2D MXene Nanosheets with ROS Scavenging Ability Effectively Delay Osteoarthritis Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Ti3C2Tx Nanosheets Characterization
2.3. Cell Viability Test
2.4. Lived/Dead Cell Staining
2.5. Antioxidant Efficiency of Ti3C2Tx Nanosheets
2.6. Quantitative Real-Time Polymerase Chain Reaction
2.7. Immunofluorescence Staining
2.8. RNA Sequencing
2.9. Western Blotting
2.10. Induction of Rat OA Model
2.11. Statistical Analysis
3. Results
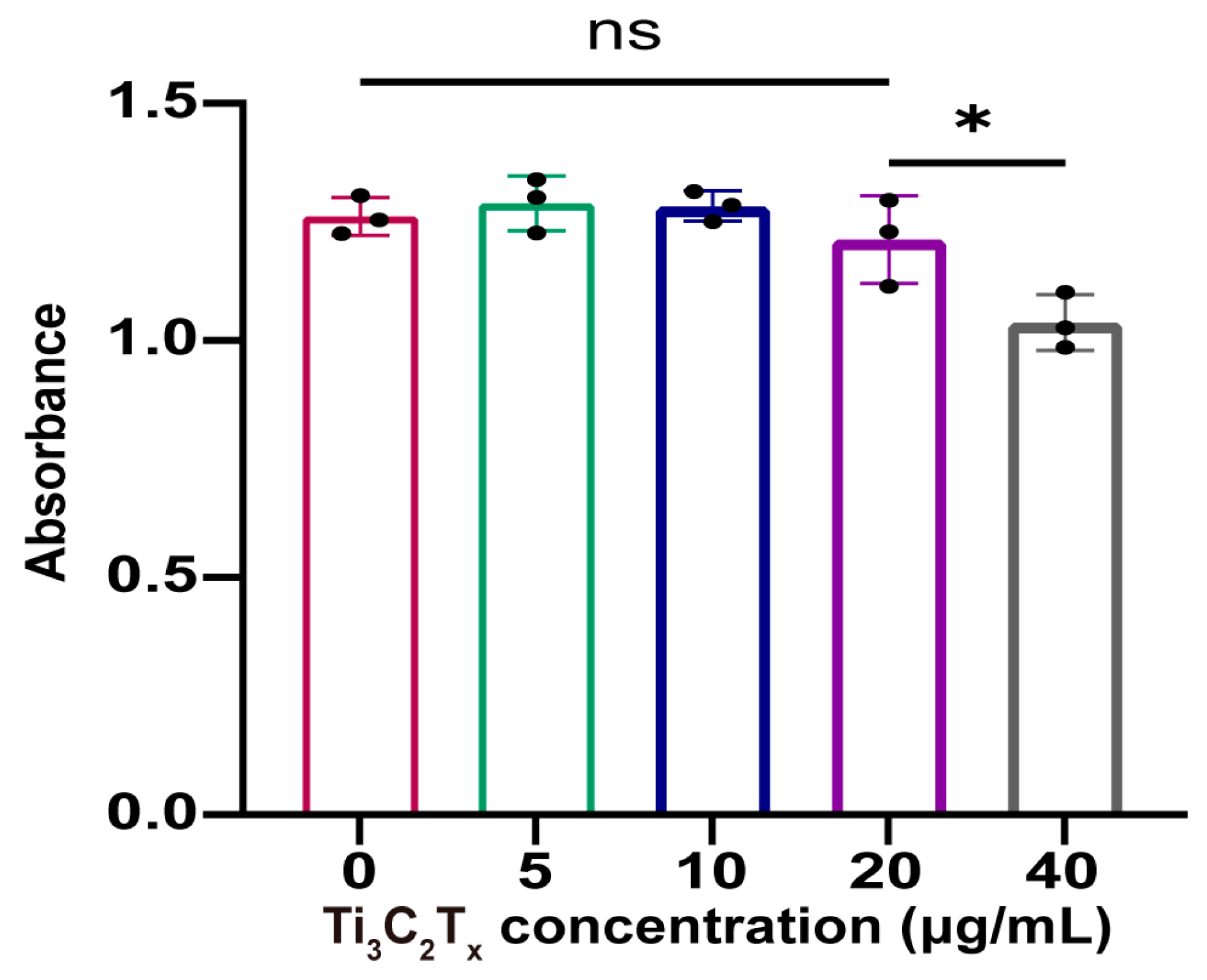
3.1. Characterization of Ti3C2Tx Nanosheets
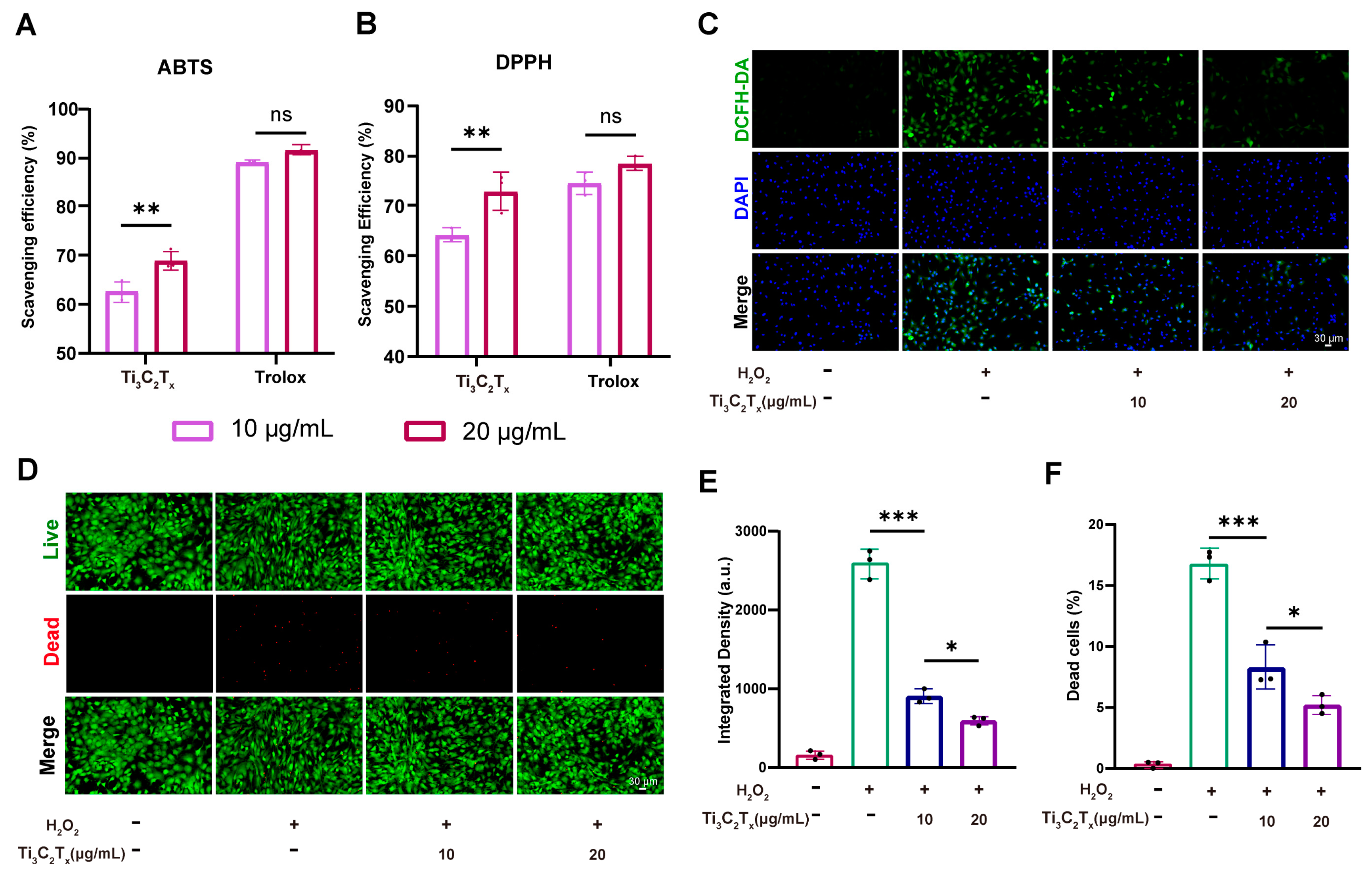
3.2. Cytocompatibility and ROS Scavenging Ability of Ti3C2Tx Nanosheets
3.3. Ti3C2Tx Nanosheets Restored the Cellular Homeostasis and Inhibited Inflammation
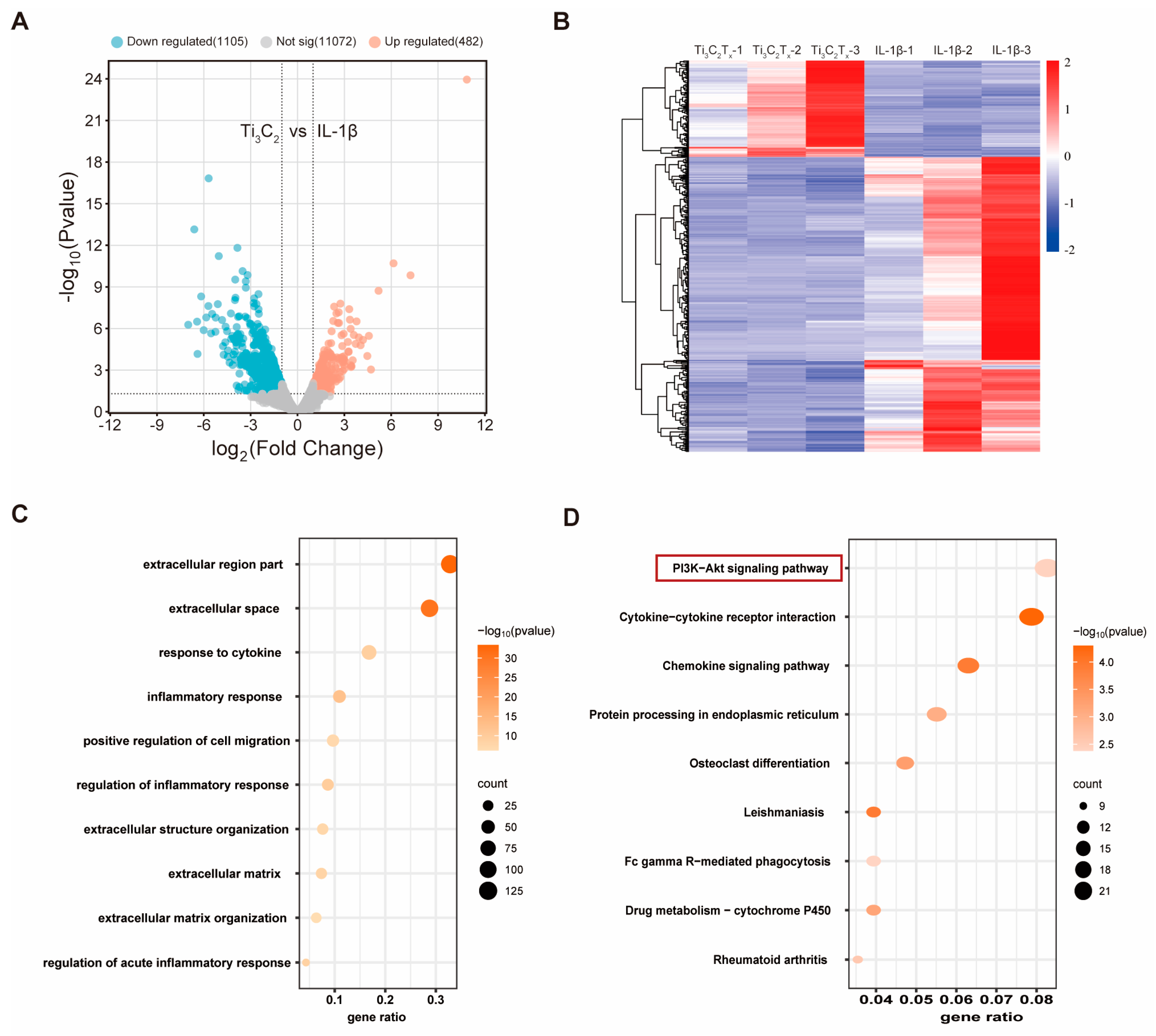
3.4. RNA Sequencing Analysis of Ti3C2Tx Nanosheets against IL-1β-Challenged Chondrocytes
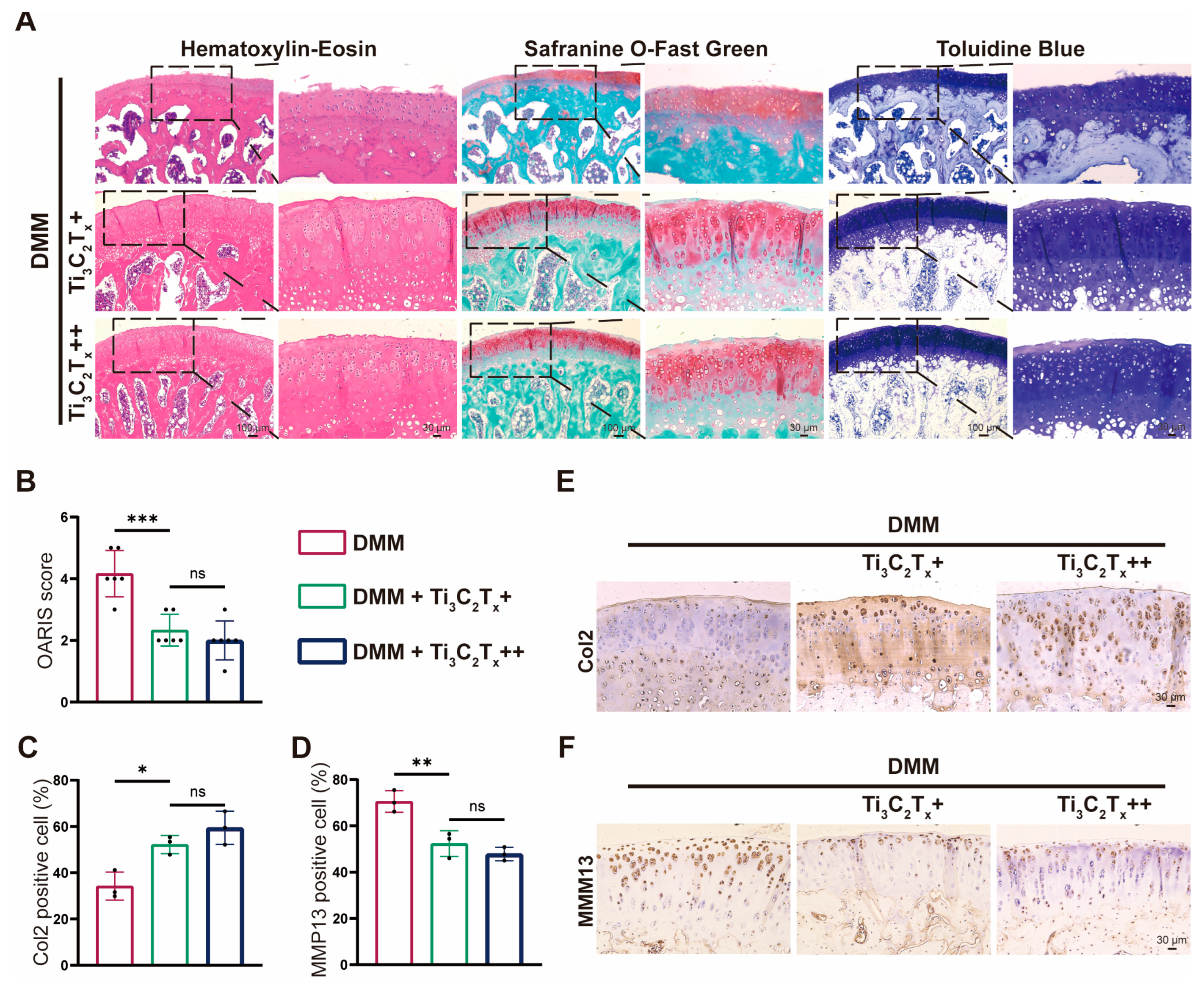
3.5. Ti3C2Tx Nanosheets Effectively Ameliorated OA Progression In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sohn, H.S.; Choi, J.W.; Jhun, J.; Kwon, S.P.; Jung, M.; Yong, S.; Na, H.S.; Kim, J.H.; Cho, M.L.; Kim, B.S. Tolerogenic nanoparticles induce type II collagen-specific regulatory T cells and ameliorate osteoarthritis. Sci. Adv. 2022, 8, eabo5284. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Sun, Q.; Dong, Y.; Lu, H.; Li, W.; Lin, Z.; Li, K.; Cheng, J.; Liu, Z.; Qi, J.; et al. Calcitriol-Loaded Multifunctional Nanospheres with Superlubricity for Advanced Osteoarthritis Treatment. ACS Nano 2023, 17, 12842–12861. [Google Scholar] [CrossRef]
- Boer, C.G.; Hatzikotoulas, K.; Southam, L.; Stefánsdóttir, L.; Zhang, Y.; Coutinho de Almeida, R.; Wu, T.T.; Zheng, J.; Hartley, A.; Teder-Laving, M.; et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell 2021, 184, 4784–4818.e4717. [Google Scholar] [CrossRef]
- Weng, Q.; Chen, Q.; Jiang, T.; Zhang, Y.; Zhang, W.; Doherty, M.; Xie, J.; Liu, K.; Li, J.; Yang, T.; et al. Global burden of early-onset osteoarthritis, 1990–2019: Results from the Global Burden of Disease Study 2019. Ann. Rheum. Dis. 2024, 83, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Hunter, D.; Zeng, C.; Lei, G. The gut microbiome-joint axis in osteoarthritis. Sci. Bull. 2023, 68, 759–762. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Yang, J.; Han, Y.; Lin, J.; Zhu, Y.; Wang, F.; Deng, L.; Zhang, H.; Xu, X.; Cui, W. Ball-Bearing-Inspired Polyampholyte-Modified Microspheres as Bio-Lubricants Attenuate Osteoarthritis. Small 2020, 16, e2004519. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Zhao, W.; Wang, H.; Sun, Y.; Chen, Y.; Luo, J.; Deng, L.; Xu, X.; Cui, W.; et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021, 6, 3596–3607. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Y.; Liu, Y.; Liu, Q.; Deng, S.; Liu, Y.; Cui, X.; Liang, J.; Zhang, X.; Fan, Y.; et al. Injectable Microgels with Hybrid Exosomes of Chondrocyte-Targeted FGF18 Gene-Editing and Self-Renewable Lubrication for Osteoarthritis Therapy. Adv. Mater. 2024, 36, e2312559. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Liu, Q.; Yan, J.; Pan, D.; Wang, L.; Xu, Y.; Wang, F.; Liu, Y.; Li, X.; et al. ROS-Responsive Boronate-Stabilized Polyphenol-Poloxamer 188 Assembled Dexamethasone Nanodrug for Macrophage Repolarization in Osteoarthritis Treatment. Adv. Healthc. Mater. 2021, 10, e2100883. [Google Scholar] [CrossRef]
- Yu, H.; Ren, P.; Pan, X.; Zhang, X.; Ma, J.; Chen, J.; Sheng, J.; Luo, H.; Lu, H.; Chen, G. Intracellular Delivery of Itaconate by Metal-Organic Framework-Anchored Hydrogel Microspheres for Osteoarthritis Therapy. Pharmaceutics 2023, 15, 724. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.C.; Wang, S.; Padera, R.F., Jr.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ye, T.; Hu, F.; Chen, K.; Li, B.; Qiu, M.; Chen, Z.; Sun, Y.; Ye, W.; Wang, H.; et al. Urchin-like ceria nanoparticles for enhanced gene therapy of osteoarthritis. Sci. Adv. 2023, 9, eadf0988. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, C.; Xiong, R.; Lin, Y.; Cui, Z.; Tu, Y.; Zhang, M.; Sa, B.; Shao, J. Multifunctional Theranostic 2D Vanadium Carbidel for Enhanced Cancer Immunotherapy. Adv. Funct. Mater. 2024, 2406529. [Google Scholar] [CrossRef]
- Adomaviciute-Grabusove, S.; Popov, A.; Ramanavicius, S.; Sablinskas, V.; Shevchuk, K.; Gogotsi, O.; Baginskiy, I.; Gogotsi, Y.; Ramanavicius, A. Monitoring Ti(3)C(2)T(x) MXene Degradation Pathways Using Raman Spectroscopy. ACS Nano 2024, 18, 13184–13195. [Google Scholar] [CrossRef]
- Vasyukova, I.A.; Zakharova, O.V.; Kuznetsov, D.V.; Gusev, A.A. Synthesis, Toxicity Assessment, Environmental and Biomedical Applications of MXenes: A Review. Nanomaterials 2022, 12, 1797. [Google Scholar] [CrossRef]
- Murali, A.; Lokhande, G.; Deo, K.A.; Brokesh, A.; Gaharwar, A.K. Emerging 2D Nanomaterials for Biomedical Applications. Mater. Today 2021, 50, 276–302. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, S.; Vijayan, V.; Lee, J.S.; Park, S.S.; Cui, X.; Chung, I.; Lee, J.; Ahn, S.K.; Kim, J.R.; et al. ROS- and pH-Responsive Polydopamine Functionalized Ti(3)C(2)T(x) MXene-Based Nanoparticles as Drug Delivery Nanocarriers with High Antibacterial Activity. Nanomaterials 2022, 12, 4392. [Google Scholar] [CrossRef]
- Soleymaniha, M.; Shahbazi, M.A.; Rafieerad, A.R.; Maleki, A.; Amiri, A. Promoting Role of MXene Nanosheets in Biomedical Sciences: Therapeutic and Biosensing Innovations. Adv. Healthc. Mater. 2019, 8, e1801137. [Google Scholar] [CrossRef]
- Feng, W.; Han, X.; Hu, H.; Chang, M.; Ding, L.; Xiang, H.; Chen, Y.; Li, Y. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nat. Commun. 2021, 12, 2203. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.Y.; Li, J.M.; Peng, L.M.; Tang, C.Y.; Zha, X.J.; Ke, K.; Yang, M.B.; Su, B.H.; Yang, W. Redox-Mediated Artificial Non-Enzymatic Antioxidant MXene Nanoplatforms for Acute Kidney Injury Alleviation. Adv. Sci. 2021, 8, e2101498. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Guo, Y.; Xie, C.; Li, S.; Liu, Z.; Lei, B. Photoactivated MXene Nanosheets for Integrated Bone-Soft Tissue Therapy: Effect and Potential Mechanism. ACS Nano 2023, 17, 7229–7240. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhao, J.; Li, B.; Cai, P.; Loh, X.J.; Xu, C.; Chen, P.; Kai, D.; Zheng, L. Implantable and degradable antioxidant poly(ε-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials 2020, 230, 119601. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Shen, J.; Cai, Z.; Zhao, C.; Chen, H.; Luo, X.; Hu, N.; Cui, W.; Huang, W. Injectable hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis. Sci. Adv. 2022, 8, eabl6449. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Sun, H.; Zhang, H.; Kang, H.; Wang, P.; Xin, Q.; Ding, C.; Xie, J.; Li, J. Mimicking Antioxidases and Hyaluronan Synthase: A Zwitterionic Nanozyme for Photothermal Therapy of Osteoarthritis. Adv. Mater. 2023, 35, e2303299. [Google Scholar] [CrossRef]
- Lu, H.; Wei, J.; Liu, K.; Li, Z.; Xu, T.; Yang, D.; Gao, Q.; Xiang, H.; Li, G.; Chen, Y. Radical-Scavenging and Subchondral Bone-Regenerating Nanomedicine for Osteoarthritis Treatment. ACS Nano 2023, 17, 6131–6146. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, D.; Pan, J.; Liu, D.; Fu, J.; Fan, H. Lubricating MXenzyme-based hybrid hydrogel reverses oxidative damage to alleviate osteoarthritis. Chem. Eng. J. 2024, 482, 148815. [Google Scholar] [CrossRef]
- Kang, M.S.; Jang, H.J.; Jo, H.J.; Raja, I.S.; Han, D.W. MXene and Xene: Promising frontier beyond graphene in tissue engineering and regenerative medicine. Nanoscale Horiz. 2023, 9, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xia, L.; Ye, X.; Xu, J.; Liu, T.; Wang, L.; Zhang, S.; Feng, W.; Du, D.; Chen, Y. Ultrathin Niobium Carbide MXenzyme for Remedying Hypertension by Antioxidative and Neuroprotective Actions. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303539. [Google Scholar] [CrossRef]
- Arra, M.; Swarnkar, G.; Ke, K.; Otero, J.E.; Ying, J.; Duan, X.; Maruyama, T.; Rai, M.F.; O’Keefe, R.J.; Mbalaviele, G.; et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 2020, 11, 3427. [Google Scholar] [CrossRef]
- Yu, B.; Sun, W.; Lin, J.; Fan, C.; Wang, C.; Zhang, Z.; Wang, Y.; Tang, Y.; Lin, Y.; Zhou, D. Using Cu-Based Metal-Organic Framework as a Comprehensive and Powerful Antioxidant Nanozyme for Efficient Osteoarthritis Treatment. Adv. Sci. 2024, 11, e2307798. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.B.; Tuan, R.S. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: Identifying molecular targets. PM R 2011, 3 (Suppl. 1), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef]
- Varga, G.; Gattorno, M.; Foell, D.; Rubartelli, A. Redox distress and genetic defects conspire in systemic autoinflammatory diseases. Nat. Rev. Rheumatol. 2015, 11, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Wang, Y.; Hemmings, B.A.; Rüegg, C.; Xue, G. PKB/Akt-dependent regulation of inflammation in cancer. Semin. Cancer Biol. 2018, 48, 62–69. [Google Scholar] [CrossRef]
- Cravero, J.D.; Carlson, C.S.; Im, H.J.; Yammani, R.R.; Long, D.; Loeser, R.F. Increased expression of the Akt/PKB inhibitor TRB3 in osteoarthritic chondrocytes inhibits insulin-like growth factor 1-mediated cell survival and proteoglycan synthesis. Arthritis Rheum. 2009, 60, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Rosa, S.C.; Rufino, A.T.; Judas, F.; Tenreiro, C.; Lopes, M.C.; Mendes, A.F. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: Modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthr. Cartil. 2011, 19, 719–727. [Google Scholar] [CrossRef]
- Iwasa, K.; Hayashi, S.; Fujishiro, T.; Kanzaki, N.; Hashimoto, S.; Sakata, S.; Chinzei, N.; Nishiyama, T.; Kuroda, R.; Kurosaka, M. PTEN regulates matrix synthesis in adult human chondrocytes under oxidative stress. J. Orthop. Res. 2014, 32, 231–237. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, S.; Li, Y.; Xu, J.; Yan, W.; Guo, B.; Xu, J.; Jiang, L.; Zhang, Y.; Wei, H.; et al. Engineered MgO nanoparticles for cartilage-bone synergistic therapy. Sci. Adv. 2024, 10, eadk6084. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Li, Y.; Zhang, C.; Fang, G. Recent Advances in Photothermal Therapy at Near-Infrared-II Based on 2D MXenes. Small 2024, 20, e2305645. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Rethinasabapathy, M.; Jeon, S.; Jeong, J.; Kim, E.; Lee, S.; Kim, S.; Kim, G.; Ha, Y.; Bae, E.; et al. Role of reactive oxygen species in the toxicity of two-dimensional nanomaterials: A study on layered Ti3C2 MXenes. Nano Today 2023, 51, 101925. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Ma, N.L.; Morsin, M.; Nayan, N.; Ahmad, M.K.; Tee, K.S. Cytotoxicity of MXene-based nanomaterials for biomedical applications: A mini review. Environ. Res. 2021, 201, 111592. [Google Scholar] [CrossRef]
- Lee, G.S.; Yun, T.; Kim, H.; Kim, I.H.; Choi, J.; Lee, S.H.; Lee, H.J.; Hwang, H.S.; Kim, J.G.; Kim, D.W.; et al. Mussel Inspired Highly Aligned Ti(3)C(2)T(x) MXene Film with Synergistic Enhancement of Mechanical Strength and Ambient Stability. ACS Nano 2020, 14, 11722–11732. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Wang, T.; Fang, X.; Xu, T.; Li, J.; Jing, S.; Chen, G.; Liu, Y.; Sheng, G. 2D MXene Nanosheets with ROS Scavenging Ability Effectively Delay Osteoarthritis Progression. Nanomaterials 2024, 14, 1572. https://doi.org/10.3390/nano14191572
Zhao H, Wang T, Fang X, Xu T, Li J, Jing S, Chen G, Liu Y, Sheng G. 2D MXene Nanosheets with ROS Scavenging Ability Effectively Delay Osteoarthritis Progression. Nanomaterials. 2024; 14(19):1572. https://doi.org/10.3390/nano14191572
Chicago/Turabian StyleZhao, Hongqi, Tianqi Wang, Xuan Fang, Tao Xu, Jian Li, Shaoze Jing, Guangzi Chen, Yang Liu, and Gaohong Sheng. 2024. "2D MXene Nanosheets with ROS Scavenging Ability Effectively Delay Osteoarthritis Progression" Nanomaterials 14, no. 19: 1572. https://doi.org/10.3390/nano14191572







