Synthetic Extracellular Matrix of Polyvinyl Alcohol Nanofibers for Three-Dimensional Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Post-Crosslinking of Nanofibers with HCl Vapor
2.4. Assay of Nanofiber Morphology
2.5. Light Transmittance
2.6. Fourier Transform Infrared Spectra
2.7. Water Contact Angle Measurements
2.8. Release Assay of Fluorescence from Florescence-Conjugated Peptide-Blended Nanofibers
2.9. Cell Culture
2.10. Culture of BMDCs on PVA NM
2.11. Morphological Assay of Cultured Cells by SEM
2.12. Laser Confocal Microscopy
2.13. Live Cell Imaging
2.14. Cell Proliferation Assay
2.15. Statistical Analysis
3. Results
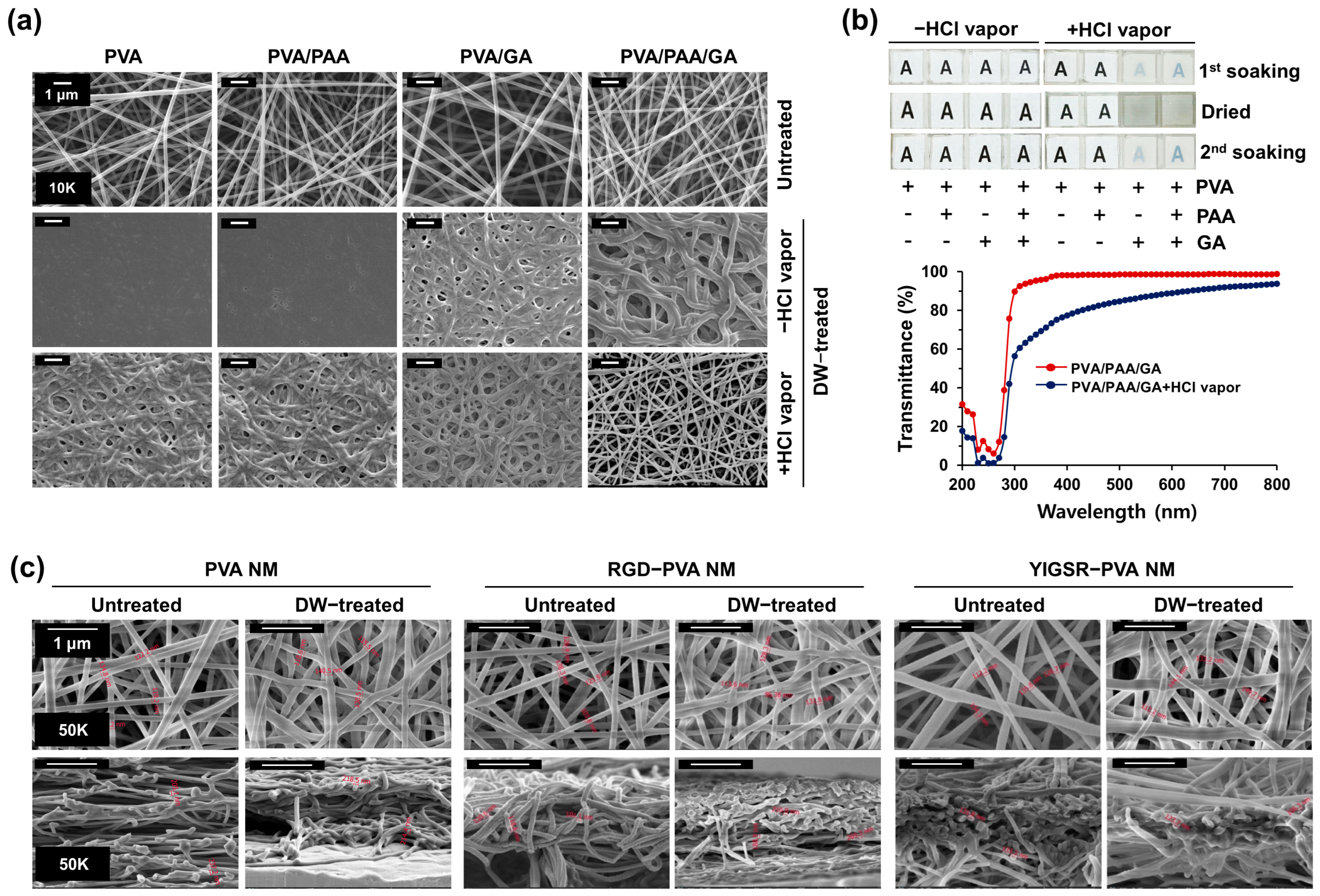
3.1. Preparation of Water-Stable PVA Nanofibers
3.2. Optical Transparency of Water-Stable PVA Nanofibers
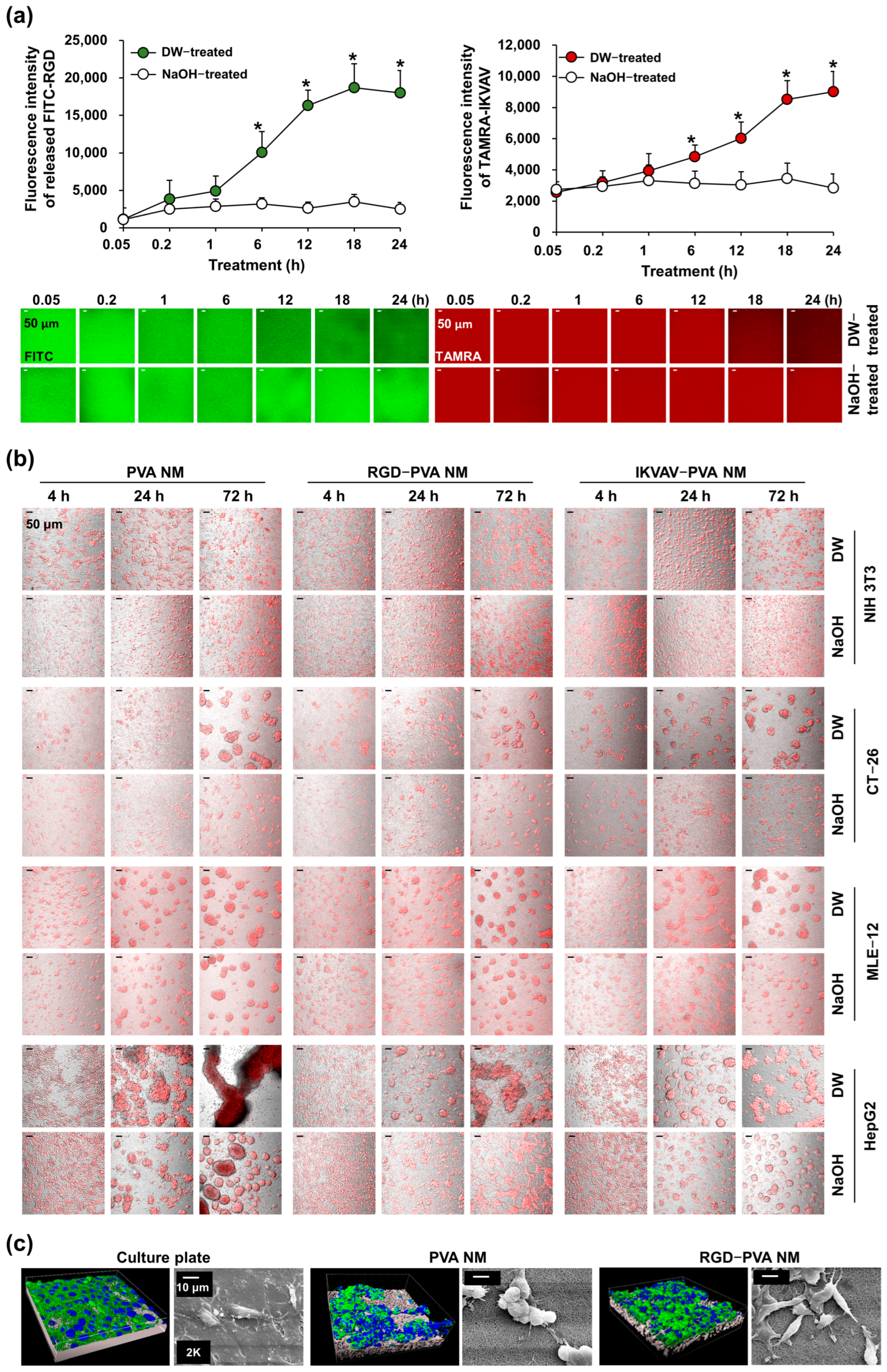
3.3. Production of Peptide-Blended PVA Nanofibers
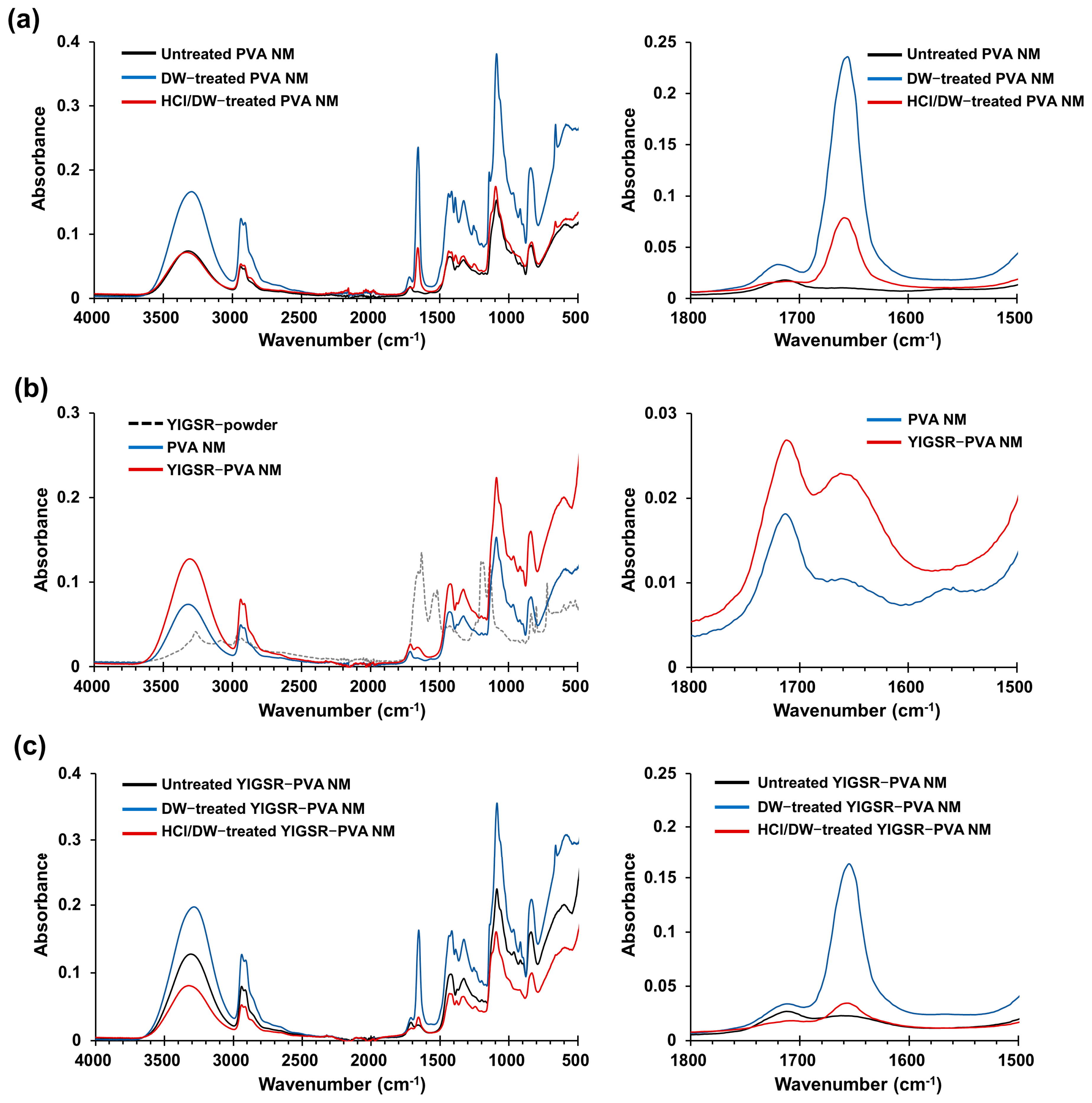
3.4. Fourier Transform Infrared Spectra
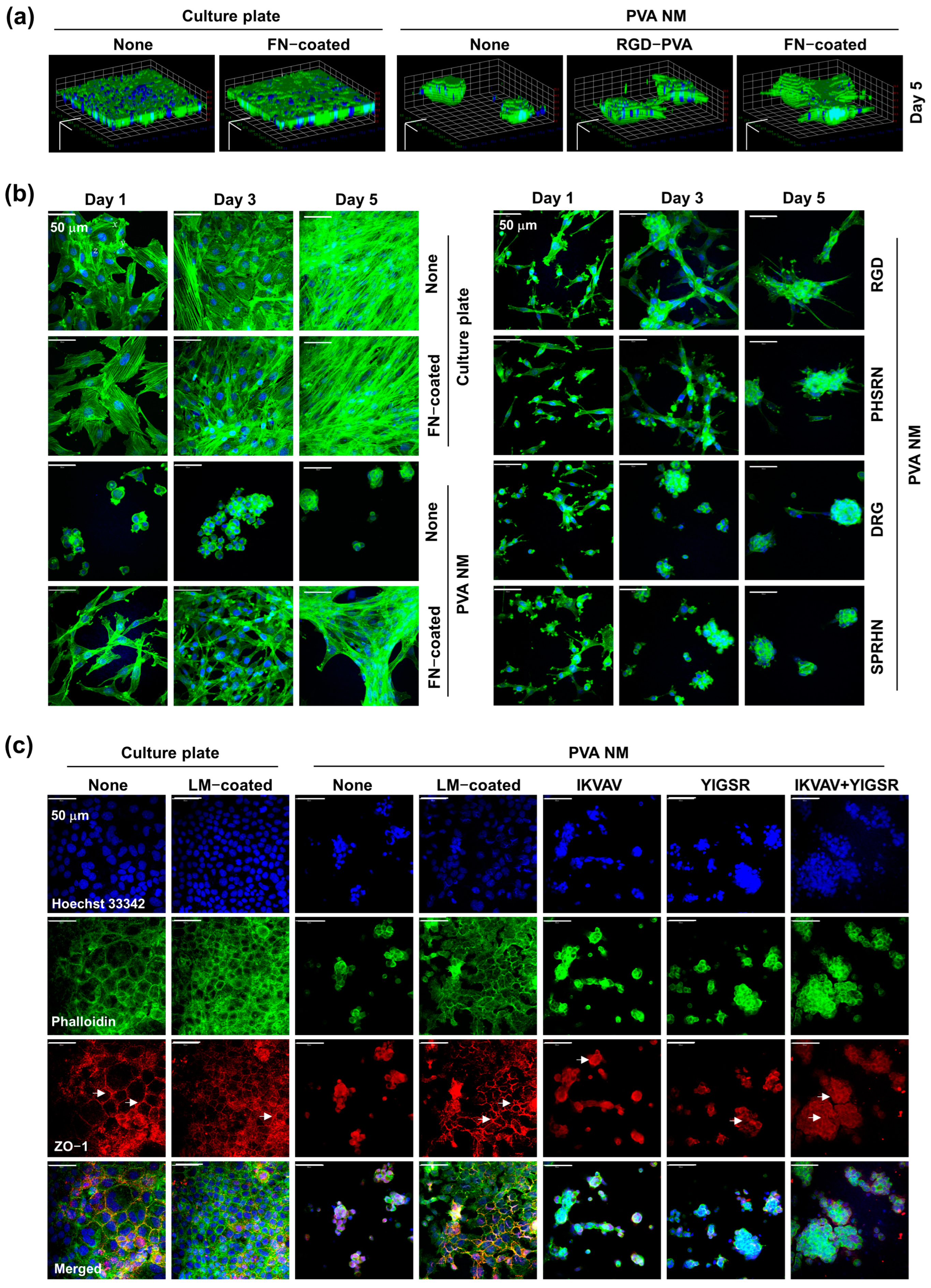
3.5. Cell Culture on PVA NMs
3.6. Cell Culture on Peptide-Blended PVA NMs
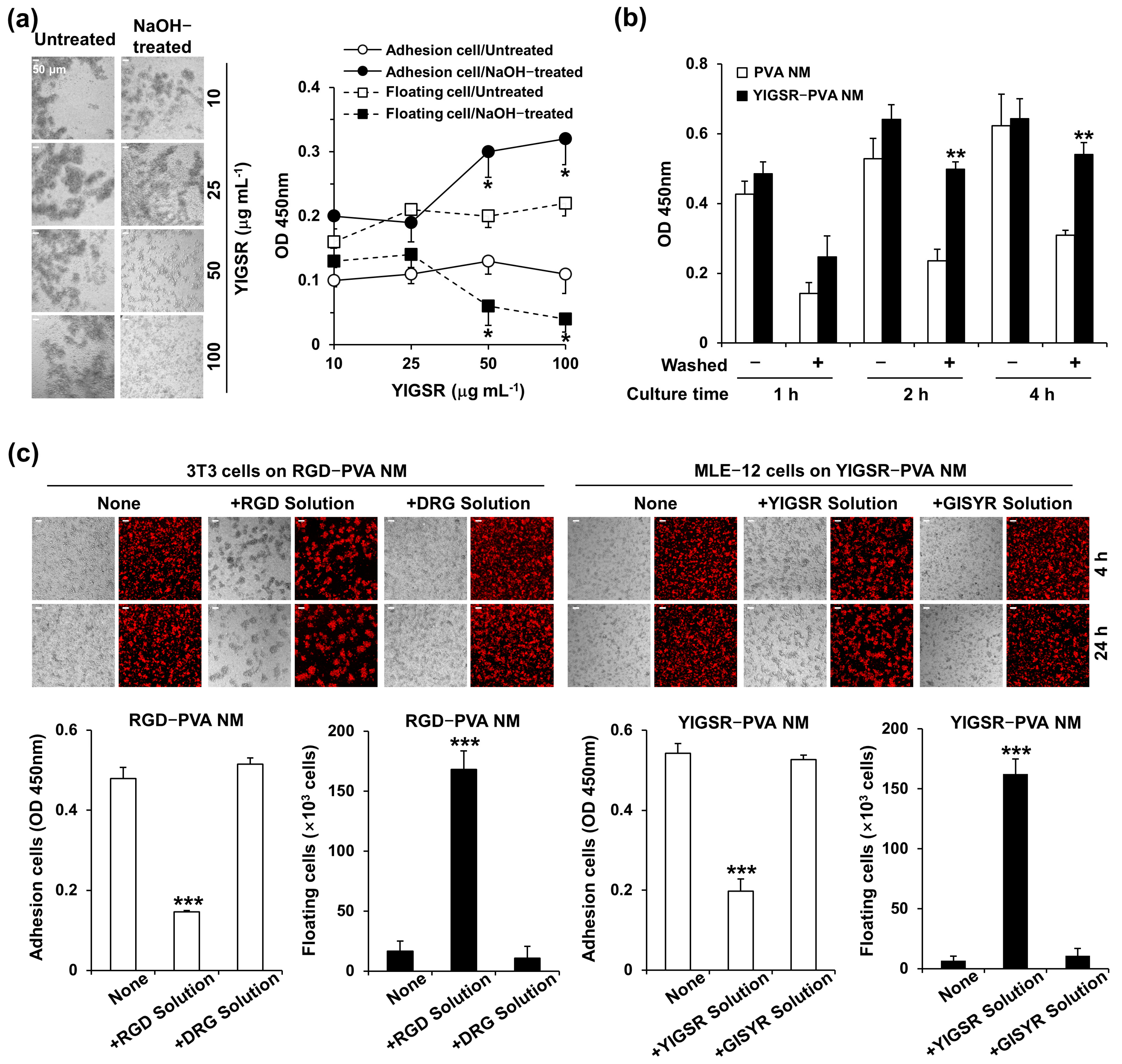
3.7. Treatment of Peptide-Blended PVA NMs with NaOH
3.8. Cell Culture on NaOH-Treated PVA NMs Blended with Peptides
3.9. Adhesion of Seeded Cells to Peptide-Retained PVA NMs
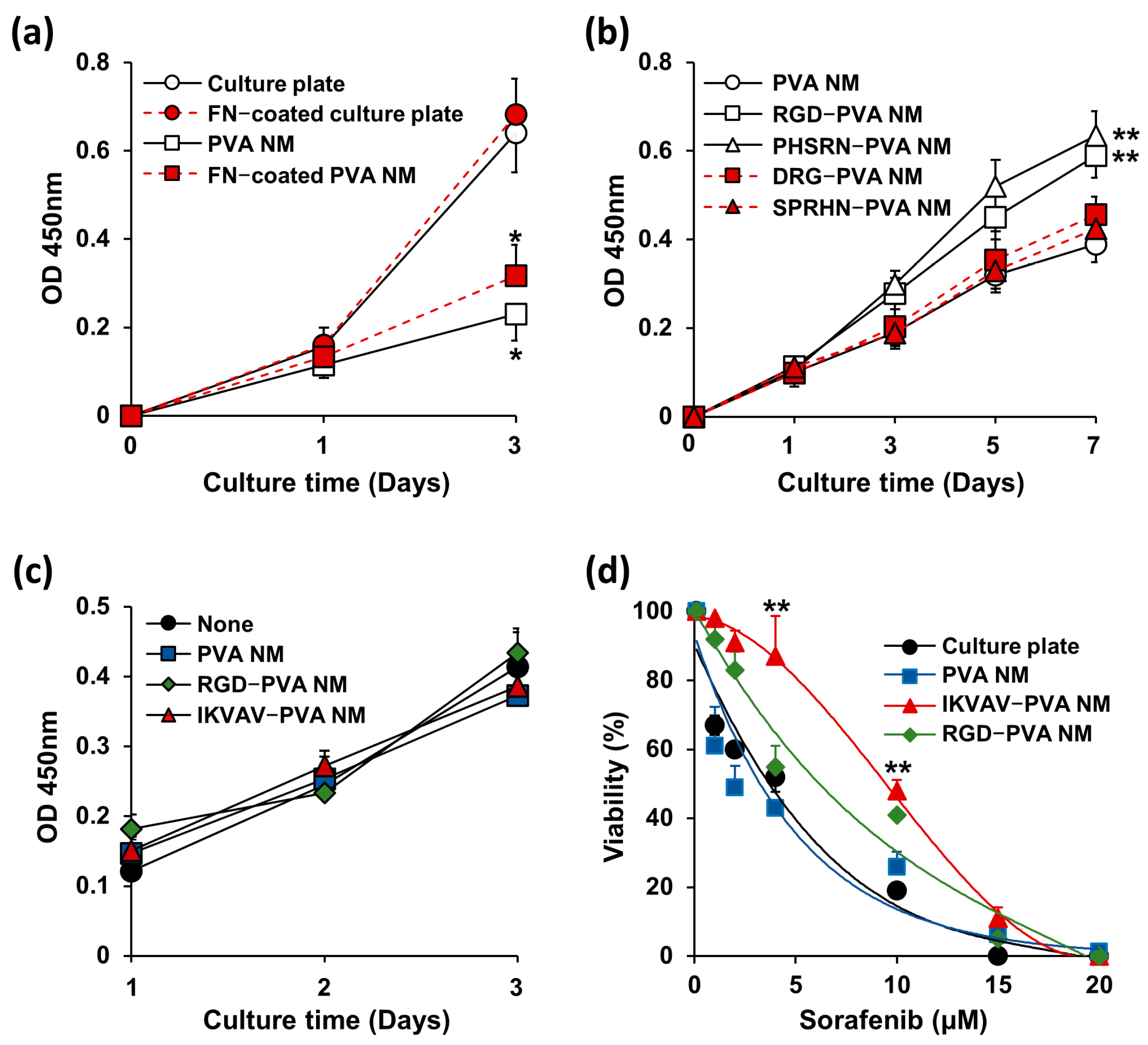
3.10. Comparison of Cell Growth on Peptide-Blended PVA NM and ECM-Coated PVA NM
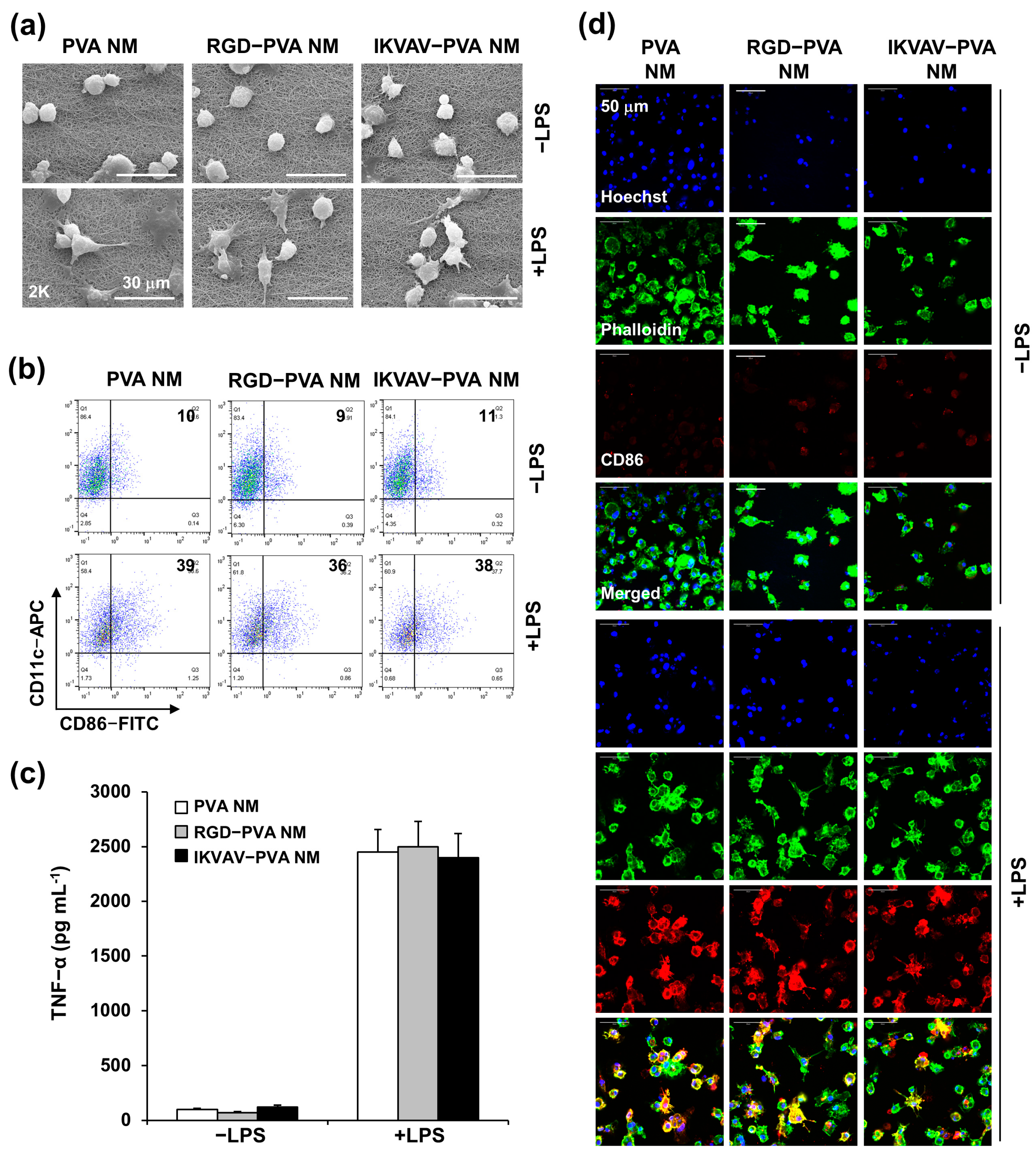
3.11. Bioinert of Peptide-Blended PVA NM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rosso, F.; Giordano, A.; Barbarisi, M.; Barbarisi, A. From cell-ECM interactions to tissue engineering. J. Cell Physiol. 2004, 199, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Stocco, T.D.; Bassous, N.J.; Zhao, S.; Granato, A.E.C.; Webster, T.J.; Lobo, A.O. Nanofibrous scaffolds for biomedical applications. Nanoscale 2018, 10, 12228–12255. [Google Scholar] [CrossRef]
- Gao, X.; Han, S.; Zhang, R.; Liu, G.; Wu, J. Progress in electrospun composite nanofibers: Composition, performance and applications for tissue engineering. J. Mater. Chem. B 2019, 7, 7075–7089. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymer 2023, 15, 2418. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; John, J.V.; McCarthy, A.; Xie, J. New forms of electrospun nanofiber materials for biomedical applications. J. Mater. Chem. B 2020, 8, 3733–3746. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef]
- Chen, J.; Rong, F.; Xie, Y. Fabrication, Microstructures and Sensor Applications of Highly Ordered Electrospun Nanofibers: A Review. Materials 2023, 16, 3310. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Zahra, F.T.; Quick, Q.; Mu, R. Electrospun PVA fibers for drug delivery: A review. Polymers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Kamaraj, M.; Moghimi, N.; Chen, J.; Morales, R.; Chen, S.; Khademhosseini, A.; John, J.V. New dimensions of electrospun nanofiber material designs for biotechnological uses. Trends Biotechnol. 2024, 42, 631–647. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.G.; Liu, Y.; Liu, Y.N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef]
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 349–364. [Google Scholar] [CrossRef]
- Phutane, P.; Telange, D.; Agrawal, S.; Gunde, M.; Kotkar, K.; Pethe, A. Biofunctionalization and Applications of Polymeric Nanofibers in Tissue Engineering and Regenerative Medicine. Polymers 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Linh, N.T.; Min, Y.K.; Song, H.Y.; Lee, B.T. Fabrication of polyvinyl alcohol/gelatin nanofiber composites and evaluation of their material properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 184–191. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Hu, K.-H.; Wei, Z.-H. Comparison of cell behavior on pva/pva-gelatin electrospun nanofibers with random and aligned configuration. Sci. Rep. 2016, 6, 37960. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, G.; Tang, J.; Cheng, R.; Shen, X.; Gu, Y.; Wu, L.; Xi, K.; Zhao, Y.; Cui, W.; et al. ECM-inspired micro/nanofibers for modulating cell function and tissue generation. Sci. Adv. 2020, 6, eabc2036. [Google Scholar] [CrossRef] [PubMed]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef]
- Bucci, R.; Vaghi, F.; Erba, E.; Romanelli, A.; Gelmi, M.L.; Clerici, F. Peptide grafting strategies before and after electrospinning of nanofibers. Acta Biomater. 2021, 122, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.L.; Soares, D.G.; Anovazzi, G.; Mendes Soares, I.P.; Hebling, J.; de Souza Costa, C.A. Development of fibronectin-loaded nanofiber scaffolds for guided pulp tissue regeneration. J. Biomed. Mater. Res. 2021, 109, 1244–1258. [Google Scholar] [CrossRef]
- Samokhin, Y.; Varava, Y.; Diedkova, K.; Yanko, I.; Husak, Y.; Radwan-Pragłowska, J.; Pogorielova, O.; Janus, Ł.; Pogorielov, M.; Korniienko, V. Fabrication and Characterization of Electrospun Chitosan/Polylactic Acid (CH/PLA) Nanofiber Scaffolds for Biomedical Application. J. Funct. Biomater. 2023, 14, 414. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Vineis, C.; Cruz-Maya, I.; Tonetti, C.; Guarino, V.; Varesano, A. Wool Keratin Nanofibers for Bioinspired and Sustainable Use in Biomedical Field. J. Funct. Biomater. 2022, 14, 5. [Google Scholar] [CrossRef]
- Alves, M.H.; Jensen, B.E.; Smith, A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-based nanofibrous electrospun scaffolds for tissue engineering applications. Polymers 2019, 12, 7. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.; Mohamad, A.B.; Al-Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Park, J.-C.; Ito, T.; Kim, K.-O.; Kim, K.-W.; Kim, B.-S.; Khil, M.-S.; Kim, H.-Y.; Kim, I.-S. Electrospun poly(vinyl alcohol) nanofibers: Effects of degree of hydrolysis and enhanced water stability. Polym. J. 2010, 42, 273–276. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Tang, C.; Saquing, C.D.; Harding, J.R.; Khan, S.A. In situ cross-linking of electrospun poly(vinyl alcohol) nanofibers. Macromolecules 2010, 43, 630–637. [Google Scholar] [CrossRef]
- Han, W.H.; Wang, Q.Y.; Kang, Y.Y.; Shi, L.R.; Long, Y.; Zhou, X.; Hao, C.C. Cross-linking electrospinning. Nanoscale 2023, 15, 15513–15551. [Google Scholar] [CrossRef] [PubMed]
- Miraftab, M.; Saifullah, A.N.; Cay, A. Physical stabilisation of electrospun poly(vinyl alcohol) nanofibres: Comparative study on methanol and heat-based crosslinking. J. Mater. Sci. 2015, 50, 1943–1957. [Google Scholar] [CrossRef]
- Weis, C.; Odermatt, E.K.; Kressler, J.; Funke, Z.; Wehner, T.; Freytag, D. Poly(vinyl alcohol) membranes for adhesion prevention. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70, 191–202. [Google Scholar] [CrossRef]
- Oh, Y.S.; Choi, M.H.; Shin, J.I.; Maza, P.A.M.A.; Kwak, J.Y. Coculturing of endothelial and cancer cells in a nanofibrous scaffold-based two-layer system. Int. J. Mol. Sci. 2020, 21, 4128. [Google Scholar] [CrossRef]
- Kim, T.E.; Kim, C.G.; Kim, J.S.; Jin, S.; Yoon, S.; Bae, H.R.; Kim, J.H.; Jeong, Y.H.; Kwak, J.Y. Three-dimensional culture and interaction of cancer cells and dendritic cells in an electrospun nano-submicron hybrid fibrous scaffold. Int. J. Nanomed. 2016, 11, 823–835. [Google Scholar]
- Koo, O.M.; Fiske, J.D.; Yang, H.; Nikfar, F.; Thakur, A.; Scheer, B.; Adams, M.L. Investigation into stability of poly(vinyl alcohol)-based Opadry® II films. AAPS PharmSciTech 2011, 12, 746–754. [Google Scholar] [CrossRef]
- Desai, S.D.; Kundu, I.; Swamy, N.P.; Crull, G.B.; Pan, D.; Zhao, J.; Shah, R.P.; Venkatesh, C.; Vig, B.; Varia, S.A.; et al. Cross-linking of poly (vinyl alcohol) films under acidic and thermal stress. Eur. J. Pharm. Sci. 2020, 152, 105429. [Google Scholar] [CrossRef]
- Aota, S.; Nomizu, M.; Yamada, K.M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 1994, 269, 24756–24761. [Google Scholar] [CrossRef]
- Tashiro, K.; Sephel, G.C.; Weeks, B.; Sasaki, M.; Martin, G.R.; Kleinman, H.K.; Yamada, Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Chem. 1989, 264, 16174–16182. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.P.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Crosslinked electrospun PVA nanofibrous membranes: Elucidation of their physicochemical, physicomechanical and molecular disposition. Biofabrication 2012, 4, 025002. [Google Scholar] [CrossRef]
- Wang, Y.; Hsieh, Y.-L. Crosslinking of polyvinyl alcohol (PVA) fibrous membranes with glutaraldehyde and PEG diacylchloride. J. Appl. Polym. Sci. 2010, 116, 3249–3255. [Google Scholar] [CrossRef]
- Baştürk, E.; Demir, S.; Danış, Ö.; Kahraman, M.V. Covalent immobilization of α-amylase onto thermally crosslinked electrospun PVA/PAA nanofibrous hybrid membranes. J. Appl. Polym. Sci. 2013, 127, 349–355. [Google Scholar] [CrossRef]
- Kumeta, K.; Nagashima, I.; Matsui, S.; Mizoguchi, K. Crosslinking reaction of poly(vinyl alcohol) with poly(acrylic acid) (PAA) by heat treatment: Effect of neutralization of PAA. J. Appl. Polym. Sci. 2003, 90, 2420–2427. [Google Scholar] [CrossRef]
- Destaye, A.G.; Lin, C.K.; Lee, C.K. Glutaraldehyde vapor cross-linked nanofibrous PVA mat with in situ formed silver nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Chen, C.S. Deconstructing Dimensionality. Science 2013, 339, 402–404. [Google Scholar] [CrossRef]
- Chen, M.; Patra, P.K.; Warner, S.B.; Bhowmick, S. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 2007, 13, 579–587. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yao, D.; Jiang, J.; Guo, X.; Gao, Y.; Li, Q.; Shen, C. Effects of aligned and random fibers with different diameter on cell behaviors. Coll. Surf. B Biointerfaces 2018, 171, 461–467. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- Siadat, S.M.; Silverman, A.A.; DiMarzio, C.A.; Ruberti, J.W. Measuring collagen fibril diameter with differential interference contrast microscopy. J. Struct. Biol. 2021, 213, 107697. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.A.; Horwitz, A.F. Cell surface receptors for extracellular matrix molecules. Annu. Rev. Cell Biol. 1987, 3, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Mortisen, D.J.; Henry, S.M.; Anseth, K.S. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res. 2001, 57, 217–223. [Google Scholar] [CrossRef]
- Goldvaser, M.; Epstein, E.; Rosen, O.; Jayson, A.; Natan, N.; Ben-Shalom, T.; Saphier, S.; Katalan, S.; Shoseyov, O. Poly(vinyl alcohol)-methacrylate with CRGD peptide: A photocurable biocompatible hydrogel. J. Tissue Eng. Regen. Med. 2022, 16, 140–150. [Google Scholar] [CrossRef]
- Serrano, M.C.; Portolés, M.T.; Vallet-Regí, M.; Izquierdo, I.; Galletti, L.; Comas, J.V.; Pagani, R. Vascular endothelial and smooth muscle cell culture on NaOH-treated poly(ε-caprolactone) films: A preliminary study for vascular graft development. Macromol. Biosci. 2005, 5, 415–423. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.-M.; Lee, S.J.; Lee, S.G.; Jeong, Y.-K.; Kim, S.E.; Lee, S.C. Surface hydrolysis of fibrous poly(ε-caprolactone) scaffolds for enhanced osteoblast adhesion and proliferation. Macromol. Res. 2007, 15, 424–429. [Google Scholar] [CrossRef]
- Chen, F.; Lee, C.N.; Teoh, S.H. Nanofibrous modification on ultra-thin poly(e-caprolactone) membrane via electrospinning. Mater. Sci. Eng. C 2007, 27, 325–332. [Google Scholar] [CrossRef]
- Park, G.E.; Pattison, M.A.; Park, K.; Webster, T.J. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials 2005, 26, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Niklason, L.; Langer, R. Surface hydrolysis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells. J. Biomed. Mater. Res. 1998, 42, 417–424. [Google Scholar] [CrossRef]
- Perego, G.; Preda, P.; Pasquinelli, G.; Curti, T.; Freyrie, A.; Cenni, E. Functionalization of poly-(L-lactic-co-epsilon-caprolactone): Effects of surface modification on endothelial cell proliferation and hemocompatibility [corrected]. J. Biomater. Sci. Polym. Ed. 2003, 14, 1057–1075. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.X.T.; Sun, G.-M.; Tran, H.V.A.; Jeong, Y.H.; Slama, P.; Chang, Y.-C.; Lee, I.-J.; Kwak, J.-Y. Synthetic Extracellular Matrix of Polyvinyl Alcohol Nanofibers for Three-Dimensional Cell Culture. J. Funct. Biomater. 2024, 15, 262. https://doi.org/10.3390/jfb15090262
Tran TXT, Sun G-M, Tran HVA, Jeong YH, Slama P, Chang Y-C, Lee I-J, Kwak J-Y. Synthetic Extracellular Matrix of Polyvinyl Alcohol Nanofibers for Three-Dimensional Cell Culture. Journal of Functional Biomaterials. 2024; 15(9):262. https://doi.org/10.3390/jfb15090262
Chicago/Turabian StyleTran, Thi Xuan Thuy, Gyu-Min Sun, Hue Vy An Tran, Young Hun Jeong, Petr Slama, Young-Chae Chang, In-Jeong Lee, and Jong-Young Kwak. 2024. "Synthetic Extracellular Matrix of Polyvinyl Alcohol Nanofibers for Three-Dimensional Cell Culture" Journal of Functional Biomaterials 15, no. 9: 262. https://doi.org/10.3390/jfb15090262






