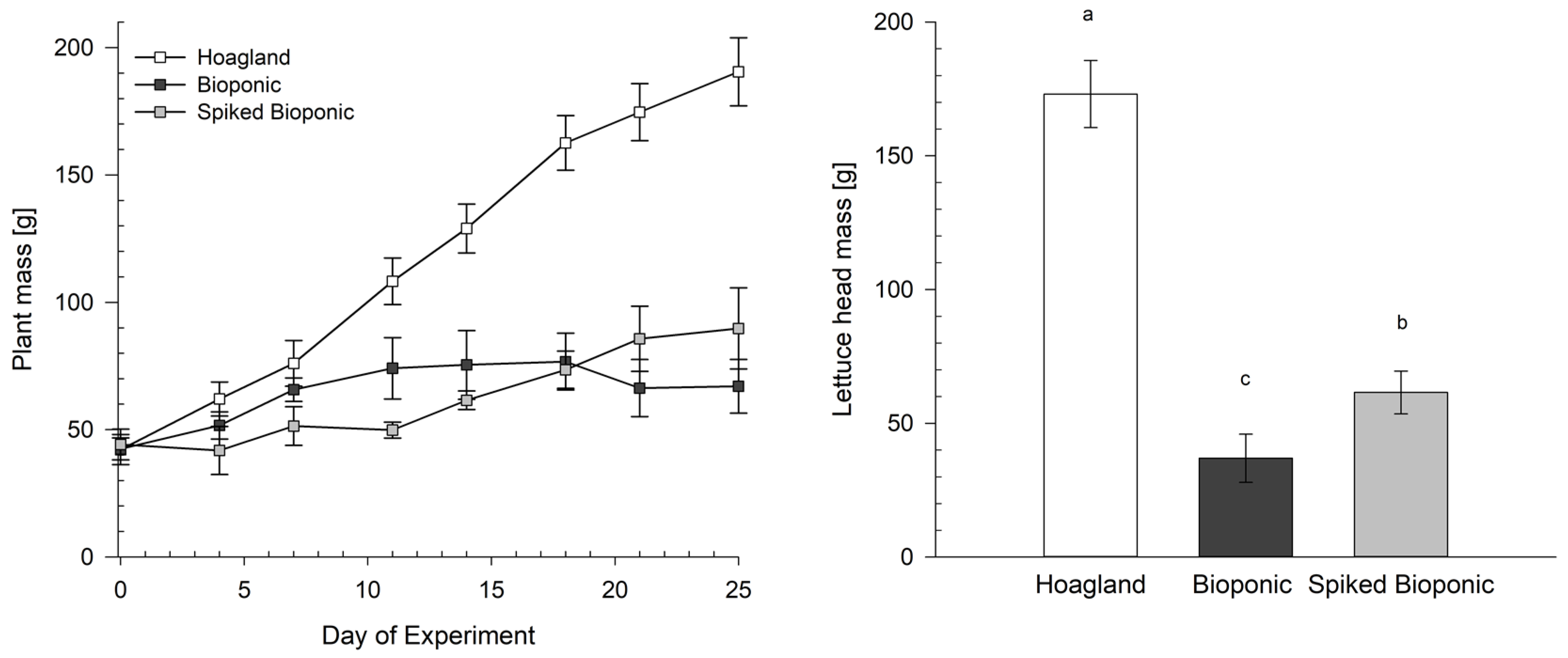
3.2. Testing of the Hoagland, Bioponic and Spiked Bioponic Solutions on Lettuce
Plant Development
4. Discussion
4.1. Bioponic Stock Solution of N, P and K
4.2. Nutrient Composition of the Bioponic and the Spiked Bioponic Solution
4.3. Plant Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tchakerian, V.P. Hydrology, Floods and Droughts|Deserts and Desertification. In Encyclopedia of Atmospheric Sciences; Elsevier: Amsterdam, The Netherlands, 2015; pp. 185–192. ISBN 9780123822253. [Google Scholar]
- Hjort, A. Traditional Land Use in Marginal Drylands. Ecol. Bull. 1976, 24, 43–53. [Google Scholar]
- Safari, J.G.; Kirwa, M.K.; Mandara, C.G. Food insecurity in pastoral communities of Ngorongoro conservation area, Tanzania. Agric. Food Secur. 2022, 11, 36. [Google Scholar] [CrossRef]
- Gebre, A.; Reddy, P.S.; Mulugeta, A.; Sedik, Y.; Kahssay, M. Prevalence of Malnutrition and Associated Factors among Under-Five Children in Pastoral Communities of Afar Regional State, Northeast Ethiopia: A Community-Based Cross-Sectional Study. J. Nutr. Metab. 2019, 2019, 9187609. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, M.; Sayer, J.; Sinclair, F.; Aw-Hassan, A.; Bossio, D.; Craufurd, P.; El Mourid, M.; Haddad, N.; Hoisington, D.; Johnson, N.; et al. An integrated agro-ecosystem and livelihood systems approach for the poor and vulnerable in dry areas. Food Secur. 2013, 5, 751–767. [Google Scholar] [CrossRef]
- Lee, C.; Schaaf, T. The Future of Drylands: International Scientific Conference on Desertification and Drylands Research, Tunis, Tunisia, 19–21 June 2006; Springer: Berlin/Heidelberg, Germany; UNESCO: Dordrecht, The Netherlands; Paris, France, 2008; ISBN 9781402069697. [Google Scholar]
- World Food Programme. WFP Innovation Accelerator-Report 2021; WFP: Rome, Italy, 2021. [Google Scholar]
- Kechasov, D.; Verheul, M.J.; Paponov, M.; Panosyan, A.; Paponov, I.A. Organic Waste-Based Fertilizer in Hydroponics Increases Tomato Fruit Size but Reduces Fruit Quality. Front. Plant Sci. 2021, 12, 680030. [Google Scholar] [CrossRef]
- Krishnasamy, K.; Nair, J.; Bäuml, B. Hydroponic system for the treatment of anaerobic liquid. Water Sci. Technol. 2012, 65, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Liedl, B.E.; Cummins, M.; Young, A.; Williams, M.L. Hydroponic Lettuce Production Using Liquid Effluent from Poultry Waste Bioremediation as a Nutrient Source. Acta Hortic. 2004, 659, 721–728. [Google Scholar] [CrossRef]
- Szekely, I.; Zeaiter, Z.; Jijakli, M.H. Development of a Simple Bioponic Method Using Manure and Offering Comparable Lettuce Yield than Hydroponics. Water 2023, 15, 2335. [Google Scholar] [CrossRef]
- Bergstrand, K.-J.; Asp, H.; Hultberg, M. Utilizing Anaerobic Digestates as Nutrient Solutions in Hydroponic Production Systems. Sustainability 2020, 12, 10076. [Google Scholar] [CrossRef]
- Garland, J.L.; Mackowiak, C.L.; Strayer, R.F.; Finger, B.W. Integration of waste processing and biomass production systems as part of the KSC breadboard project. Adv. Space Res. 1997, 20, 1821–1826. [Google Scholar] [CrossRef]
- Phibunwatthanawong, T.; Riddech, N. Liquid organic fertilizer production for growing vegetables under hydroponic condition. Int. J. Recycl. Org. Waste Agric. 2019, 8, 369–380. [Google Scholar] [CrossRef]
- Mowa, E. Organic manure for vegetable production under hydroponic conditions in arid Namibia. Int. Sci. Technol. J. Namib. 2015, 5, 3–12. [Google Scholar]
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watanabe, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial mineralization of organic nitrogen into nitrate to allow the use of organic fertilizer in hydroponics. Soil Sci. Plant Nutr. 2011, 57, 190–203. [Google Scholar] [CrossRef]
- Wongkiew, S.; Koottatep, T.; Polprasert, C.; Prombutara, P.; Jinsart, W.; Khanal, S.K. Bioponic system for nitrogen and phosphorus recovery from chicken manure: Evaluation of manure loading and microbial communities. Waste Manag. 2021, 125, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Shammas, N.K.; Hung, Y.-T. Biosolids Treatment Processes; Humana Press: Totowa, NJ, USA, 2007; ISBN 9781588293961. [Google Scholar]
- Mupambwa, H.A.; Namwoonde, A.S.; Liswaniso, G.M.; Hausiku, M.K.; Ravindran, B. Biogas digestates are not an effective nutrient solution for hydroponic tomato (Lycopersicon esculentum L.) production under a deep water culture system. Heliyon 2019, 5, e02736. [Google Scholar] [CrossRef] [PubMed]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-15942-9. [Google Scholar]
- Pelayo Lind, O.; Hultberg, M.; Bergstrand, K.-J.; Larsson-Jönsson, H.; Caspersen, S.; Asp, H. Biogas Digestate in Vegetable Hydroponic Production: pH Dynamics and pH Management by Controlled Nitrification. Waste Biomass Valorization 2020, 12, 123–133. [Google Scholar] [CrossRef]
- Gartmann, F.; Hügly, J.; Krähenbühl, N.; Brinkmann, N.; Schmautz, Z.; Smits, T.H.M.; Junge, R. Bioponics—An Organic Closed-Loop Soilless Cultivation System: Yields and Characteristics Compared to Hydroponics and Soil Cultivation. Agronomy 2023, 13, 1436. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Wu, S.; Feng, X.; Wittmeier, A. Microwave Digestion of Plant and Grain Reference Materials in Nitric Acid or a Mixture of Nitric Acid and Hydrogen Peroxide for the Determination of Multi-elements by Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom. 1997, 12, 797–806. [Google Scholar] [CrossRef]
- Matsushita, N.; Matoh, I. Characterization of Na+ exclusion mechanisms of salt-tolerant reed plants in comparison with salt-sensitive rice plants. Physiol. Plant. 1991, 83, 170–176. [Google Scholar] [CrossRef]
- Abfallwirtschaftsbetrieb Esslingen, Deutschland, Kompostwerk Kirchheim u.T. GmbH. RAL-Gütesicherung Kompost Anlage Kirchheim, Jahreszeugnis 2022. Available online: https://www.awb-es.de/medias/Jahreserzeugnis-G%C3%BCtekompost-2022_71846719ed57a63d2a427515e0a91087.pdf (accessed on 8 April 2024).
- Ødegaard, H.; Rusten, B.; Westrum, T. A new moving bed biofilm reactor-applications and results. Water Sci. Technol. 1994, 29, 157–165. [Google Scholar] [CrossRef]
- Epple, M.; Enax, J. Moderne Zahnpflege aus chemischer Sicht. Chem. Nserer Zeit 2018, 52, 218–228. [Google Scholar] [CrossRef]
- Schaad, P.; Poumier, F.; Voegel, J.C.; Gramain, P. Analysis of calcium hydroxyapatite dissolution in non-stoichiometric solutions. Physicochem. Eng. Asp. 1997, 121, 217–228. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates, Inc.: Sunderland, UK, 2006. [Google Scholar]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower, 7th ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781439878699. [Google Scholar]
- Savvas, D.; Passam, H.C.; Olympios, C.; Nasi, E.; Moustaka, E.; Mantzos, N.; Barouchas, P. Effects of Ammonium Nitrogen on Lettuce Grown on Pumice in a Closed Hydroponic System. HortScience 2006, 41, 1667–1673. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Makaka, G.; Simon, M.; Okoh, A.I. An Overview of the Control of Bacterial Pathogens in Cattle Manure. Int. J. Environ. Res. Public Health 2016, 13, 843. [Google Scholar] [CrossRef] [PubMed]
- Narihiro, T.; Sekiguchi, Y. Microbial communities in anaerobic digestion processes for waste and wastewater treatment: A microbiological update. Curr. Opin. Biotechnol. 2007, 18, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen mineralization from organic amendments is variable but predictable. J. Environ. Qual. 2020, 49, 483–495. [Google Scholar] [CrossRef]
- Calderón, F.J.; McCarty, G.W.; Reeves, J.B. Analysis of manure and soil nitrogen mineralization during incubation. Biol. Fertil. Soils 2005, 41, 328–336. [Google Scholar] [CrossRef]
- Gale, E.S.; Sullivan, D.M.; Cogger, C.G.; Bary, A.I.; Hemphill, D.D.; Myhre, E.A. Estimating plant-available nitrogen release from manures, composts, and specialty products. J. Environ. Qual. 2006, 35, 2321–2332. [Google Scholar] [CrossRef]
- Roberts, H.; Palmeiro, B.S. Toxicology of aquarium fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2008, 11, 359–374. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Ako, P.A.E.; Adebanjo, A.S.; Fadipe, A.L.; Ndamitso, M.M. Extractability of Potassium from Some Organic Manures in Aqueous Medium and the Effect of pH, Time and Concentration. J. Appl. Sci. Environ. Manag. 2003, 7, 51–56. [Google Scholar] [CrossRef]
- Stefanakis, A.; Akratos, C.S.; Tsihrintzis, V.A. Vertical Flow Constructed Wetlands: Eco-Engineering Systems for Wastewater and Sludge Treatment, 1st ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2014; ISBN 9780124046122. [Google Scholar]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Barker, A.V. Ammonium accumulation and ethylene evolution by tomato infected with root-knot nematode and grown under different regimes of plant nutrition. Commun. Soil Sci. Plant Anal. 1999, 30, 175–182. [Google Scholar] [CrossRef]
- Barker, A.V. Foliar ammonium accumulation as an index of stress in plants. Commun. Soil Sci. Plant Anal. 1999, 30, 167–174. [Google Scholar] [CrossRef]
- van Beusichem, M.L.; Kirkby, E.A.; Baas, R. Influence of Nitrate and Ammonium Nutrition on the Uptake, Assimilation, and Distribution of Nutrients in Ricinus communis. Plant Physiol. 1988, 86, 914–921. [Google Scholar] [CrossRef]
- Claussen, W.; Lenz, F. Effect of ammonium or nitrate nutrition on net photosynthesis, growth, and activity of the enzymes nitrate reductase and glutamine synthetase in blueberry, raspberry and strawberry. Plant Soil 1999, 208, 95–102. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, C.L.; Garland, J.L.; Strayer, R.F.; Finger, B.W.; Wheeler, R.M. Comparison of aerobically treated and untreated crop residue as a source odf recycled nutrients in a recirculating hydroponic system. Adv. Space Res. 1996, 18, 281–287. [Google Scholar] [CrossRef]
- Biesalski, H.K. Hidden Hunger; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-33949-3. [Google Scholar]
- Breś, W.; Kleiber, T.; Markiewicz, B.; Mieloszyk, E.; Mieloch, M. The Effect of NaCl Stress on the Response of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 244. [Google Scholar] [CrossRef]
- Gunes, A.; Alpaslan, M.; Inal, A. Critical nutrient concentrations and antagonistic and synergistic relationships among the nutrients of NFT-grown young tomato plants. J. Plant Nutr. 1998, 21, 2035–2047. [Google Scholar] [CrossRef]
- Williams, K.A.; Nelson, J.S. Challenges of using organic fertilizers in hydroponic production systems. Acta Hortic. 2016, 365–370. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into biological apatite: Physiochemical properties and preparation approaches. BioMed Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef] [PubMed]
- Payus, C.; David, O.; Yan, M.P. Bone Meal As Alterantive Treatment For Acidic And Metal Contaminated Acid Mine Drainage Water Effluent: Lab Scale. Am. J. Environ. Sci. 2014, 10, 61–73. [Google Scholar] [CrossRef]
- Atkin, K.; Nichols, M.A. Organic Hydroponics. Acta Hortic. 2004, 648, 121–127. [Google Scholar] [CrossRef]
- Lam, V.P.; Kim, S.J.; Park, J.S. Optimizing the Electrical Conductivity of a Nutrient Solution for Plant Growth and Bioactive Compounds of Agastache rugosa in a Plant Factory. Agronomy 2020, 10, 76. [Google Scholar] [CrossRef]
- Sutton, J.C.; Sopher, C.R.; Owen-Going, T.N.; Liu, W.; Grodzinski, B.; Hall, J.C.; Benchimol, R.L. Etiology and epidemiology of Pythium root rot in hydroponic crops: Current knowledge and perspectives. Summa Phytopathol. 2006, 32, 307–321. [Google Scholar] [CrossRef]
- Hartz, T.K.; Johnstone, P.R.; Williams, E.; Smith, R.F. Establishing Lettuce Leaf Nutrient Optimum Ranges Through DRIS Analysis. HortScience 2007, 42, 143–146. [Google Scholar] [CrossRef]


| Organic Residue | N | P | K | DM |
|---|---|---|---|---|
| [% DM] | [% FM] | |||
| Blood meal | 15.5 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.1 | 92.1 ± 0.2 |
| Bone meal | 7.0 ± 1.3 | 20.1 ± 0.3 | 0.0 ± 0.0 | 95.2 ± 0.1 |
| Potato peel | / | / | 2.4 ± 0.0 | 13.2 ± 0.2 |
| Macronutrients | Micronutrients | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References | NH4+-N | NO3−-N | PO43−-P | K | Ca | Mg | S | B | Fe | Zn | Cu | Mn | Mo | |
| mg L−1 | mg L−1 | |||||||||||||
| Jones and Shive (1921) [31] | 39 | 204 | 65 | 102 | 292 | 172 | 227 | / | 0.80 | / | / | / | / | |
| Hoagland and Arnon (1938) [32] | 14 | 196 | 31 | 234 | 160 | 48 | 64 | 0.50 | 0.60 | 0.05 | 0.02 | 0.50 | 0.01 | |
| Withrow (1948) [33] | A | 28 | 70 | 63 | 390 | 200 | 96 | 607 | 0.50 | 2.00 | 0.05 | 0.02 | 0.30 | / |
| B | 28 | 140 | 63 | 390 | 200 | 96 | 447 | 0.50 | 1.00 | 0.05 | 0.02 | 0.30 | / | |
| C | 14 | 224 | 63 | 390 | 120 | 96 | 64 | 0.50 | 1.00 | 0.05 | 0.02 | 0.30 | / | |
| Schwarz (1968) [34] | A | 15 | 196 | 31 | 234 | 160 | 48 | 64 | / | / | / | / | / | / |
| B | 20 | 126 | 71 | 90 | 180 | 55 | 96 | / | / | / | / | / | / | |
| C | 33 | 93 | 37 | 209 | 131 | 22 | 30 | 0.46 | 1.70 | 0.09 | 0.04 | 0.80 | 0.03 | |
| D | 33 | 135 | 37 | 209 | 146 | 22 | 30 | 0.46 | 1.70 | 0.09 | 0.04 | 0.80 | 0.03 | |
| E | 33 | 177 | 37 | 209 | 146 | 22 | 30 | 0.46 | 1.70 | 0.09 | 0.04 | 0.80 | 0.03 | |
| Range | 14–39 | 70–224 | 31–71 | 90–390 | 120–292 | 22–172 | 30–607 | 0.46–0.50 | 0.60–2.00 | 0.05–0.09 | 0.02–0.04 | 0.30–0.80 | 0.01–0.03 | |
| Means | 26 | 156 | 50 | 246 | 174 | 68 | 166 | 0.48 | 1.31 | 0.07 | 0.03 | 0.54 | 0.03 | |
| Nutrient | BM Digestate | NO3− Solution | P Solution | K Solution |
|---|---|---|---|---|
| [mg L−1] | ||||
| NH4+-N | 1155.9 ± 3.3 | 30.3 ± 0.4 | 105.9 ± 1.4 | 97.4 ± 2.2 |
| NO3−-N | 0.9 ± 0.1 | 68.9 ± 1.3 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| PO43−-P | 2.5 ± 0.0 | 0.0 ± 0.0 | 166.0 ± 2.9 | 8.6 ± 0.2 |
| K | 86.4 ± 0.0 | 47.9 ± 0.0 | 36.0 ± 3.1 | 2520.0 ± 20.4 |
| Ca | 180.5 ± 0.5 | 77.2 ± 0.2 | 429.5 ± 0.5 | 27.3 ± 1.9 |
| Mg | 81.1 ± 0.1 | 11.9 ± 0.2 | 15.4 ± 0.0 | 130.5 ± 5.5 |
| S | 7.4 ± 0.3 | 3.9 ± 0.2 | 3.7 ± 0.0 | 82.8 ± 4.7 |
| B | 0.50 ± 0.0 | 0.41 ± 0.0 | 0.48 ± 0.0 | 1.29 ± 0.2 |
| Fe | 4.94 ± 0.2 | 0.52 ± 0.0 | 4.05 ± 0.0 | 62.80 ± 2.7 |
| Zn | 0.90 ± 0.0 | 0.79 ± 0.0 | 0.87 ± 0.0 | 2.10 ± 0.1 |
| Cu | 0.00 ± 0.0 | 0.12 ± 0.0 | 0.01 ± 0.0 | 0.01 ± 0.0 |
| Mn | 0.30 ± 0.0 | 0.78 ± 0.0 | 0.24 ± 0.0 | 1.56 ± 0.0 |
| Mo | 0.01 ± 0.0 | 0.01 ± 0.0 | 0.00 ± 0.0 | 0.10 ± 0.0 |
| Na | 25.30 ± 1.2 | 21.70 ± 0.5 | 19.70 ± 0.9 | 11.00 ± 2.8 |
| Nutrients | NO3−-Solution | P-Solution | K-Solution | |||
|---|---|---|---|---|---|---|
| mg L−1 | Δ | mg L−1 | Δ | mg L−1 | Δ | |
| NH4+-N | 2.7 ± 0.0 | −91.1% | 75.8 ± 1.1 | −28.4% | 101.5 ± 2.7 | 4.2% |
| NO3−-N | 7.4 ± 0.1 | −89.3% | 0.2 ± 0.0 | −33.3% | 0.3 ± 0.0 | 0.0% |
| PO43−-P | 0.1 ± 0.0 | 0.0% | 193.6 ± 2.5 | 16.6% | 6.4 ± 0.1 | −25.6% |
| K | 37.6 ± 0.1 | −21.5% | 39.8 ± 1.7 | 10.6% | 2507.8 ± 27.8 | −0.5% |
| Element | Hoagland | Bioponic | Spiked Bioponic |
|---|---|---|---|
| [g kg−1] | |||
| C | 400.2 ± 4.3b | 420.2 ± 2.6a | 409.8 ± 4.4ab |
| N | 49.5 ± 1.2a | 33.9 ± 5.0b | 40.4 ± 7.6ab |
| P | 8.3 ± 0.7a | 4.9 ± 0.7ab | 4.4 ± 0.5b |
| K | 49.8 ± 4.1a | 44.4 ± 2.2a | 55.0 ± 10.5a |
| Ca | 13.2 ± 1.4a | 8.5 ± 1.4a | 12.6 ± 1.5a |
| Mg | 2.9 ± 0.1a | 1.7 ± 0.1b | 1.8 ± 0.3ab |
| S | 2.7 ± 0.1a | 1.8 ± 0.4a | 1.8 ± 0.2a |
| [mg kg−1] | |||
| B | 27.8 ± 3.0a | 22.1 ± 2.8a | 28.1 ± 4.9a |
| Fe | 80.3 ± 9.6a | 83.2 ± 34.6ab | 57.3 ± 5.6b |
| Zn | 33.6 ± 5.7a | 22.9 ± 4.7ab | 18.4 ± 0.8b |
| Cu | 4.5 ± 0.6a | 5.7 ± 1.8a | 4.2 ± 0.8a |
| Mn | 21.2 ± 1.0a | 22.7 ± 6.5a | 16.8 ± 4.9a |
| Mo | 10.9 ± 1.7a | 7.6 ± 0.4ab | 7.4 ± 1.1b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heintze, S.; Beckett, M.; Kriem, L.S.; Germer, J.; Asch, F. A Low-Tech Approach to Mobilize Nutrients from Organic Residues to Produce Bioponic Stock Solutions. Agriculture 2024, 14, 928. https://doi.org/10.3390/agriculture14060928
Heintze S, Beckett M, Kriem LS, Germer J, Asch F. A Low-Tech Approach to Mobilize Nutrients from Organic Residues to Produce Bioponic Stock Solutions. Agriculture. 2024; 14(6):928. https://doi.org/10.3390/agriculture14060928
Chicago/Turabian StyleHeintze, Sebastian, Marc Beckett, Lukas Simon Kriem, Jörn Germer, and Folkard Asch. 2024. "A Low-Tech Approach to Mobilize Nutrients from Organic Residues to Produce Bioponic Stock Solutions" Agriculture 14, no. 6: 928. https://doi.org/10.3390/agriculture14060928





