Development of a Site-Specific Kinetic Model for Chlorine Decay and the Formation of Chlorination By-Products in Seawater
Abstract
:1. Introduction
1.1. Chlorination

1.2. Model Development
2. Purposes of This Article
3. Materials and Methods
3.1. Materials and Analysis: Water Chemistry
3.2. Chlorine Measurement
3.3. Determination of Decay Rate
3.4. Determination of CBP Formation Rate
3.5. Time Series Data Management and Error Checking
4. Results and Discussion
- (1)
- Chlorine is subject to both a rapid first order decay and a slower first order decay;
- (2)
- The pH, salinity and TOC/DOC content of the Arabian Gulf waters remain nearly constant for all samples: pH (8 ± 0.2), salinity (40–43 ppt) and TOC/DOC (3 ± 0.5 mg/L).
4.1. Chlorine Decay Model
4.2. CBP Formation Model
4.3. Curve Fitting
4.4. Chlorine Decay and CBPs Formation with Different Initial Chlorine Dosing Levels

4.5. Chlorine Decay and CBP Formation at Different Temperatures

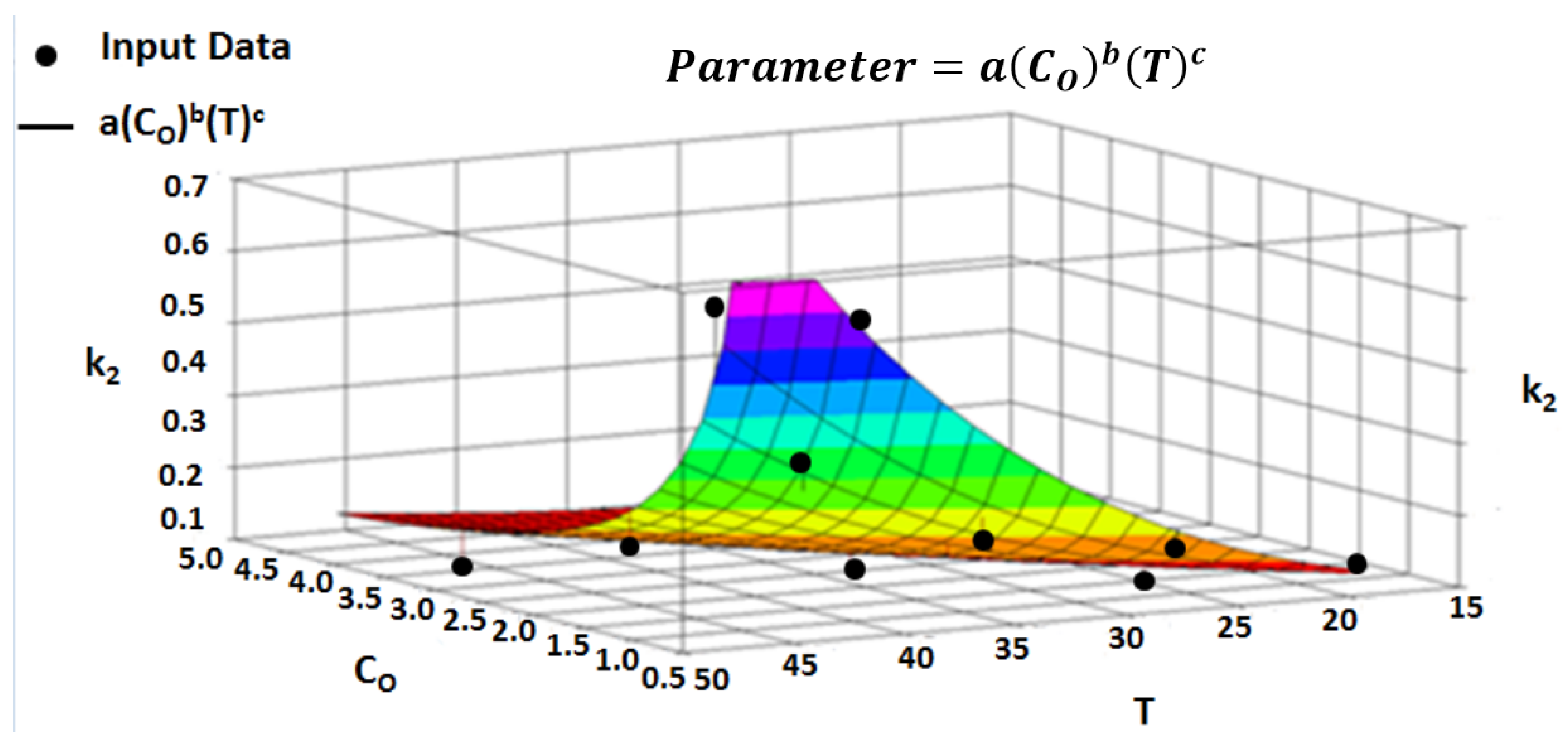
4.6. Multivariate Dependencies
4.7. Multivariate Parameter Equation
4.8. Multivariate Regression


| Parameter | |||
|---|---|---|---|
| 1.550 × 101 | −2.737 × 10−1 | −1.149 × 100 | |
| (h−1) | 5.416 × 10−4 | −5.524 × 10−1 | 2.442 × 100 |
| (h−1) | 4.612 × 10−7 | −1.277 × 100 | 3.287 × 100 |
| parameter | |||
|---|---|---|---|
| 1.090 × 10−1 | −6.932 × 10−1 | −2.330 × 10−1 | |
| 2.872 × 10−3 | 1.965 × 10−1 | 8.955 × 10−1 | |

4.9. Effect of Water Composition

4.10. Comparison with Previous Study

- Actual intake water was used, resulting in chlorination of water that consisted of NOM representative of the ambient RLIC waters.
- Use of the actual intake water indicated the small variability of pH and salinity. This resulted in exclusion of pH and salinity as control variables.
- In the present study, a range of 19–47 °C was used for temperature and a range of 0.38–5.00 mg/L was used for chlorine dosage. These wider ranges were more representative of actual operations and site-specific ambient conditions, which helps cover all possibilities for the predominant control variables, resulting in improved analysis.
- Each lab run was done so that better temporal resolution was achieved during the early parts of the run with measurements every 15 min for the first hour and every 30 min for the next hour. This higher resolution provided additional data to help with studying the fast reaction after the initial dosing.
- Each lab run was also run for a much longer time during the present study, which assured increased data during the latter part of the run when the slow reaction is dominant. Data during the slow reaction is crucial, as the water dosed with chlorine enters the RLIC water during this period. It is highly important to put more emphasis on the slow reaction phase, as the interaction with NOM in the ambient water and the resulting environmental impacts would occur during this period.
- The increased number of data during the slow reaction phase () provided increased quality of fit during this phase. Due to the longer period, the quality of fit could be evaluated for up to several days, as compared to the previous relationships.


5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, G.T.F.; Davidson, J.A. The fate of chlorine in seawater. Water Res. 1977, 11, 971–978. [Google Scholar] [CrossRef]
- Stanbro, W.D. The Chemistry of Power Plant Chlorination; John Hopkins University, Applied Physics Laboratory: Laurel, MD, USA, 1983; pp. 1–25. [Google Scholar]
- Shames Al-Din, A.M.; Arain, A.M.; Hammoud, A.A. On the chlorination of seawater. Desalination 2000, 129, 53–62. [Google Scholar] [CrossRef]
- Fayad, N.M.; Iqbal, S. Chlorination by-products of Arabian Gulf seawater. Bull. Environ. Contam. Toxicol. 1987, 78, 475–482. [Google Scholar] [CrossRef]
- Shames Al-Din, A.M.; Arain, A.M.; Hammoud, A.A. A contribution to the problem of trihalomethane formation from the Arabian Gulf water. Desalination 1991, 85, 13–32. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.; Khodoury, A.; Bensalah, N. Formation of trihalomethanes during seawater chlorination. J. Environ. Pollut. 2010, 1, 456–465. [Google Scholar] [CrossRef]
- Eppley, R.W.; Roger, E.H.; Williams, P.M. Chlorine reaction with seawater constituents and the inhibition of photosynthesis of natural phytoplankton. Estuar. Coast. Mar. Sci. 1976, 4, 147–161. [Google Scholar] [CrossRef]
- Agus, A.; Koutchkov, N.; Sedlak, D. Disinfection by-products and their potential impact on the quality of water produced by desalination systems: A literature review. Desalination 2009, 237, 214–237. [Google Scholar] [CrossRef]
- Adenekan, A.E.; Kolluru, V.S.; Smith, J.P. Transport and fate of chlorinated by-products associated with cooling water discharges. In Proceedings of the 1st Annual Gas Processing Symposium; Alfadala, H., Rex Reklaitis, G.V., El-Halwagi, M.M., Eds.; Elsevier: Berlin, Germany, 2009. [Google Scholar]
- Febbo, E.; Kolluru, V.S.; Prakash, S. Numerical Modeling of Thermal Plume and Residual Chlorine Fate in Coastal Waters of the Arabian Gulf. In Proceedings of the SPE/APPEA International Conference on Health, Safety, and Environment in Oil and Gas Exploration and Production, Perth, Australia, 11–13 September 2012.
- Saeed, S.; Deba, N.; Campbell, R.; Kolluru, V.S.; Prakash, S.; Febbo, E. Cooling Water Discharge to the Qatar Marine Zone-Laboratory Determination of Chlorine Decay Kinetics as Input to Numerical Fate and Transport Model; Qatar Foundation Research Forum: Doha, Qatar, 2013. [Google Scholar]
- Rook, J.J. Chlorination reactions of fulvic acids in natural waters. Environ. Sci. Techol. 1977, 11, 478–482. [Google Scholar] [CrossRef]
- Clark, R.M.; Sivaganesan, M. Predicting chlorine residual and formation of TTHMs in drinking water. J. Environ. Eng. 1998, 124, 1203–1210. [Google Scholar] [CrossRef]
- Chowdhury, S.; Champagne, P. An Investigation on Parameter for Modeling THMs Formation. Glob. NEST J. 2008, 10, 80–91. [Google Scholar]
- Haas, C.N.; Karra, S.B. Kinetics of wastewater chlorine demand exertion. J. Water Pollut. Control Fed. 1984, 56, 170–173. [Google Scholar]
- Sohn, J.; Amy, G.; Cho, J.; Lee, Y.; Yoon, Y. Disinfectant decay and disinfection by-products formation model development: Chlorination and ozonation by-products. Water Res. 2004, 38, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Fabbrincino, M.; Korshin, C.V. Modeling disinfection by-product formation in bromide containing water. J. Hazard. Mater. 2009, 168, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; West, J.R.; Barker, R.A.; Forster, C.F. Modelling of chlorine decay in municipal water supplies. Water Res. 1999, 33, 2735–2746. [Google Scholar] [CrossRef]
- Clark, R.M. Chlorine demand and TTHM formation kinetics: A second-order model. J. Environ. Eng. 1998, 124, 16–24. [Google Scholar] [CrossRef]
- Jadas-Hécart, A.; El Morer, A.; Stitou, M.; Bouillot, P.; Legube, B. Chlorine demand of a treated water [Modelisation de la demande en chlore d’une eau traitee]. Water Res. 1992, 26, 1073–1084. [Google Scholar] [CrossRef]
- Ventresque, C.; Bablon, G.; Legube, B.; Jadas-Hécart, A.; Dore, M. Development of chlorine demand kinetics in a drinking water treatment plant. Water Chlorination Chem. Environ. Impact Health Eff. 1990, 6, 715–728. [Google Scholar]
- Saeed, S.; Deba, N.; Cambell, R.; Prakash, S.; Kolluru, V.S. Laboratory experiments to validate 3D numerical modeling of chlorine decay in industrial cooling water discharge. In Proceedings of the Society of Environmental Toxicology and Chemistry (SETAC) Europe 23rd Annual Meeting: Building a Better Future: Responsible Innovation and Environmental Protection, Glasgow, UK, 12–16 May 2013.
- Kolluru, V.; Prakash, S.; Febbo, E. Modeling the Fate and Transport of Residual Chlorine and Chlorine By-Products (CBP) in Coastal Waters of the Arabian Gulf. In Environmental Science and Technology, Proceedings of the Fifth International Conference on Environmental Science and Technology, Houston, TX, USA, 25–29 June 2012; Sorial, G.A., Hong, J., Eds.; American Science Press: Houston, TX, USA, 2012; pp. 385–391. [Google Scholar]
- HACH. AutoCATTM9000 Amperometric Titrator User Manual for Chlorine, Chlorine Dioxide, Chlorite, Sulfite and Total Oxidants; HACH: Loveland, CO, USA, 2007. [Google Scholar]
- Stock, J.T. Amperometric Titrations. Anal. Chem. 1972, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Chang, P.C.; Chao, S.H.; Lin, Y.L. Relationship between chlorine consumption and chlorination by-products. Chemosphere 2009, 64, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Buttrick, D.; Tobiason, J.E.; Ahlfeld, D.P. Modeling as an Operational Tool for an Unfiltered Surface Water Supply. In Proceedings of the American Water Works Association Annual Conference, San Francisco, CA, USA, 12–16 June 2005.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, S.; Prakash, S.; Deb, N.; Campbell, R.; Kolluru, V.; Febbo, E.; Dupont, J. Development of a Site-Specific Kinetic Model for Chlorine Decay and the Formation of Chlorination By-Products in Seawater. J. Mar. Sci. Eng. 2015, 3, 772-792. https://doi.org/10.3390/jmse3030772
Saeed S, Prakash S, Deb N, Campbell R, Kolluru V, Febbo E, Dupont J. Development of a Site-Specific Kinetic Model for Chlorine Decay and the Formation of Chlorination By-Products in Seawater. Journal of Marine Science and Engineering. 2015; 3(3):772-792. https://doi.org/10.3390/jmse3030772
Chicago/Turabian StyleSaeed, Suhur, Shwet Prakash, Nandita Deb, Ross Campbell, Venkat Kolluru, Eric Febbo, and Jennifer Dupont. 2015. "Development of a Site-Specific Kinetic Model for Chlorine Decay and the Formation of Chlorination By-Products in Seawater" Journal of Marine Science and Engineering 3, no. 3: 772-792. https://doi.org/10.3390/jmse3030772





