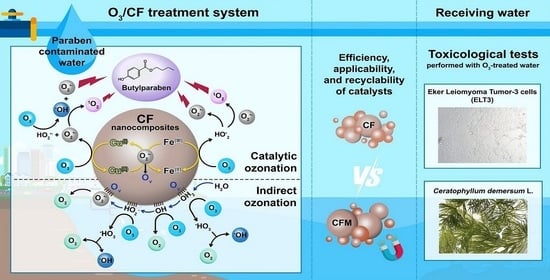
Fabrication of Ternary Nanoparticles for Catalytic Ozonation to Treat Parabens: Mechanisms, Efficiency, and Effects on Ceratophyllum demersum L. and Eker Leiomyoma Tumor-3 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Synthesis
2.2. Typical Experimental Setup
2.3. Operational and Influential Effect Experiments
2.4. Scavenging Experiments
2.5. Recyclability Testing
2.6. Toxicological Assays
3. Results and Discussion
3.1. Nanocomposite Selection
3.2. CF Nanocomposite Properties
3.2.1. Physical and Chemical Characteristics
3.2.2. Element Compositions of CF Nanocomposites
3.3. Enhanced Ozone Degradation Performance
3.4. Influential Effects on Degradation Efficiency
3.5. Material Recyclability
3.6. Proposed Mechanisms
3.6.1. Enhanced Ozonation Mechanisms
3.6.2. Degradates of Paraben Catalytic Ozonation
3.7. Toxicological Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reichert, G.; Mizukawa, A.; Antonelli, J.; de Almeida Brehm Goulart, F.; Filippe, T.C.; de Azevedo, J.C.R. Determination of Parabens, Triclosan, and Lipid Regulators in a Subtropical Urban River: Effects of Urban Occupation. Water Air Soil Pollut. 2020, 231, 133. [Google Scholar] [CrossRef]
- Terasaki, M.; Makino, M. Determination of chlorinated by-products of parabens in swimming pool water. Int. J. Environ. Anal. Chem. 2008, 88, 911–922. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence and human exposure of parabens and their chlorinated derivatives in swimming pools. Environ. Sci. Pollut. Res. 2015, 22, 17987–17997. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.R.; Gonzalez, T.; Cuerda-Correa, E.M.; Muñoz-Peña, M.J. Combating paraben pollution in surface waters with a variety of photocatalyzed systems: Looking for the most efficient technology. Open Chem. 2019, 17, 1317–1327. [Google Scholar] [CrossRef]
- Ramaswamy, B.R.; Kim, J.-W.; Isobe, T.; Chang, K.-H.; Amano, A.; Miller, T.W.; Siringan, F.P.; Tanabe, S. Determination of preservative and antimicrobial compounds in fish from Manila Bay, Philippines using ultra high performance liquid chromatography tandem mass spectrometry, and assessment of human dietary exposure. J. Hazard. Mater. 2011, 192, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, P.; Hansen, P.R.; Larsen, K.J.; Erratico, C.; Korsgaard, B.; Holbech, H. Vitellogenin as a biomarker for estrogenic effects in brown trout, Salmo trutta: Laboratory and field investigations. Environ. Toxicol. Chem. 2008, 27, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Hirai, H.; Kawai, S.; Nishida, T. Removal of estrogenic activity of iso-butylparaben and n-butylparaben by laccase in the presence of 1-hydroxybenzotriazole. Biodegradation 2009, 20, 533–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yin, D.; Guo, J.; Wu, H.; Gong, M.; Feng, X. Ternary catalyst Mn-Fe-Ce/Al2O3 for the ozonation of phenol pollutant: Performance and mechanism. Environ. Sci. Pollut. Res. 2021, 28, 32921–32932. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, F.; Zhu, Y.; Wei, F.; Liu, X.; Lian, C.; Wang, S. Magnetic core–shell CuFe2O4@C3N4 hybrids for visible light photocatalysis of Orange II. J. Hazard. Mater. 2015, 297, 224–233. [Google Scholar] [CrossRef]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.; Ren, Y.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]
- Yan, L.; Bing, J.; Wu, H. The behavior of ozone on different iron oxides surface sites in water. Sci. Rep. 2019, 9, 14752. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Chang, S.H.; Hung, P.C.; Hwang, J.F.; Chang, M.B. Catalytic oxidation of gaseous PCDD/Fs with ozone over iron oxide catalysts. Chemosphere 2008, 71, 388–397. [Google Scholar] [CrossRef]
- Devulapelli, V.G.; Sahle-Demessie, E. Catalytic oxidation of dimethyl sulfide with ozone: Effects of promoter and physico-chemical properties of metal oxide catalysts. Appl. Catal. A Gen. 2008, 348, 86–93. [Google Scholar] [CrossRef]
- Li, R.; Cai, M.; Xie, Z.; Zhang, Q.; Zeng, Y.; Liu, H.; Liu, G.; Lv, W. Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B Environ. 2019, 244, 974–982. [Google Scholar] [CrossRef]
- Liu, B.; Song, W.; Wu, H.; Liu, Z.; Teng, Y.; Sun, Y.; Xu, Y.; Zheng, H. Degradation of norfloxacin with peroxymonosulfate activated by nanoconfinement Co3O4@CNT nanocomposite. Chem. Eng. J. 2020, 398, 125498. [Google Scholar] [CrossRef]
- Angkaew, A.; Chokejaroenrat, C.; Sakulthaew, C.; Mao, J.; Watcharatharapong, T.; Watcharenwong, A.; Imman, S.; Suriyachai, N.; Kreetachat, T. Two facile synthesis routes for magnetic recoverable MnFe2O4/g-C3N4 nanocomposites to enhance visible light photo-Fenton activity for methylene blue degradation. J. Environ. Chem. Eng. 2021, 9, 105621. [Google Scholar] [CrossRef]
- Xian, G.; Kong, S.; Li, Q.; Zhang, G.; Zhou, N.; Du, H.; Niu, L. Synthesis of Spinel Ferrite MFe2O4 (M = Co, Cu, Mn, and Zn) for Persulfate Activation to Remove Aqueous Organics: Effects of M-Site Metal and Synthetic Method. Front. Chem. 2020, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Su, C.; Miao, J.; Zhong, Y.; Shao, Z.; Wang, S.; Sun, H. Insights into perovskite-catalyzed peroxymonosulfate activation: Maneuverable cobalt sites for promoted evolution of sulfate radicals. Appl. Catal. B Environ. 2018, 220, 626–634. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhang, X.; Li, B.; Guo, R.; Cheng, Q.; Cheng, X. Synthesis of magnetic CuO/MnFe2O4 nanocompisite and its high activity for degradation of levofloxacin by activation of persulfate. Chem. Eng. J. 2019, 360, 848–860. [Google Scholar] [CrossRef]
- Dhanda, R.; Kidwai, M. Magnetically separable CuFe2O4/reduced graphene oxide nanocomposites: As a highly active catalyst for solvent free oxidative coupling of amines to imines. RSC Adv. 2016, 6, 53430–53437. [Google Scholar] [CrossRef]
- Wang, L.; Ma, X.; Huang, G.; Lian, R.; Huang, J.; She, H.; Wang, Q. Construction of ternary CuO/CuFe2O4/g-C3N4 composite and its enhanced photocatalytic degradation of tetracycline hydrochloride with persulfate under simulated sunlight. J. Environ. Sci. 2022, 112, 59–70. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, S.; Ding, J.; Deng, Z.; Guo, L.; Zhong, Q. Enhanced catalytic ozonation for NOx removal with CuFe2O4 nanoparticles and mechanism analysis. J. Mol. Catal. A Chem. 2016, 424, 153–161. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Masilko, J.; Kalina, L.; Tkacz, J.; Hajdúchová, M.; Enev, V. Structural, dielectric, electrical and magnetic properties of CuFe2O4 nanoparticles synthesized by honey mediated sol–gel combustion method and annealing effect. J. Mater. Sci. Mater. Electron. 2017, 28, 6245–6261. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Zhang, Z.; Cao, J. Highly Sensitive Acetone Gas Sensor Based on g-C3N4 Decorated MgFe2O4 Porous Microspheres Composites. Sensors 2018, 18, 2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Zhai, X.; Sun, T. Sustainable and efficient removal of paraben, oxytetracycline and metronidazole using magnetic porous biochar composite prepared by one step pyrolysis. Sep. Purif. Technol. 2022, 293, 121120. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Ozonation of parabens in aqueous solution: Kinetics and mechanism of degradation. Chemosphere 2010, 81, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef]
- Haag, W.R.; Hoigné, J. Ozonation of water containing chlorine or chloramines. Reaction products and kinetics. Water Res. 1983, 17, 1397–1402. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. The role of hydroxyl radical reactions in ozonation processes in aqueous solutions. Water Res. 1976, 10, 377–386. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Liu, X.; Jiang, X.; Zhang, Z.; Zhang, T.; Zhang, L. The investigation of synergistic and competitive interaction between dye Congo red and methyl blue on magnetic MnFe2O4. Chem. Eng. J. 2014, 246, 88–96. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J.; Zhou, Q. Heat-activated persulfate oxidation of atrazine: Implications for remediation of groundwater contaminated by herbicides. Chem. Eng. J. 2015, 263, 45–54. [Google Scholar] [CrossRef]
- Sadrnourmohamadi, M.; Gorczyca, B. Effects of ozone as a stand-alone and coagulation-aid treatment on the reduction of trihalomethanes precursors from high DOC and hardness water. Water Res. 2015, 73, 171–180. [Google Scholar] [CrossRef]
- APSP. ANSI/APSP/ICC-11 2019 American National Standard for Water Quality in Public Pools and Spas. Available online: https://issuu.com/thephta/docs/apsp-11_2019 (accessed on 25 March 2022).
- Kwon, B.G.; Lee, J.H. A Kinetic Method for HO2•/O2•− Determination in Advanced Oxidation Processes. Anal. Chem. 2004, 76, 6359–6364. [Google Scholar] [CrossRef]
- Sharma, J.; Mishra, I.M.; Kumar, V. Degradation and mineralization of Bisphenol A (BPA) in aqueous solution using advanced oxidation processes: UV/H2O2 and UV/S2O82− oxidation systems. J. Environ. Manag. 2015, 156, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.-F. Occurrence, fate and behavior of parabens in aquatic environments: A review. Water Res. 2015, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Geara, D.; Lorgeoux, C.; Bressy, A.; Zedek, S.; Rocher, V.; El Samrani, A.; Chebbo, G.; Moilleron, R. First assessment of triclosan, triclocarban and paraben mass loads at a very large regional scale: Case of Paris conurbation (France). Sci. Total Environ. 2014, 493, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Daghrir, R.; Dimboukou-Mpira, A.; Seyhi, B.; Drogui, P. Photosonochemical degradation of butyl-paraben: Optimization, toxicity and kinetic studies. Sci. Total Environ. 2014, 490, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Su, T.; Xie, X.; Qin, Z.; Ji, H. The Adsorption of Ozone on the Solid Catalyst Surface and the Catalytic Reaction Mechanism for Organic Components. ChemistrySelect 2020, 5, 15092–15116. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.; Peng, S.; Feng, Y.; Liu, Z. Enhanced mineralization of dimethyl phthalate by heterogeneous ozonation over nanostructured Cu-Fe-O surfaces: Synergistic effect and radical chain reactions. Sep. Purif. Technol. 2019, 209, 588–597. [Google Scholar] [CrossRef]
- Wang, X.; Min, J.; Li, S.; Zhu, X.; Cao, X.; Yuan, S.; Zuo, X.; Deng, X. Sono-assisted synthesis of CuO nanorods–graphene oxide as a synergistic activator of persulfate for bisphenol A removal. J. Environ. Chem. Eng. 2018, 6, 4078–4083. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.-K. Improving heterogeneous photo-Fenton catalytic degradation of toluene under visible light irradiation through Ba-doping in BiFeO3 nanoparticles. J. Mol. Catal. A Chem. 2016, 425, 199–207. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Nie, Y.; Yang, M.; Qu, J. Mechanism of Catalytic Ozonation in Fe2O3/Al2O3@SBA-15 Aqueous Suspension for Destruction of Ibuprofen. Environ. Sci. Technol. 2015, 49, 1690–1697. [Google Scholar] [CrossRef]
- Chuang, L.C.; Luo, C.H. Photocatalytic degradation of parabens in aquatic environment: Kinetics and degradation pathway. Kinet. Catal. 2015, 56, 412–418. [Google Scholar] [CrossRef]
- Asgari, E.; Esrafili, A.; Rostami, R.; Farzadkia, M. O3, O3/UV and O3/UV/ZnO for abatement of parabens in aqueous solutions: Effect of operational parameters and mineralization/biodegradability improvement. Process Saf. Environ. Prot. 2019, 125, 238–250. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.-L.; Li, J.; He, X.-J.; Cai, J.-C. Bioaccumulation and tolerance characteristics of a submerged plant (Ceratophyllum demersum L.) exposed to toxic metal lead. Ecotoxicol. Environ. Saf. 2015, 122, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Güzel Bayülken, D.; Ayaz Tüylü, B. In vitro genotoxic and cytotoxic effects of some paraben esters on human peripheral lymphocytes. Drug Chem. Toxicol. 2019, 42, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Moldéus, P. Mechanism of p-Hydroxybenzoate Ester-induced Mitochondrial Dysfunction and Cytotoxicity in Isolated Rat Hepatocytes. Biochem. Pharmacol. 1998, 55, 1907–1914. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattanateeradetch, A.; Sakulthaew, C.; Angkaew, A.; Sutjarit, S.; Poompoung, T.; Lin, Y.-T.; Harris, C.E.; Comfort, S.; Chokejaroenrat, C. Fabrication of Ternary Nanoparticles for Catalytic Ozonation to Treat Parabens: Mechanisms, Efficiency, and Effects on Ceratophyllum demersum L. and Eker Leiomyoma Tumor-3 Cells. Nanomaterials 2022, 12, 3573. https://doi.org/10.3390/nano12203573
Pattanateeradetch A, Sakulthaew C, Angkaew A, Sutjarit S, Poompoung T, Lin Y-T, Harris CE, Comfort S, Chokejaroenrat C. Fabrication of Ternary Nanoparticles for Catalytic Ozonation to Treat Parabens: Mechanisms, Efficiency, and Effects on Ceratophyllum demersum L. and Eker Leiomyoma Tumor-3 Cells. Nanomaterials. 2022; 12(20):3573. https://doi.org/10.3390/nano12203573
Chicago/Turabian StylePattanateeradetch, Apiladda, Chainarong Sakulthaew, Athaphon Angkaew, Samak Sutjarit, Thapanee Poompoung, Yao-Tung Lin, Clifford E. Harris, Steve Comfort, and Chanat Chokejaroenrat. 2022. "Fabrication of Ternary Nanoparticles for Catalytic Ozonation to Treat Parabens: Mechanisms, Efficiency, and Effects on Ceratophyllum demersum L. and Eker Leiomyoma Tumor-3 Cells" Nanomaterials 12, no. 20: 3573. https://doi.org/10.3390/nano12203573