Graphene Oxide and Carbon Nanotubes-Based Polyvinylidene Fluoride Membrane for Highly Increased Water Treatment
Abstract
:1. Introduction
2. Experiments and Method
2.1. Materials and Fabrication Process of GMP Membrane
2.2. Production of GMP Filter Module
3. Results and Discussions
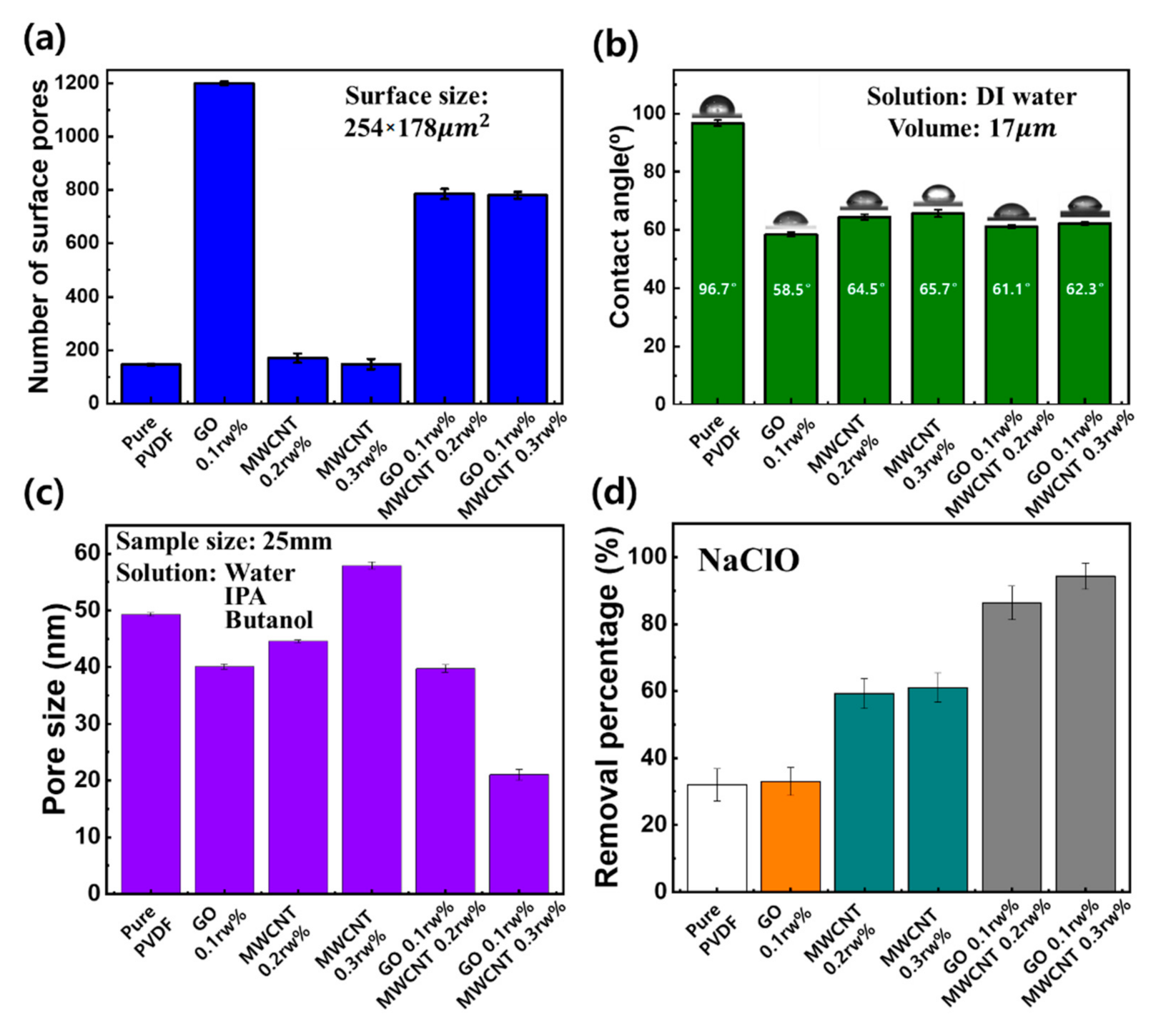
3.1. Characterization of GMP Membranes
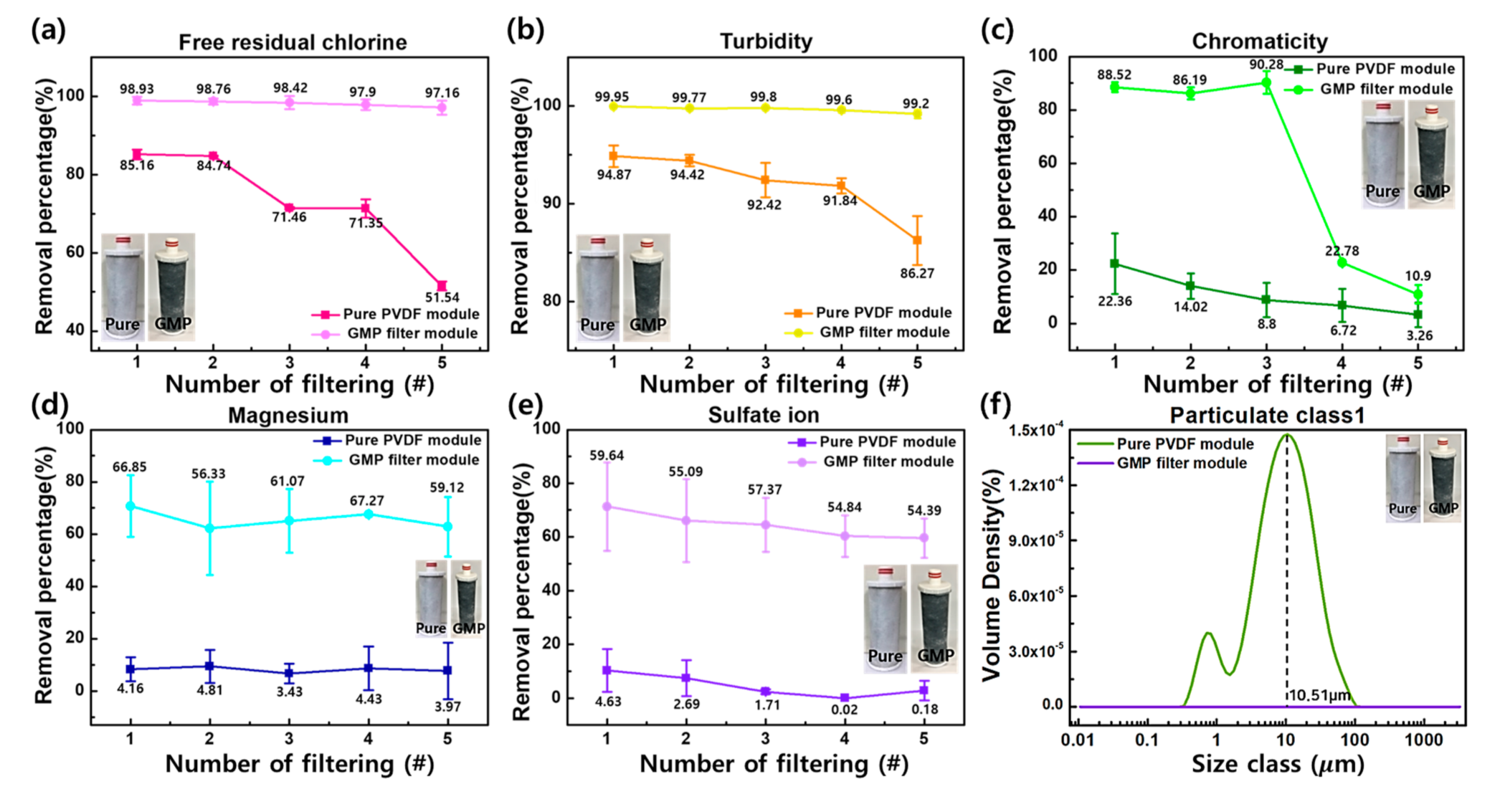
3.2. GMP Filter Module Filtration Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kummu, M.; Ward, P.J.; Moel, H.D.; Varis, I. Is physical water scarcity a new phenomenon? Global assessment of water shortage over the last two millennia. Environ. Res. Lett. 2010, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.O.; Lee, K.K. Treatment of Refractory Dye Wastewater Using AOPs. J. Korean Geo.-Environ. Soc. 2006, 7, 21–29. [Google Scholar]
- Cho, K.H.; Kim, Y.B. The trend and prospect of advanced water treatment process using ozonizer. Proc. KIIEE Annu. conf. 1998, 1, 242–244. [Google Scholar]
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; McGrath, J.E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]
- Yu, L.Y.; Xu, Z.L.; Shen, H.M.; Yang, H. Preparation and characterization of PVDF–SiO2 composite hollow fiber UF membrane by sol-gel method. J. Membr. Sci. 2009, 337, 257–265. [Google Scholar] [CrossRef]
- Lee, Y.M.; Shim, J.K. Separation Membranes in Water Treatment and their Applications. Polym. Sci. Technol. 1999, 10, 198–206. [Google Scholar]
- Kang, J.S.; Kim, J.P. Tech-Trend for Membrane in Water and Wastewater Treatment. Polym. Sci. Technol. 2005, 16, 424–435. [Google Scholar]
- Guan, Y.F.; Huang, B.C.; Qian, C.; Wang, L.F.; Yu, H.Q. Improved PVDF membrane performance by doping extracellular polymeric substances of activated sludge. Water Res. 2017, 113, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.Y.; He, Y.; Zhan, Y.Q.; Zhang, L.; Pan, Y.; Zhang, C.L.; Yu, Z.X. Novel polyvinylidene fluoride nanofiltration membrane blended with functionalized halloysite nanotubes for dye and heavy metal ions removal. J. Hazard. Mater. 2016, 317, 60–72. [Google Scholar] [CrossRef]
- Tian, X.Z.; Xue, J.F. Poly (vinylidene fluoride-co-hexafluoropropene) (PVDF-HFP) membranes for ethyl acetate removal from water. J. Hazard. Mater. 2008, 153, 128–135. [Google Scholar] [CrossRef]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.J.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the Solubility and Stability of Polyvinylidene Fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Tan, X.; Li, K.; Teo, W.K. Removal of 1,1,1-trichloroethane from water using a polyvinylidene fluoride hollow fiber membrane module: Vacuum membrane distillation operation. Sep. Purif. Technol. 2006, 52, 301–309. [Google Scholar] [CrossRef]
- Malakootian, M.; Mahvi, A.H.; Fatehizadeh, A.; Ehrampoush, M.H. Efficiency of calcium and magnesium removal by nanofiltration membrane from synthetic water under different operating conditions. Toloo-e-Behdasht 2011, 9, 1–9. [Google Scholar]
- Chen, Y.; Xu, W.; Zhu, H.; Wei, D.; He, F.; Wang, D.; Du, B.; Wei, Q. Effect of turbidity on micropollutant removal and membrane fouling by MIEX/ultrafiltration hybrid process. Chemosphere 2019, 216, 488–498. [Google Scholar] [CrossRef]
- Lee, M.J.; Ong, C.S.; Lau, W.J.; Ng, B.C.; Ismail, A.F.; Lai, S.O. Degradation of PVDF-based composite membrane and its impacts on membrane intrinsic and separation properties. J. Polym. Eng. 2016, 36, 261–268. [Google Scholar] [CrossRef]
- Adem, E.; Rickards, J.; Burillo, G.; Avalos-Borja, M. Changes in poly-vinylidene fluoride produced by electron irradiation. Radiat. Phys. Chem. 1999, 54, 637–641. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H.; Dahlan, K.Z.M. Investigation of electron irradiation induced-changes in poly(vinylidene fluoride) films. Polym. Degrad. Stab. 2002, 75, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Jaleh, B.; Gavar, N.; Fakhri, P.; Muensit, N.; Taheri, S.M. Characteristics of PVDF membranes irradiated by electron beam. Membranes 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, A.S.; Gual, M.R.; Pereira, C.; Faria, L.O. Thermal analysis for study of the gamma radiation effects in poly(vinylidene fluoride). Radiat. Phys. Chem. 2015, 116, 345–348. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly (vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, Y.J.; Yu, Q.S.; Deng, B.L. Preparation and characterization of polyamide thin-film composite (TFC) membranes on plasma-modified polyvinylidene fluoride (PVDF). J. Membr. Sci. 2009, 344, 71–81. [Google Scholar] [CrossRef]
- Chang, I.S.; Clech, P.L.; Jefferson, B.; Judd, S. Membrane Fouling in Membrane Bioreactors for Wastewater Treatment. J. Environ. Eng. 2002, 125, 1018–1029. [Google Scholar] [CrossRef]
- Zhao, X.Z.; Xuan, H.X.; Chen, Y.L.; He, C.J. Preparation and characterization of superior antifouling PVDF membrane with extremely ordered and hydrophilic surface layer. J. Membr. Sci. 2015, 494, 48–56. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with ionic liquid modified nano-TiO2 particles. J. Membr. Sci. 2013, 427, 259–269. [Google Scholar] [CrossRef]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly Hydrophilic Polyvinylidene Fluoride (PVDF) Ultrafiltration Membranes via P ostfabrication Grafting of Surface-Tailored Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, C.; He, X.; Lyu, M.; Wang, S.; Wang, L. Processable graphene oxide-embedded titanate nanofiber membranes with improved filtration performance. J. Hazard. Mater. 2017, 325, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alramadhan, S.A.; Hammud, H.H. Graphene nickel silica supported nanocomposites as an efficient purifier for water treatment. Appl. Nanosci. 2021, 11, 273–291. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Shan, M.; Li, Y.; Li, B.; Niu, J.; Zhou, B.; Qian, X. Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2014, 458, 1–13. [Google Scholar] [CrossRef]
- Fazelabdolabadi, B.; Khodadadi, A.A.; Sedaghatzadeh, M. Thermal and rheological properties improvement of drilling fluids using functionalized carbon nanotubes. Appl. Nanosci. 2015, 5, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, P.; Vijayakumar, R.P. Synthesis of UMCNOs from MWCNTs and analysis of its structure and properties for wastewater treatment applications. Appl. Nanosci. 2018, 8, 1989–2000. [Google Scholar] [CrossRef]
- Xie, P.; Lannoy, C.F.d.; Ma, J.; Wang, Z.; Wang, S.; Li, J.; Wiesner, M.R. Improved chlorine tolerance of a polyvinyl pyrrolidone-polysulfone membrane enabled by carboxylated carbon nanotubes. Water Res. 2016, 104, 497–506. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Qian, Y.L.; Zhu, B.K.; Xu, Y.Y. Modification of porous poly (vinylidene fluoride) membrane using amphiphilic polymers with different structures in phase inversion process. J. Membr. Sci. 2008, 310, 567–576. [Google Scholar] [CrossRef]
- Zhao, X.; Sui, K.; Wu, W.; Liang, H.; Li, Y.; Wu, Z.; Xia, Y. Synthesis and properties of amphiphilic block polymer functionalized multi-walled carbon nanotubes and nanocomposites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 758–764. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Yuan, X.S.; Geng, H.Z.; Wang, L.D.; Jing, L.C.; Gu, Z.Z. High conductive PPY–CNT surface-modified PES membrane with anti-fouling property. Appl. Nanosci. 2018, 8, 1597–1606. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors-Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Organic Fouling and Chemical Cleaning of Nanofiltration Membranes: Measurements and Mechanism. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.T.; Xu, C.X.; Geng, H.Z.; Ji, Q.; Wang, L.; He, B.; Jiang, Y.; Kong, J.; Lia, J. Multifunctional PVDF/CNT/GO mixed matrix membranes for ultrafiltration and fouling detection. J. Hazard. Mater. 2020, 384, 120978. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, J.; Lim, T.; Cheng, H.; Hu, J.; Kim, S.; Jung, W. Graphene Oxide and Carbon Nanotubes-Based Polyvinylidene Fluoride Membrane for Highly Increased Water Treatment. Nanomaterials 2021, 11, 2498. https://doi.org/10.3390/nano11102498
Chae J, Lim T, Cheng H, Hu J, Kim S, Jung W. Graphene Oxide and Carbon Nanotubes-Based Polyvinylidene Fluoride Membrane for Highly Increased Water Treatment. Nanomaterials. 2021; 11(10):2498. https://doi.org/10.3390/nano11102498
Chicago/Turabian StyleChae, Jungryeong, Taeuk Lim, Hao Cheng, Jie Hu, Sunghoon Kim, and Wonsuk Jung. 2021. "Graphene Oxide and Carbon Nanotubes-Based Polyvinylidene Fluoride Membrane for Highly Increased Water Treatment" Nanomaterials 11, no. 10: 2498. https://doi.org/10.3390/nano11102498





