Bright CsPbBr3 Perovskite Nanocrystals with Improved Stability by In-Situ Zn-Doping
Abstract
:1. Introduction
2. Materials and Methods
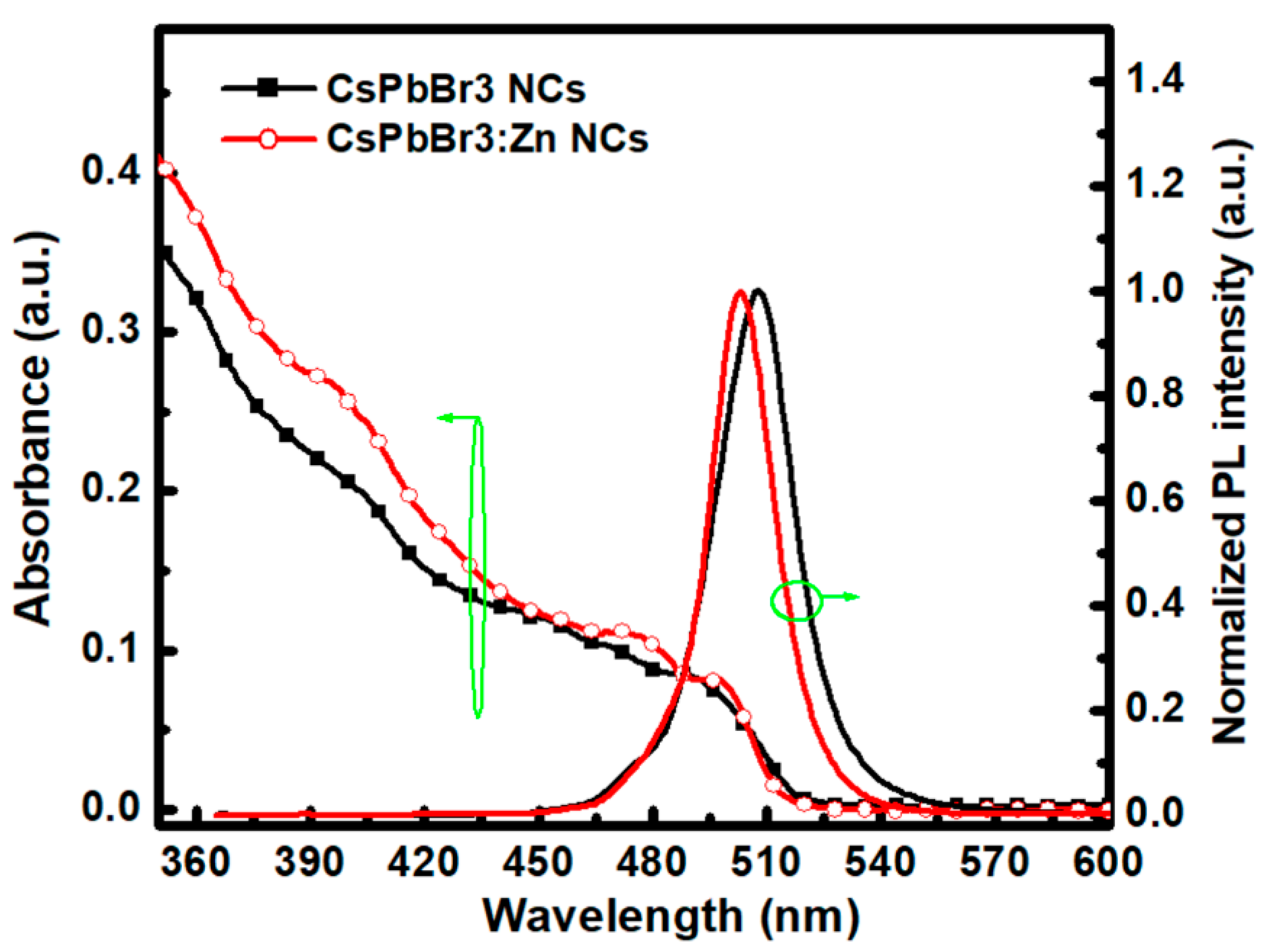
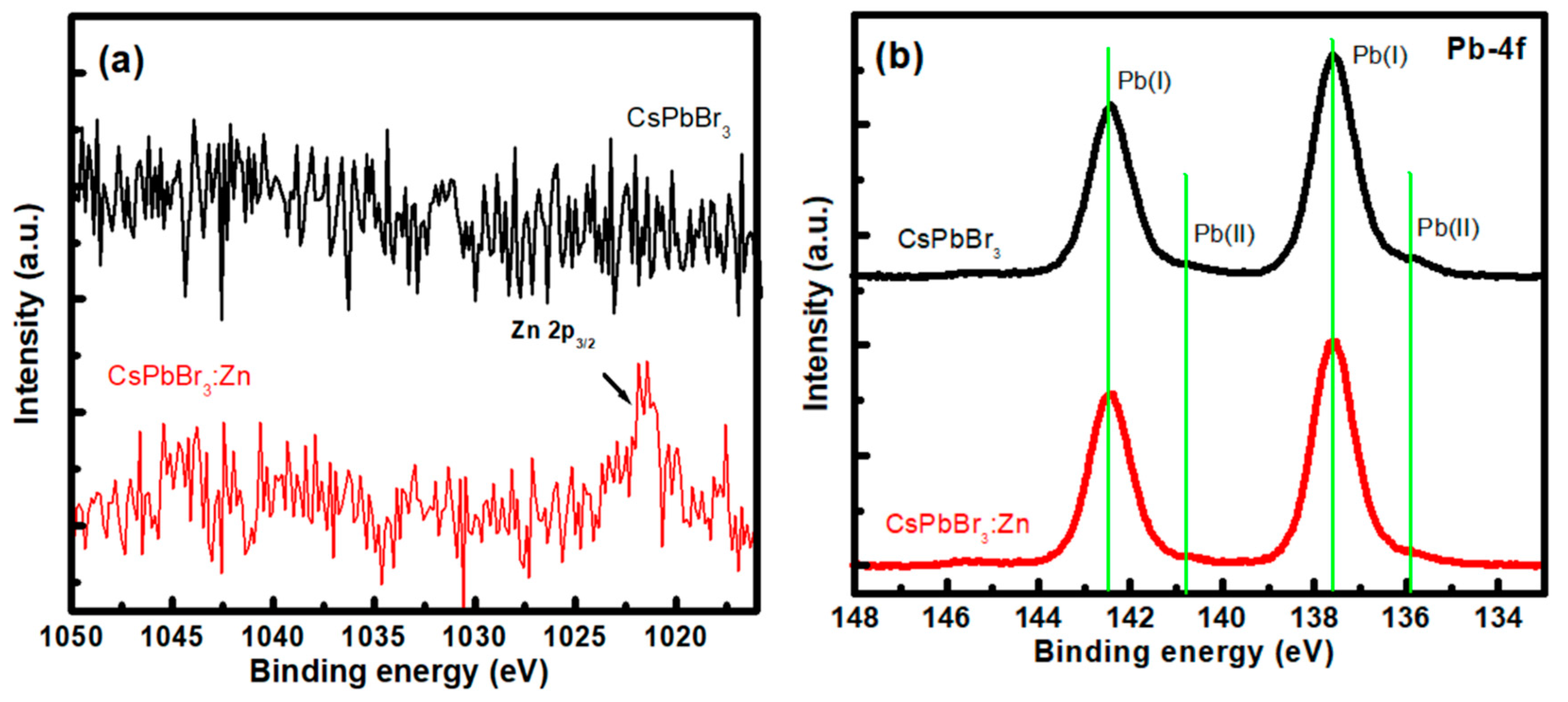
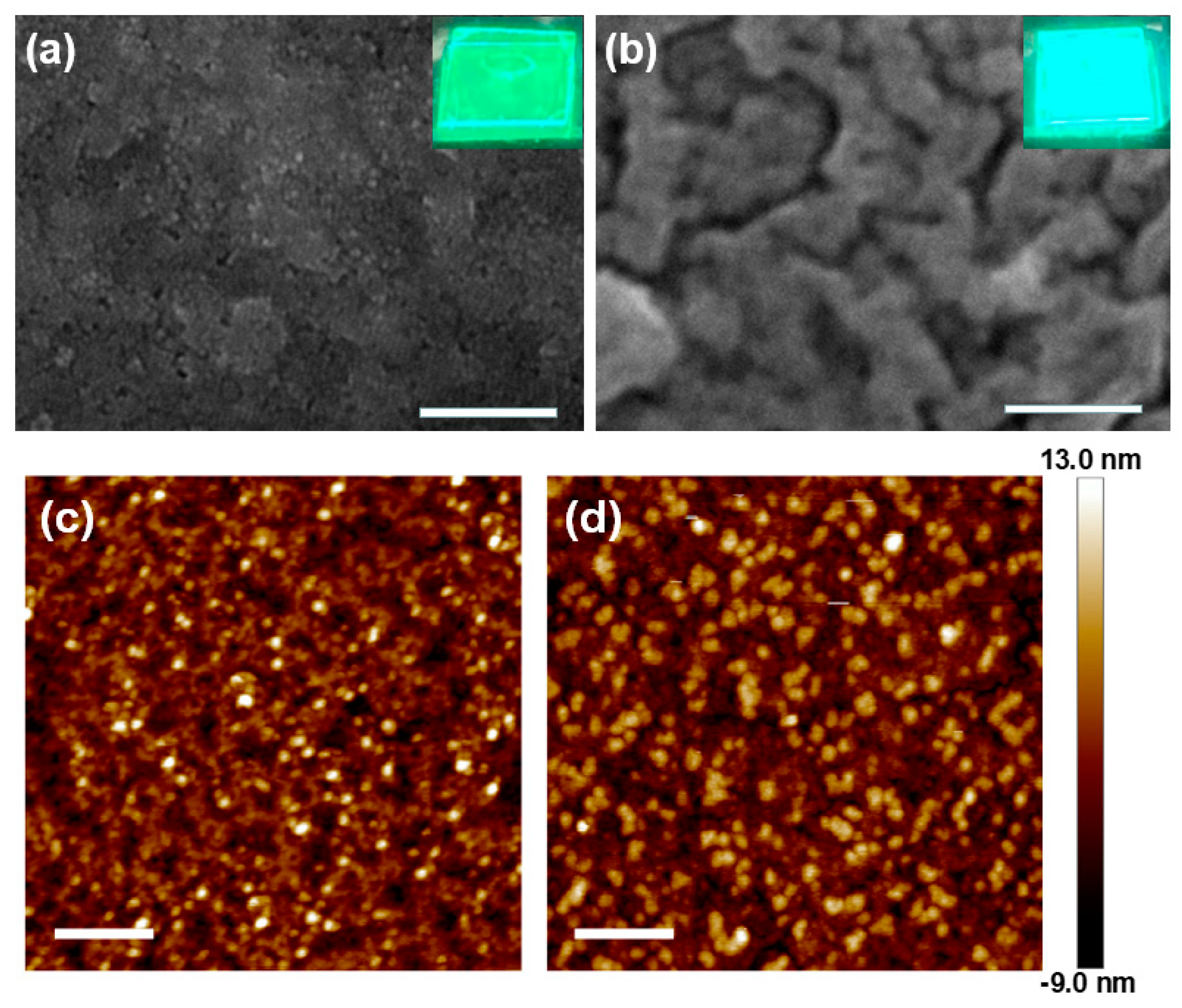
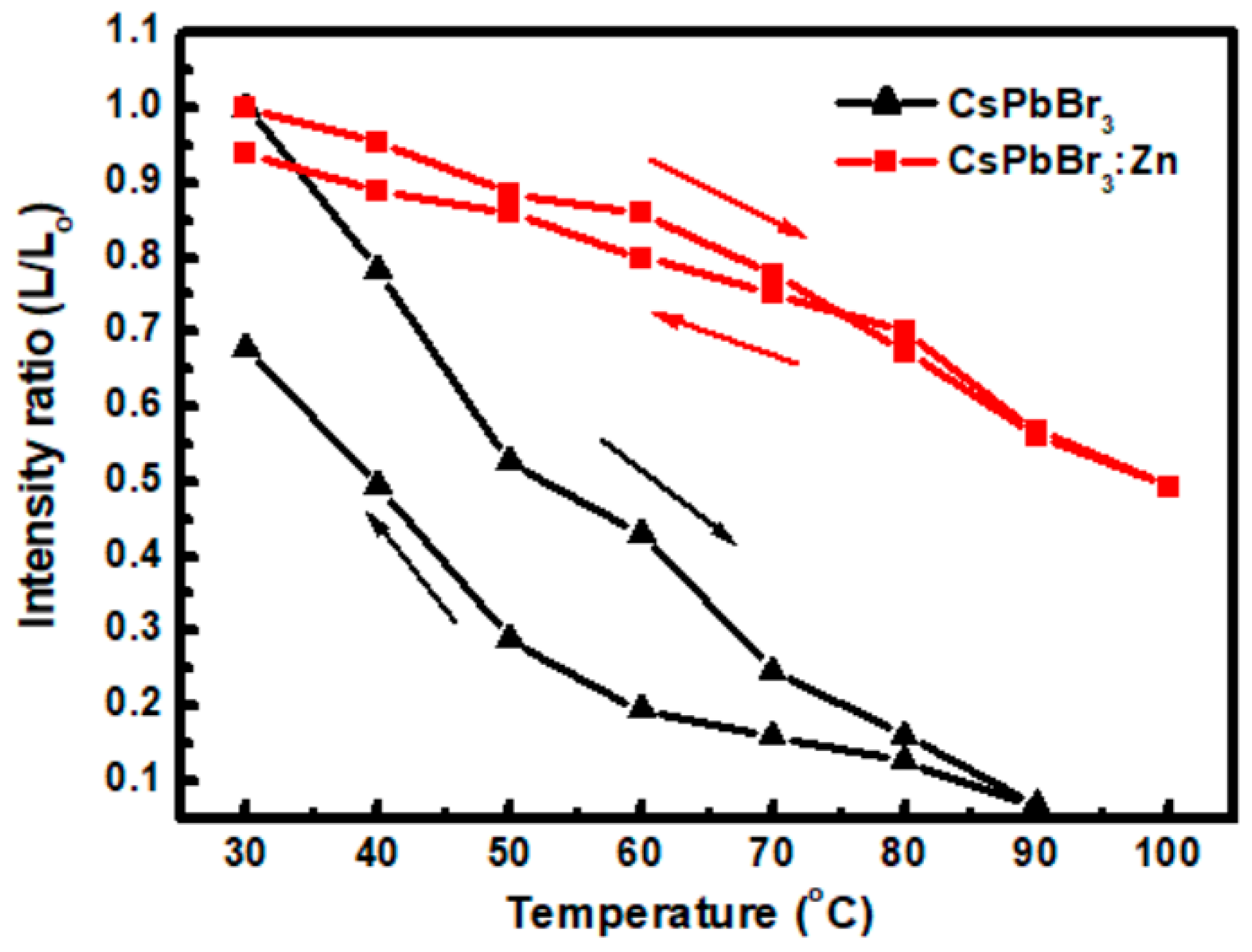
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiba, T.; Ishikawa, S.; Sato, J.; Takahashi, Y.; Ebe, H.; Ohisa, S.; Kido, J. Blue perovskite nanocrystal light-emitting devices via the ligand exchange with adamantane diamine. Adv. Opt. Mater. 2020, 8, 2000289. [Google Scholar] [CrossRef]
- Sahli, F.; Werner, J.; Kamino, B.A.; Boccard, M.; Nicolay, S.; Jeangros, Q.; Niesen, B.; Ballif, C. Fully textured monolithic perovskite/silicon tandem solar cells with 25.2% power conversion efficiency. Nat. Mater. 2018, 17, 820. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiu, W.; Peng, X.; Gu, Q.; Ma, F.; Yip, H.L.; Hou, L.; Qi, Z.; Su, S.J. Perovskite Light-Emitting Diodes with EQE Exceeding 28% through a Synergetic Dual-Additive Strategy for Defect Passivation and Nanostructure Regulation. Adv. Mater. 2021, 33, 2103268. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, S.J.; Liu, M.H.M. Hybridization of CsPbBr1.5I1.5 perovskite quantum dots with 9,9-dihexylfluorene co-oligomer for white electroluminescence. Org. Electron. 2017, 44, 6–10. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wu, C.C.; Wu, C.L.; Lin, C.W. CsPbBr3 perovskite powder, a robust and mass-producible single-source precursor: Synthesis, characterization, and optoelectronic applications. ACS Omega 2019, 4, 8081. [Google Scholar] [CrossRef] [Green Version]
- Chew, G.G.; Huang, W.W.; Chou, T.H.; Rasal, A.S.; Chang, J.Y. Brightly luminescent (NH4)xCs1-xPbBr3 quantum dots for in vitro imaging and efficient photothermal ablation therapy. J. Comp. Interface Sci. 2022, 605, 500. [Google Scholar]
- Tseng, Z.L.; Huang, Y.S.; Liu, Y.L.; Wu, T.L.; Wei, Y.J. Tetraoctylammonium bromide-passivated CsPbI3−xBrx perovskite nanoparticles with improved stability for efficient red light-emitting diodes. J. Alloys Comp. 2022, 897, 163182. [Google Scholar] [CrossRef]
- Gutiérrez Álvarez, S.; Lin, W.; Abdellah, M.; Meng, J.; Žídek, K.; Pullerits, T.; Zheng, K. Charge Carrier Diffusion Dynamics in Multisized Quaternary Alkylammonium-Capped CsPbBr3 Perovskite Nanocrystal Solids. ACS Appl. Mater. Interfaces 2021, 13, 44742. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, S.H.; Wu, C.L.; Wang, Z.H.; Yang, C.C. Cs4PbBr6/CsPbBr3 nanocomposites for all-inorganic electroluminescent perovskite light-emitting diodes. ACS Appl. Nano Mater. 2020, 3, 11760. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of metal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Huang, C.; Bi, Z.; Yu, H.; Shi, S.; Xu, X. Two-dimensional Bi2OS2 doping improves the performance and stability of perovskite solar cells. Chem. Eng. J. 2021, 420, 127700. [Google Scholar] [CrossRef]
- Vashishtha, P.; Griffith, B.E.; Brown, A.A.M.; Hooper, T.J.N.; Fang, Y.; Ansari, M.S.; Bruno, A.; White, T.; Hanna, J.V. Performance Enhanced Light-Emitting Diodes Fabricated from Nanocrystalline CsPbBr3 with In Situ Zn2+ Addition. ACS Appl. Electron. Mater. 2020, 2, 4002. [Google Scholar] [CrossRef]
- Ma, H.H.; Imran, M.; Dang, Z.; Hu, Z. Growth of Metal Halide Perovskite, from Nanocrystal to Micron-Scale Crystal: A Review. Crystals 2018, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Zu, Y.; Dai, J.; Li, L.; Yuan, F.; Chen, X.; Feng, Z.; Li, K.; Hou, X.; Ju, M.; Wu, Z. Ultra-stable CsPbBr3 nanocrystals with near-unity photoluminescence quantum yield via postsynthetic surface engineering. J. Mater. Chem. A 2019, 7, 26116. [Google Scholar] [CrossRef]
- Dang, Z.; Shamsi, J.; Palazon, F.; Imran, M.; Akkerman, Q.A.; Park, S.; Bertoni, G.; Prato, M.; Brescia, R.; Manna, L. In Situ Transmission Electron Microscopy Study of Electron Beam-Induced Transformations in Colloidal Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2017, 11, 2124. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I.J. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content. J. Am. Chem. Soc. 2016, 138, 14954. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Pan, G.; Chen, X.; Li, D.; Xu, W.; Bai, X.; Song, H. Cerium and Ytterbium Codoped Halide Perovskite Quantum Dots: A Novel and Efficient Downconverter for Improving the Performance of Silicon Solar Cells. Adv. Mater. 2017, 29, 1704149. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Han, B.; Zeng, H. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2016, 29, 1603885. [Google Scholar] [CrossRef]
- Chen, J.; Žídek, K.; Chábera, P.; Liu, D.; Cheng, P.; Nuuttila, L.; Al-Marri, M.J.; Lehtivuori, H.; Messing, M.E.; Han, K.; et al. Size-And Wavelength-Dependent Two-Photon Absorption Cross-Section of CsPbBr3 Perovskite Quantum Dots. J. Phys. Chem. Lett. 2017, 8, 2316. [Google Scholar] [CrossRef]
- Kajal, S.; Kim, J.; Shin, Y.S.; Singh, A.N.; Myung, C.W.; Kim, J.Y.; Kim, K.S. Unfolding the Influence of Metal Doping on Properties of CsPbI3 Perovskite. Small Methods 2020, 4, 2000296. [Google Scholar] [CrossRef]
- Bao, Z.; Wang, W.; Tsai, H.; Wang, H.; Chen, S.; Liu, R.S. Photo-/electro-luminescence enhancement of CsPbX3 (X = Cl, Br, or I) perovskite quantum dots via thiocyanate surface modification. J. Mater. Chem. C 2020, 8, 1065. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, R.; Wan, W.; Jing, X.; Wen, T.; Ye, S. Stabilizing CsPbBr3 quantum dots with conjugated aromatic ligands and their regulated optical behaviors. Chem. Eng. J. 2020, 289, 124453. [Google Scholar] [CrossRef]
- Nakahara, S.; Tahara, H.; Yumoto, G.; Kawawaki, T.; Saruyama, M.; Sato, R.; Teranishi, T.; Kanemitsu, Y. Suppression of Trion Formation in CsPbBr3 Perovskite Nanocrystals by Postsynthetic Surface Modification. J. Phys. Chem. C 2018, 38, 22188. [Google Scholar] [CrossRef]
- Akbali, B.; Topcu, G.; Guner, T.; Ozcan, M.; Demir, M.M.; Sahin, H. CsPbBr3 perovskites: Theoretical and experimental investigation on water-assisted transition from nanowire formation to degradation. Phys. Rev. Mater. 2018, 2, 034601. [Google Scholar] [CrossRef] [Green Version]
- Ghaithan, H.M.; Alahmed, Z.A.; Qaid, S.M.H.; Hezam, M.; Aldwayyan, A.S. Density Functional Study of Cubic, Tetragonal, and Orthorhombic CsPbBr3 Perovskite. ACS Omega 2020, 5, 7468. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.-T.; Li, Z.-R.; Chang, S.-P.; Ansay, A.; Wang, Z.-H.; Huang, C.-Y. Bright CsPbBr3 Perovskite Nanocrystals with Improved Stability by In-Situ Zn-Doping. Nanomaterials 2022, 12, 759. https://doi.org/10.3390/nano12050759
Zeng Y-T, Li Z-R, Chang S-P, Ansay A, Wang Z-H, Huang C-Y. Bright CsPbBr3 Perovskite Nanocrystals with Improved Stability by In-Situ Zn-Doping. Nanomaterials. 2022; 12(5):759. https://doi.org/10.3390/nano12050759
Chicago/Turabian StyleZeng, Yong-Tang, Zhan-Rong Li, Sheng-Po Chang, Arjun Ansay, Zi-Hao Wang, and Chun-Yuan Huang. 2022. "Bright CsPbBr3 Perovskite Nanocrystals with Improved Stability by In-Situ Zn-Doping" Nanomaterials 12, no. 5: 759. https://doi.org/10.3390/nano12050759





