Dietary Wheat Gluten Alters the Gut Microbiome and Plasma Taurine Levels in European Sea Bass (Dicentrarchus labrax)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish System Maintenance and Care
2.2. Diet Preparation
2.3. Blood and Tissue Sampling and Analysis
2.4. Plasma Analysis
2.5. Plasma Taurine Analysis by HPLC
2.6. Gliadin SDS-PAGE Electrophoresis and Immunoblotting
2.7. IgM and IgT Immunoblotting
2.8. Microbiome Analysis
2.8.1. DNA Extraction
2.8.2. PCR and Agarose Gel Electrophoresis
2.8.3. Sequencing
2.9. Statistics
3. Results
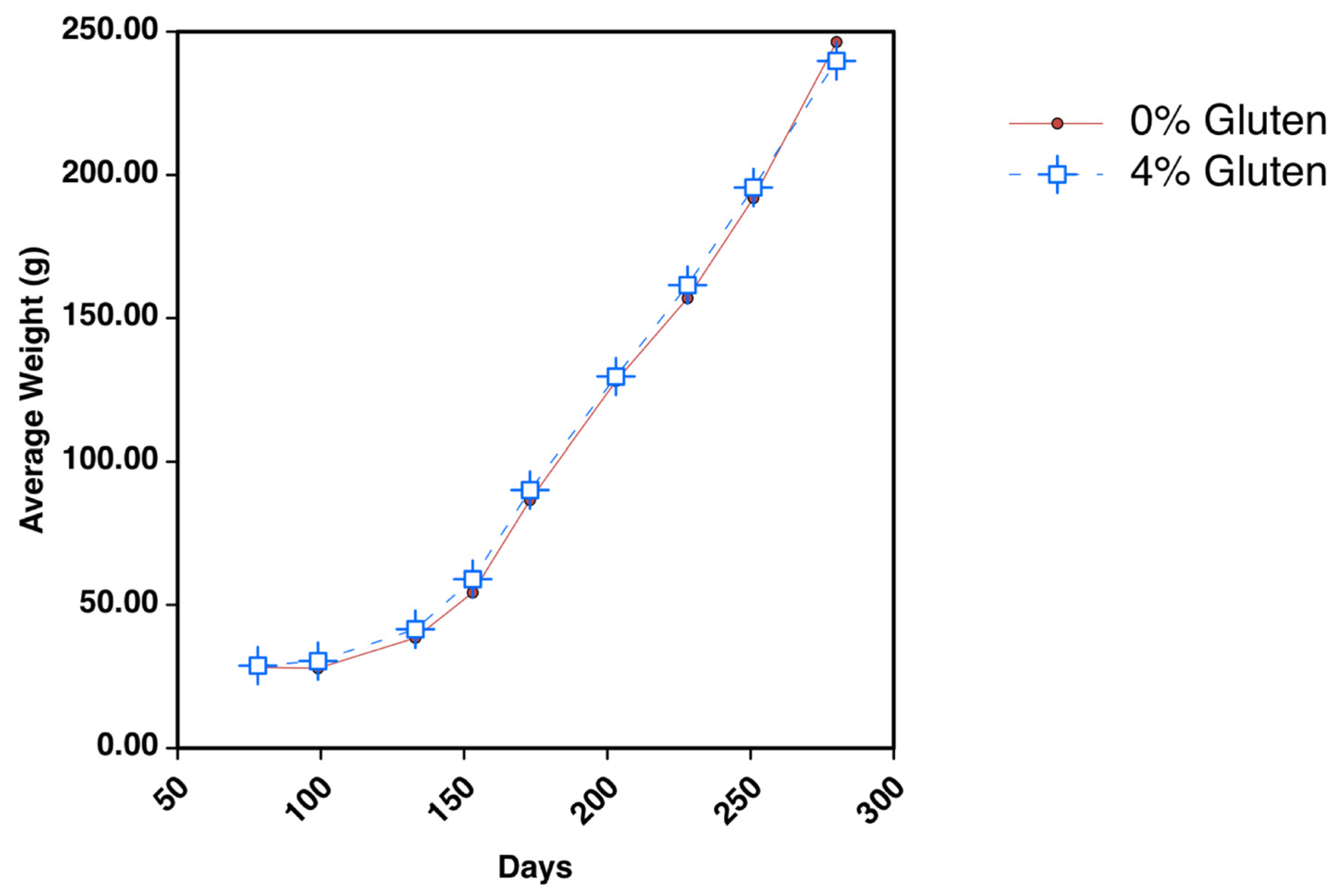
3.1. Growth Data
3.2. Plasma Analysis
3.3. Body and Tissue Weights
3.4. Plasma Taurine Analysis
3.5. Gliadin Immunoblotting
3.6. IgT and IgM
3.7. Intestinal Microbiome Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, R.L.; Hasan, M.R. A limited supply of fishmeal: Impact on future increases in global aquaculture production. Trends Food Sci. Technol. 2012, 27, 120–128. [Google Scholar] [CrossRef]
- Rust, M.B.; Barrows, F.T.; Hardy, R.W.; Lazur, A.; Naughten, K.; Silverstein, J. The Future of Aquafeeds. In NOAA Tech.; 2011 Memorandum NMFS F/SPO-124. Available online: https://repository.library.noaa.gov/view/noaa/4300 (accessed on 8 May 2023).
- Hardy, R.W. New developments in aquatic feed ingredients, and potential of enzyme supplements. In Proceedings of the Avances en Nutrition Acuicola V. Memorias del V Simposium Internacional de Nutricion Acuicola, Mérida, Mexico, 19–22 November 2000; pp. 216–226. [Google Scholar]
- Apper-Bossard, E.; Feneuil, A.; Wagner, A.; Respondek, F. Use of vital wheat gluten in aquaculture feeds. Aquat. Biosyst. 2013, 9, 21. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Gujral, N.; Freeman, H.J.; Thomson, A.B.R. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M. Coeliac disease: Dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2002, 2, 647–655. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Corazza, G.R. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Bagni, M. Cultured Aquatic Species Information Programme. Dicentrarchus Labrax; FAO Fisheries and Aquaculture Department: Rome, Italy; Available online: https://www.fao.org/fishery/en/culturedspecies/dicentrarchus_labrax/en (accessed on 8 May 2023).
- Kaushik, S.; Covès, D.; Dutto, G.; Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Geay, F.; Ferraresso, S.; Zambonino-Infante, J.L.; Bargelloni, L.; Quentel, C.; Vandeputte, M.; Kaushik, S.; Cahu, C.L.; Mazurais, D. Effects of the total replacement of fish-based diet with plant-based diet on the hepatic transcriptome of two European sea bass (Dicentrarchus labrax) half-sibfamilies showing different growth rates with the plant-based diet. BMC Genom. 2011, 12, 522. [Google Scholar] [CrossRef]
- Danilova, N.; Bussmann, J.; Jekosch, K.; A Steiner, L. The immunoglobulin heavy-chain locus in zebrafish: Identification and expression of a previously unknown isotype, immunoglobulin Z. Nat. Immunol. 2005, 6, 295–302. [Google Scholar] [CrossRef]
- Hansen, J.D.; Landis, E.D.; Phillips, R.B. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc. Natl. Acad. Sci. USA 2005, 102, 6919–6924. [Google Scholar] [CrossRef]
- Xu, Z.; Parra, D.; Gómez, D.; Salinas, I.; Zhang, Y.-A.; Von Gersdorff Jørgensen, L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Oriol Sunyer, J. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef] [PubMed]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Watson, A.M.; Kissil, G.W.; Barrows, F.T.; Place, A.R. Developing a plant-based diet for cobia (Rachycentron canadum). Int. Aquafeed. 2012, 15, 34–38. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- R Development Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates. Biophys. Rev. 2017, 10, 435–443. [Google Scholar] [CrossRef]
- Picchietti, S.; Nuñez-Ortiz, N.; Stocchi, V.; Randelli, E.; Buonocore, F.; Guerra, L.; Scapigliati, G. Evolution of lymphocytes. Immunoglobulin T of the teleost sea bass (Dicentrarchus labrax): Quantitation of gene expressing and immunoreactive cells. Fish Shellfish. Immunol. 2017, 63, 40–52. [Google Scholar] [CrossRef]
- Roque, A.; Yildiz, H.Y.; Carazo, I.; Duncan, N. Physiological stress responses of sea bass (Dicentrarchus labrax) to hydrogen peroxide (H2O2) exposure. Aquaculture 2010, 304, 104–107. [Google Scholar] [CrossRef]
- Al-Khashal, M.S.; Al-Shawi, S. Effect of Salt Stress on ALT and AST Enzymes Activity and Cortisol Level in Adults of Carassius auratus. Pak. J. Nutr. 2012, 12, 97–100. [Google Scholar] [CrossRef]
- Peres, H.; Santos, S.; Oliva-Teles, A. Blood chemistry profile as indicator of nutritional status in European seabass (Dicentrarchus labrax). Fish Physiol. Biochem. 2014, 40, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Piccolo, G.; Tulli, F.; Cardinaletti, G.; Tibaldi, E. Lipid composition and metabolism of European sea bass (Dicentrarchus labrax L.) fed diets containing wheat gluten and legume meals as substitutes for fish meal. Aquaculture 2013, 376–379, 6–14. [Google Scholar] [CrossRef]
- Castro, C.; Corraze, G.; Perez-Jimenez, A.; Larroquet, L.; Cluzeaud, M.; Panserat, S.; Oliva-Teles, A. Dietary carbohydrate and lipid source affect cholesterol metabolism of European sea bass (Dicentrarchus labrax) juveniles. Br. J. Nutr. 2015, 114, 1143–1156. [Google Scholar]
- Gómez-Requeni, P.; Mingarro, M.; Calduch-Giner, J.A.; Médale, F.; Martin, S.A.M.; Houlihan, D.F.; Kaushik, S.; Pérez-Sánchez, J. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture 2004, 232, 493–510. [Google Scholar] [CrossRef]
- Schmidt, V.; Amaral-Zettler, L.; Davidson, J.; Summerfelt, S.; Good, C. Influence of Fishmeal-Free Diets on Microbial Communities in Atlantic Salmon (Salmo salar) Recirculation Aquaculture Systems. Appl. Environ. Microbiol. 2016, 82, 4470–4481. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M. A Broken Heart: Risk of Heart Disease in Boutique or Grain-Free Diets and Exotic Ingredients. Tufts University Cummings School of Veterinary Medicine. Available online: https://vetnutrition.tufts.edu/2018/06/a-broken-heart-risk-of-heart-disease-in-boutique-or-grain-free-diets-and-exotic-ingredients (accessed on 25 March 2023).
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef]
- Nylund, L.; Kaukinen, K.; Lindfors, K. The microbiota as a component of the celiac disease and non-celiac gluten sensitivity. Clin. Nutr. Exp. 2016, 6, 17–24. [Google Scholar] [CrossRef]
- Koo, H.; Hakim, J.A.; Powell, M.L.; Kumar, R.; Eipers, P.G.; Morrow, C.D.; Crowley, M.; Lefkowitz, E.J.; Watts, S.A.; Bej, A.K. Metagenomics approach to the study of the gut microbiome structure and function in zebrafish Danio rerio fed with gluten formulated diet. J. Microbiol. Methods 2017, 135, 69–76. [Google Scholar] [CrossRef]
- Savarese, M.; Tousignant, K.D.; Uno, J. The effect of wheat products on the colonization of the microbiota in the intestines of zebrafish (903.2). FASEB J. 2014, 28, 903.2. [Google Scholar]
- Gajardo, K.; Jaramillo-Torres, A.; Kortner, T.M.; Merrifield, D.L.; Tinsley, J.; Bakke, A.M.; Krogdahl, Å. Alternative Protein Sources in the Diet Modulate Microbiota and Functionality in the Distal Intestine of Atlantic Salmon (Salmo salar). Appl. Environ. Microbiol. 2017, 83, e02615-16. [Google Scholar] [CrossRef] [PubMed]






| Ingredient (g kg−1) | 0% Wheat Gluten | 4% Wheat Gluten |
|---|---|---|
| Profine VF | 28.75 | 26.75 |
| Soybean meal, 47.5% | 23.33 | 23.33 |
| Wheat flour, bagged | 15.04 | 15.04 |
| Corn gluten, 60% | 15.34 | 13.34 |
| Menhaden gold oil, top-dressed | 5.96 | 5.96 |
| Monocalcium phosphate FG | 3.95 | 3.95 |
| Lecithin FG | 3 | 3 |
| L-Lysine, 98.5% | 0.75 | 0.75 |
| Choline chloride, 70% | 0.6 | 0.6 |
| Potassium chloride (DYNA K) FG | 0.56 | 0.56 |
| DL-Methionine, 99% | 0.45 | 0.45 |
| Sodium chloride | 0.28 | 0.28 |
| Tiger C-35 | 0.2 | 0.2 |
| Premix AquaVit | 0.12 | 0.12 |
| Premix Aquamin Fish | 0.12 | 0.12 |
| Magnesium oxide FG | 0.05 | 0.05 |
| Taurine FG, 98.5% | 1.5 | 1.5 |
| Wheat gluten | 0 | 4 |
| Proximate Composition 1 | 0% Wheat Gluten | 4% Wheat Gluten |
|---|---|---|
| Protein (% DM) | 45.02 | 44.94 |
| Lipid (% DM) | 6.19 | 6.02 |
| Fiber (% DM) | 1.95 | 2.2 |
| Moisture (%) | 6.97 | 9.4 |
| Ash (% DM) | 7.75 | 7.58 |
| Taurine (%) | 1.48 | 1.54 |
| Plasma Component | 0% Wheat Gluten | 4% Wheat Gluten |
|---|---|---|
| Glucose (mg/dL) | 120.727 ± 27.335 | 113.874 ± 32.011 |
| Calcium (mg/dL) | * 11.045 ± 0.465 | * 11.75 ± 0.567 |
| Magnesium (mg/dL) | 3.545 ± 0.398 | 3.7 ± 0.25 |
| Phosphorus (mg/dL) | 7.909 ± 0.76 | 8.125 ± 0.784 |
| Triglycerides (mg/dL) | 410.364 ± 77.747 | 388 ± 129.836 |
| Cholesterol (mg/dL) | 199.545 ± 33.074 | 206.75 ± 43.702 |
| ALP (U/L) | 24.091 ± 1.486 | 25.125 ± 1.511 |
| AST (U/L) | * 47.8 ± 28.558 | * 35.25 ± 17.484 |
| Osmolality (mOsmol/kg) | 363 ± 5.773 | 368.917 ± 6.851 |
| Total Protein (g/dL) | 4.291 ± 0.241 | 4 ± 0.525 |
| Weight (g) | 0% Wheat Gluten | 4% Wheat Gluten |
|---|---|---|
| Whole body | 312.142 ± 68.727 | 311.667 ± 71.247 |
| Pyloric caeca | 0.818 ± 0.402 | 0.805 ± 0.266 |
| Anterior intestine | 0.875 ± 0.194 | 1.03 ± 0.235 |
| Middle intestine | * 0.576 ± 0.13 | * 0.932 ± 0.335 |
| Posterior intestine | 0.621 ± 0.16 | 0.753 ± 0.238 |
| Total intestine | 2.89 ± 0.605 | 3.52 ± 0.918 |
| Liver | 3.625 ± 1.188 | 3.703 ± 1.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larkin, M.E.M.; Place, A.R. Dietary Wheat Gluten Alters the Gut Microbiome and Plasma Taurine Levels in European Sea Bass (Dicentrarchus labrax). J. Mar. Sci. Eng. 2023, 11, 1022. https://doi.org/10.3390/jmse11051022
Larkin MEM, Place AR. Dietary Wheat Gluten Alters the Gut Microbiome and Plasma Taurine Levels in European Sea Bass (Dicentrarchus labrax). Journal of Marine Science and Engineering. 2023; 11(5):1022. https://doi.org/10.3390/jmse11051022
Chicago/Turabian StyleLarkin, Mary E. M., and Allen R. Place. 2023. "Dietary Wheat Gluten Alters the Gut Microbiome and Plasma Taurine Levels in European Sea Bass (Dicentrarchus labrax)" Journal of Marine Science and Engineering 11, no. 5: 1022. https://doi.org/10.3390/jmse11051022
APA StyleLarkin, M. E. M., & Place, A. R. (2023). Dietary Wheat Gluten Alters the Gut Microbiome and Plasma Taurine Levels in European Sea Bass (Dicentrarchus labrax). Journal of Marine Science and Engineering, 11(5), 1022. https://doi.org/10.3390/jmse11051022






