Enhanced Photocatalytic Paracetamol Degradation by NiCu-Modified TiO2 Nanotubes: Mechanistic Insights and Performance Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. TiO2 Nanotube Array Fabrication
2.2. Sputter Deposition of Ni, Cu and NiCu Thin Films
2.3. Characterization of Materials
2.4. Photocatalytic Tests and Mechanistic Studies
3. Results and Discussion
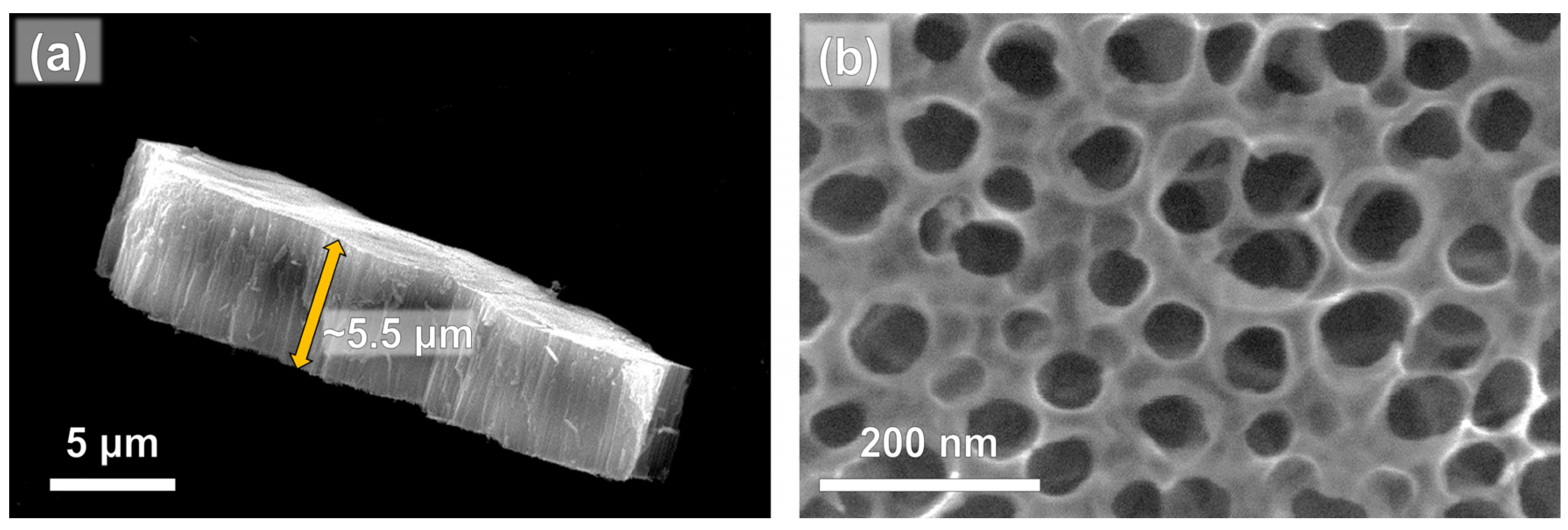
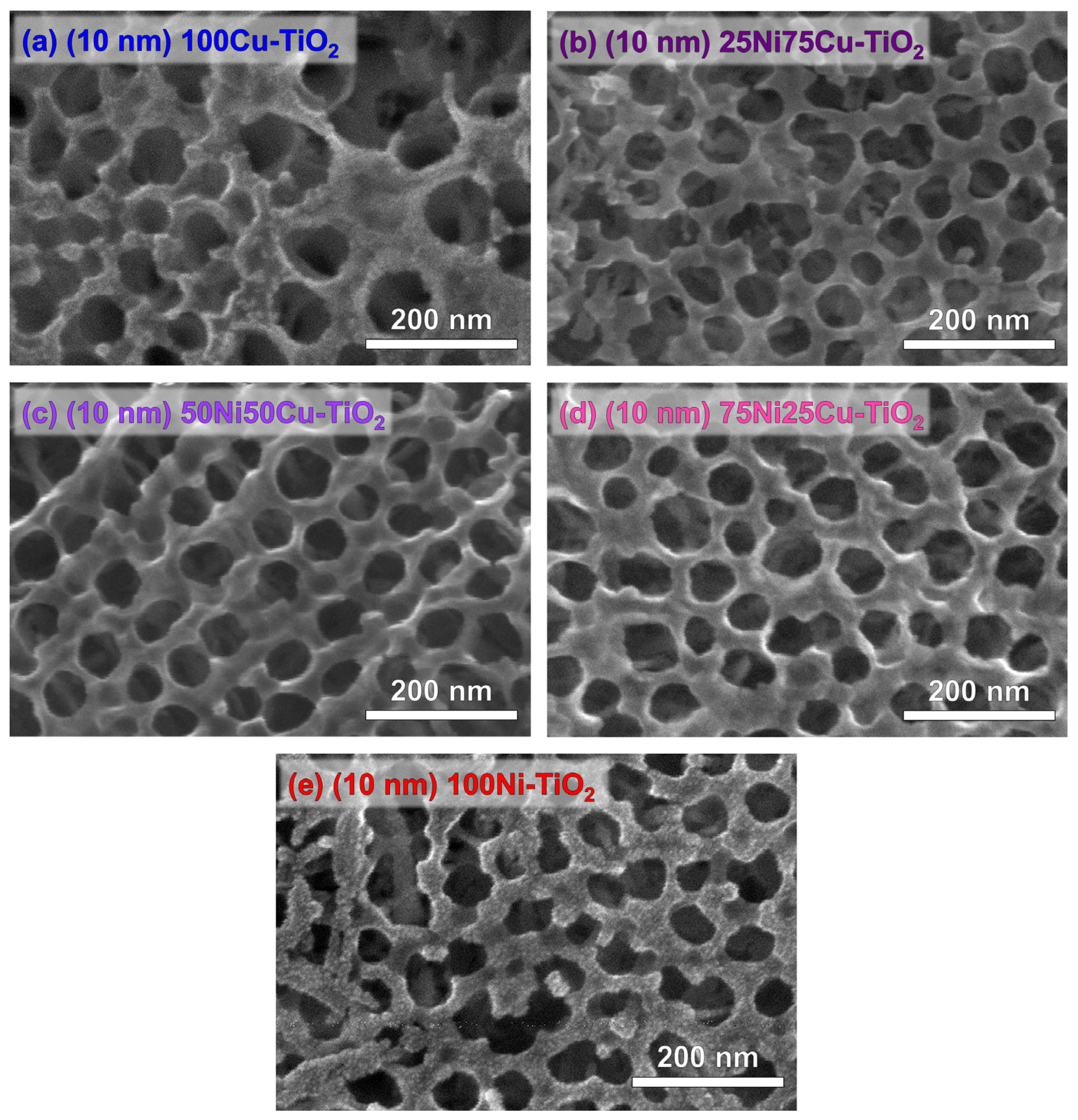
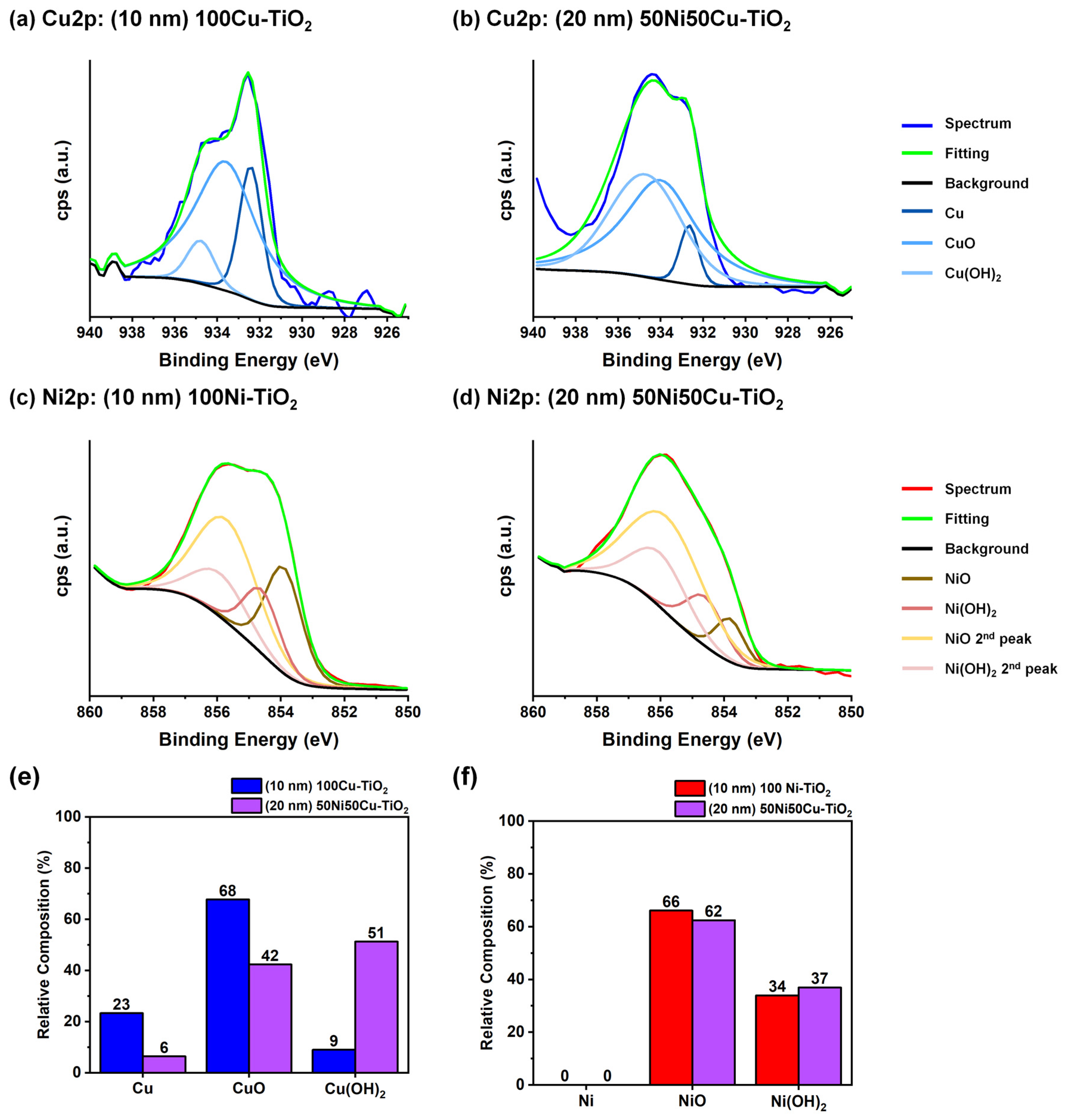
3.1. Photocatalysts Characterization
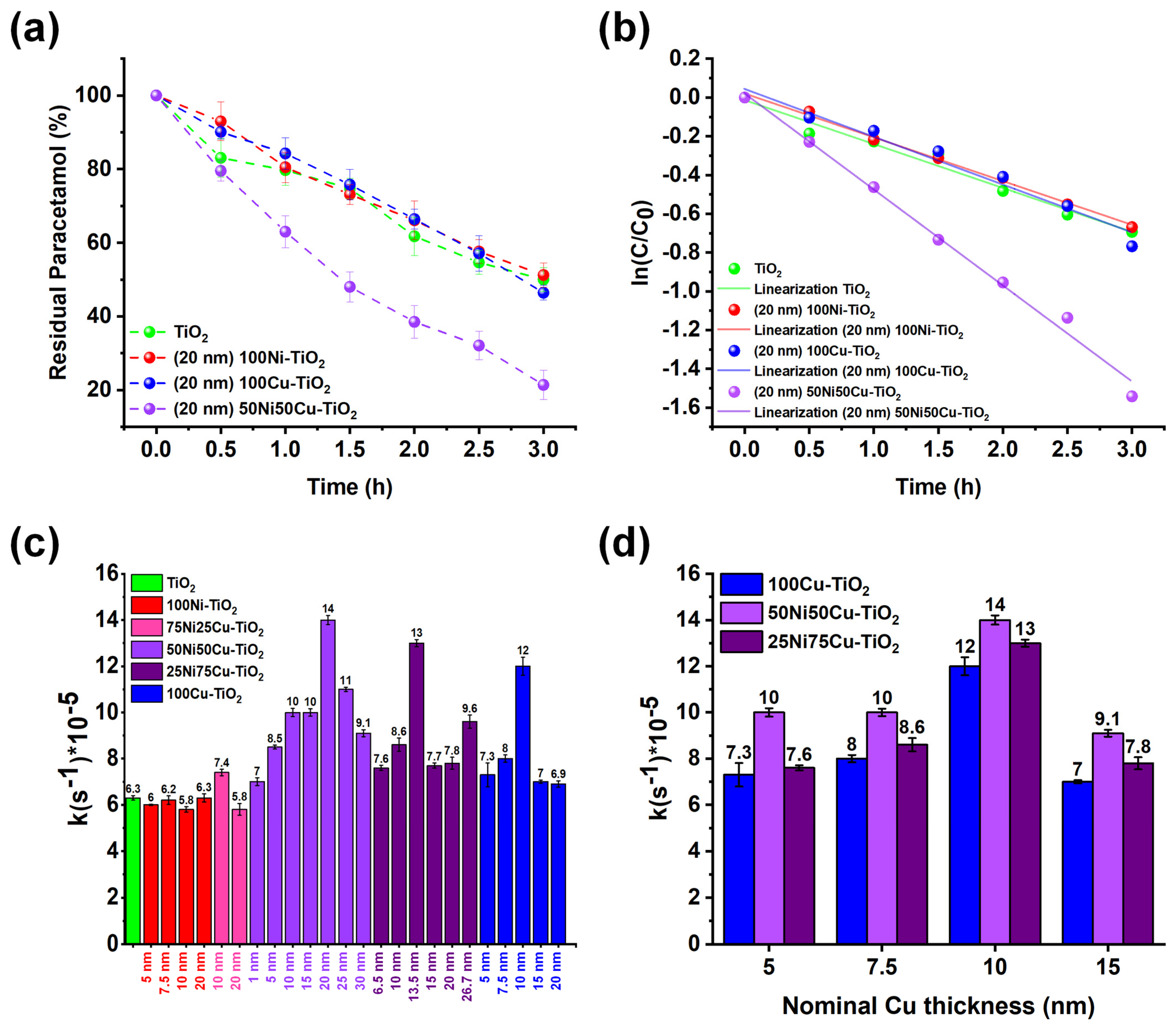
3.2. Photocatalytic Experiments: The Effect of the Co-Catalyst
3.3. Mechanistic Insights
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liyanage, C.; Yamada, K. Impact of Population Growth on the Water Quality of Natural Water Bodies. Sustainability 2017, 9, 1405. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C.; et al. Removal of Emerging Contaminants from Wastewater Using Advanced Treatments. A Review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current Research Trends on Emerging Contaminants Pharmaceutical and Personal Care Products (PPCPs): A Comprehensive Review. Sci. Total Environ. 2023, 859, 160031. [Google Scholar] [CrossRef] [PubMed]
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and Personal Care Products in Waters: Occurrence, Toxicity, and Risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef]
- Canna-Michaelidou, S.; Nicolaou, A.-S. Evaluation of the Genotoxicity Potential (by MutatoxTM Test) of Ten Pesticides Found as Water Pollutants in Cyprus. Sci. Total Environ. 1996, 193, 27–35. [Google Scholar] [CrossRef]
- Bayabil, H.K.; Teshome, F.T.; Li, Y.C. Emerging Contaminants in Soil and Water. Front. Environ. Sci. 2022, 10, 873499. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging Pollutants of Severe Environmental Concern in Water and Wastewater: A Comprehensive Review on Current Developments and Future Research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Singh, D.S.H.; Godson, P.S.; Thanga, S.G. Emerging Pollutants: Impact on Environment, Management, and Challenges. Environ. Sci. Pollut. Res. 2022, 29, 72309–72311. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Lozano-Morales, S.A.; Morales, G.; López Zavala, M.Á.; Arce-Sarria, A.; Machuca-Martínez, F. Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of PH. Processes 2019, 7, 319. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Matarangolo, M. Photocatalytic Degradation of Paracetamol under UV Irradiation Using TiO2-Graphite Composites. Catal. Today 2018, 315, 230–236. [Google Scholar] [CrossRef]
- Vieira, Y.; Spode, J.E.; Dotto, G.L.; Georgin, J.; Franco, D.S.P.; dos Reis, G.S.; Lima, E.C. Paracetamol Environmental Remediation and Ecotoxicology: A Review. Environ. Chem. Lett. 2024, 22, 2343–2373. [Google Scholar] [CrossRef]
- Lee, D.-E.; Kim, M.-K.; Danish, M.; Jo, W.-K. State-of-the-Art Review on Photocatalysis for Efficient Wastewater Treatment: Attractive Approach in Photocatalyst Design and Parameters Affecting the Photocatalytic Degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Ahmad, K.; Ghatak, H.R.; Ahuja, S.M. A Review on Photocatalytic Remediation of Environmental Pollutants and H2 Production through Water Splitting: A Sustainable Approach. Environ. Technol. Innov. 2020, 19, 100893. [Google Scholar] [CrossRef]
- Garrido, I.; Flores, P.; Hellín, P.; Vela, N.; Navarro, S.; Fenoll, J. Solar Reclamation of Agro-Wastewater Polluted with Eight Pesticides by Heterogeneous Photocatalysis Using a Modular Facility. A Case Study. Chemosphere 2020, 249, 126156. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Devanesan, S.; Asemi, N.N.; Aldawsari, M. Construction of SnO2/CuO/RGO Nanocomposites for Photocatalytic Degradation of Organic Pollutants and Antibacterial Applications. Environ. Res. 2023, 222, 115370. [Google Scholar] [CrossRef]
- Verma, M.; Haritash, A.K. Photocatalytic Degradation of Amoxicillin in Pharmaceutical Wastewater: A Potential Tool to Manage Residual Antibiotics. Environ. Technol. Innov. 2020, 20, 101072. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A Review of the Existing and Emerging Technologies in the Combination of AOPs and Biological Processes in Industrial Textile Wastewater Treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; Aguilar, C.A.; Luna, R.A.; Montalvo, C. Photocatalytic Degradation of Paracetamol: Intermediates and Total Reaction Mechanism. J. Hazard. Mater. 2012, 243, 130–138. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Wang, G. Photocatalytic Advanced Oxidation Processes for Water Treatment: Recent Advances and Perspective. Chem. Asian J. 2020, 15, 3239–3253. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Trellu, C.; Vargas, H.O.; Mousset, E.; Ganiyu, S.O.; Oturan, M.A. Electro-Fenton Process in Combination with Other Advanced Oxidation Processes: Challenges and Opportunities. Curr. Opin. Electrochem. 2023, 37, 101171. [Google Scholar] [CrossRef]
- Yacouba, Z.A.; Mendret, J.; Lesage, G.; Zaviska, F.; Brosillon, S. Removal of Organic Micropollutants from Domestic Wastewater: The Effect of Ozone-Based Advanced Oxidation Process on Nanofiltration. J. Water Process Eng. 2021, 39, 101869. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y. A Comprehensive Review on Persulfate Activation Treatment of Wastewater. Sci. Total Environ. 2022, 831, 154906. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, e1901997. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem.-Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Spanu, D.; Denisov, N.; Recchia, S.; Schmuki, P.; Altomare, M. A Dewetted-Dealloyed Nanoporous Pt Co-Catalyst Formed on TiO 2 Nanotube Arrays Leads to Strongly Enhanced Photocatalytic H 2 Production. Chem. Asian J. 2020, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Spanu, D.; Recchia, S.; Mohajernia, S.; Schmuki, P.; Altomare, M. Site-Selective Pt Dewetting on WO3-Coated TiO2 Nanotube Arrays: An Electron Transfer Cascade-Based H2 Evolution Photocatalyst. Appl. Catal. B 2018, 237, 198–205. [Google Scholar] [CrossRef]
- Pinna, M.; Binda, G.; Altomare, M.; Marelli, M.; Dossi, C.; Monticelli, D.; Spanu, D.; Recchia, S. Biochar Nanoparticles over Tio2 Nanotube Arrays: A Green Co-Catalyst to Boost the Photocatalytic Degradation of Organic Pollutants. Catalysts 2021, 11, 1048. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Rincón, G. Visible-Light-Active Noble-Metal Photocatalysts for Water Disinfection: A Review. J. Water Resour. Prot. 2019, 11, 1207–1232. [Google Scholar] [CrossRef]
- Loddo, V.; Bellardita, M.; Camera-Roda, G.; Parrino, F.; Palmisano, L. Heterogeneous Photocatalysis. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–43. [Google Scholar]
- Park, J.; Lam, S.S.; Park, Y.K.; Kim, B.J.; An, K.H.; Jung, S.C. Fabrication of Ni/TiO2 Visible Light Responsive Photocatalyst for Decomposition of Oxytetracycline. Environ. Res. 2023, 216, 114657. [Google Scholar] [CrossRef] [PubMed]
- Altomare, M.; Qin, S.; Saveleva, V.A.; Badura, Z.; Tomanec, O.; Mazare, A.; Zoppellaro, G.; Vertova, A.; Taglietti, A.; Minguzzi, A.; et al. Metastable Ni(I)-TiO2−x Photocatalysts: Self-Amplifying H 2 Evolution from Plain Water without Noble Metal Co-Catalyst and Sacrificial Agent. J. Am. Chem. Soc. 2023, 145, 26122–26132. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Liu, S.; Gao, L.; Mao, L.; Fan, Z.; Shangguan, W.; Jiang, Z. Non-Noble Metal Cu as a Cocatalyst on TiO2 Nanorod for Highly Efficient Photocatalytic Hydrogen Production. Appl. Surf. Sci. 2018, 445, 527–534. [Google Scholar] [CrossRef]
- Khazaee, Z.; Mahjoub, A.R.; Cheshme Khavar, A.H. One-Pot Synthesis of CuBi Bimetallic Alloy Nanosheets-Supported Functionalized Multiwalled Carbon Nanotubes as Efficient Photocatalyst for Oxidation of Fluoroquinolones. Appl. Catal. B 2021, 297, 120480. [Google Scholar] [CrossRef]
- Spanu, D.; Minguzzi, A.; Recchia, S.; Shahvardanfard, F.; Tomanec, O.; Zboril, R.; Schmuki, P.; Ghigna, P.; Altomare, M. An Operando X-Ray Absorption Spectroscopy Study of a NiCu-TiO2 Photocatalyst for H2 Evolution. ACS Catal. 2020, 10, 8293–8302. [Google Scholar] [CrossRef]
- Pinna, M.; Wei, A.W.W.; Spanu, D.; Will, J.; Yokosawa, T.; Spiecker, E.; Recchia, S.; Schmuki, P.; Altomare, M. Amorphous NiCu Thin Films Sputtered on TiO2 Nanotube Arrays: A Noble-Metal Free Photocatalyst for Hydrogen Evolution. ChemCatChem 2022, 14, e202201052. [Google Scholar] [CrossRef]
- Cadenhead, D.A.; Wagner, N.J. Low-Temperature Hydrogen Adsorption on Copper-Nickel Alloys. J. Phys. Chem. 1968, 72, 2775–2781. [Google Scholar] [CrossRef]
- Ishii, R.; Matsumura, K.; Sakai, A.; Sakata, T. Work Function of Binary Alloys. Appl. Surf. Sci. 2001, 169–170, 658–661. [Google Scholar] [CrossRef]
- Eastman, D.E. Photoelectric Work Functions of Transition, Rare-Earth, and Noble Metals. Phys. Rev. B 1970, 2, 1–2. [Google Scholar] [CrossRef]
- Djebbari, C.; Zouaoui, E.; Ammouchi, N.; Nakib, C.; Zouied, D.; Dob, K. Degradation of Malachite Green Using Heterogeneous Nanophotocatalysts (NiO/TiO2, CuO/TiO2) under Solar and Microwave Irradiation. SN Appl. Sci. 2021, 3, 255. [Google Scholar] [CrossRef]
- Monticelli, D.; Castelletti, A.; Civati, D.; Recchia, S.; Dossi, C. How to Efficiently Produce Ultrapure Acids. Int. J. Anal. Chem. 2019, 2019, 5180610. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntema, D.; Rijnaarts, H.H.M. Photocatalytic Degradation of Metoprolol by TiO2 Nanotube Arrays and UV-LED: Effects of Catalyst Properties, Operational Parameters, Commonly Present Water Constituents, and Photo-Induced Reactive Species. Appl. Catal. B 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Trenczek-Zajac, A.; Synowiec, M.; Zakrzewska, K.; Zazakowny, K.; Kowalski, K.; Dziedzic, A.; Radecka, M. Scavenger-Supported Photocatalytic Evidence of an Extended Type I Electronic Structure of the TiO2@Fe2O3 Interface. ACS Appl. Mater. Interfaces 2022, 14, 38255–38269. [Google Scholar] [CrossRef]
- Cha, G.; Schmuki, P.; Altomare, M. Anodic TiO2 Nanotube Membranes: Site-Selective Pt-Activation and Photocatalytic H2 Evolution. Electrochim. Acta 2017, 258, 302–310. [Google Scholar] [CrossRef]
- Nagaraj, G.; Brundha, D.; Chandraleka, C.; Arulpriya, M.; Kowsalya, V.; Sangavi, S.; Jayalakshmi, R.; Tamilarasu, S.; Murugan, R. Facile Synthesis of Improved Anatase TiO2 Nanoparticles for Enhanced Solar-Light Driven Photocatalyst. SN Appl. Sci. 2020, 2, 734. [Google Scholar] [CrossRef]
- Boonchuduang, T.; Bootchanont, A.; Klysubun, W.; Amonpattaratkit, P.; Khamkongkaeo, A.; Puncreobutr, C.; Yimnirun, R.; Lohwongwatana, B. Formation of Alpha-Case Layer During Investment Casting of Pure Ti and Ti-6Al-4V Using Comparative XRD and EXAFS Investigation. Metall. Mater. Trans. A 2020, 51, 586–596. [Google Scholar] [CrossRef]
- Rodríguez-Salinas, J.; Hernández, M.B.; Cruz, L.G.; Martínez-Romero, O.; Ulloa-Castillo, N.A.; Elías-Zúñiga, A. Enhancing Electrical and Thermal Properties of Al6061 Parts by Electrophoresis Deposition of Multi-Walled Carbon Nanotubes. Coatings 2020, 10, 656. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-Ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Loch, D.A.L.; Gonzalvo, Y.A.; Ehiasarian, A.P. Plasma Analysis of Inductively Coupled Impulse Sputtering of Cu, Ti and Ni. Plasma Sources Sci. Technol. 2017, 26, 065012. [Google Scholar] [CrossRef]
- Shimatsu, T.; Mollema, R.H.; Monsma, D.; Keim, E.G.; Lodder, J.C. Metal Bonding during Sputter Film Deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 1998, 16, 2125–2131. [Google Scholar] [CrossRef]
- Su, J.; Wang, Z.; Ma, J.; Tang, B.; Lang, X.; Jiang, M.; He, Z. Selective Bias Deposition of CuO Thin Film on Unpolished Si Wafer. Mater. Res. Express 2020, 7, 026402. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J.; Liu, Y.; Jiang, M.; Zhou, L. Parameter-Dependent Oxidation of Physically Sputtered Cu and the Related Fabrication of Cu-Based Semiconductor Films with Metallic Resistivity. Sci. China Mater. 2016, 59, 144–150. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Etzkorn, F.A. Standard Reduction Potentials by Value. In Green Chemistry: Principles and Case Studies; The Royal Society of Chemistry: London, UK, 2019; pp. 418–420. [Google Scholar]
- Wang, Z.; Lin, R.; Huo, Y.; Li, H.; Wang, L. Formation, Detection, and Function of Oxygen Vacancy in Metal Oxides for Solar Energy Conversion. Adv. Funct. Mater. 2022, 32, 2109503. [Google Scholar] [CrossRef]
- Pan, C.; Shen, H.; Liu, G.; Zhang, X.; Liu, X.; Liu, H.; Xu, P.; Chen, W.; Tian, Y.; Deng, H.; et al. CuO/TiO2 Nanobelt with Oxygen Vacancies for Visible-Light-Driven Photocatalytic Bacterial Inactivation. ACS Appl. Nano Mater. 2022, 5, 10980–10990. [Google Scholar] [CrossRef]
- Jubu, P.R.; Yam, F.K.; Igba, V.M.; Beh, K.P. Tauc-Plot Scale and Extrapolation Effect on Bandgap Estimation from UV–Vis–NIR Data – A Case Study of β-Ga2O3. J. Solid State Chem. 2020, 290, 121576. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Ruiz-López, I.I.; Ruiz-Peralta, M.d.; Tepech-Carrillo, L.; Sánchez-Cantú, M.; Moreno-Orea, J.E. Automated Method for the Determination of the Band Gap Energy of Pure and Mixed Powder Samples Using Diffuse Reflectance Spectroscopy. Heliyon 2019, 5, e01505. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Tahini, H.A.; Lin, Q.; Chen, Y.; Guan, D.; Zhou, C.; Hu, Z.; Lin, H.-J.; Chan, T.-S.; et al. Single-Phase Perovskite Oxide with Super-Exchange Induced Atomic-Scale Synergistic Active Centers Enables Ultrafast Hydrogen Evolution. Nat. Commun. 2020, 11, 5657. [Google Scholar] [CrossRef]
- Aguilar, C.A.; de la Cruz, A.; Montalvo, C.; Ruiz-Marín, A.; Oros-Ruiz, S.; Figueroa-Ramírez, S.J.; Abatal, M.; Anguebes, F.; Córdova-Quiroz, V. Effect of Kinetics on the Photocatalytic Degradation of Acetaminophen and the Distribution of Major Intermediate with Anatase-Ag Synthesized by Sol Gel under Visible Irradiation. Front. Environ. Sci. 2022, 10, 943776. [Google Scholar] [CrossRef]
- Akşit, D.; Soylu, S.P.G. Photocatalytic Degradation of Paracetamol by Semiconductor Oxides under UV and Sunlight Illumination. Turk. J. Chem. 2022, 46, 1866–1874. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Haug, C. Enhanced Photocatalytic Degradation of an Azo Textile Dye by Using TiO2/NiO Coupled Nanoparticles: Optimization of Synthesis and Operational Key Factors. Mater. Sci. Semicond. Process 2014, 27, 240–253. [Google Scholar] [CrossRef]
- Mannaa, M.A.; Qasim, K.F.; Alshorifi, F.T.; El-Bahy, S.M.; Salama, R.S. Role of NiO Nanoparticles in Enhancing Structure Properties of TiO2and Its Applications in Photodegradation and Hydrogen Evolution. ACS Omega 2021, 6, 30386–30400. [Google Scholar] [CrossRef] [PubMed]
- Singha, B.; Ray, K. Density Functional Theory Insights on Photocatalytic Ability of CuO/TiO2 and CuO/ZnO. Mater. Today Proc. 2023, 72, 451–458. [Google Scholar] [CrossRef]
- Pansri, S.; Supruangnet, R.; Nakajima, H.; Rattanasuporn, S.; Noothongkaew, S. Band Offset Determination of P-NiO/n-TiO2 Heterojunctions for Applications in High-Performance UV Photodetectors. J. Mater. Sci. 2020, 55, 4332–4344. [Google Scholar] [CrossRef]
- Santos, H.L.S.; Corradini, P.G.; Andrade, M.A.S.; Mascaro, L.H. CuO/NiOx Thin Film–Based Photocathodes for Photoelectrochemical Water Splitting. J. Solid. State Electrochem. 2020, 24, 1899–1908. [Google Scholar] [CrossRef]
- Weldekirstos, H.D.; Habtewold, B.; Kabtamu, D.M. Surfactant-Assisted Synthesis of NiO-ZnO and NiO-CuO Nanocomposites for Enhanced Photocatalytic Degradation of Methylene Blue Under UV Light Irradiation. Front. Mater. 2022, 9, 832439. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of Paracetamol in Aqueous Solutions by TiO2 Photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Photocatalytic Oxidation of Paracetamol: Dominant Reactants, Intermediates, and Reaction Mechanisms. Environ. Sci. Technol. 2009, 43, 460–465. [Google Scholar] [CrossRef]
- Jallouli, N.; Elghniji, K.; Trabelsi, H.; Ksibi, M. Photocatalytic Degradation of Paracetamol on TiO2 Nanoparticles and TiO2/Cellulosic Fiber under UV and Sunlight Irradiation. Arab. J. Chem. 2017, 10, S3640–S3645. [Google Scholar] [CrossRef]

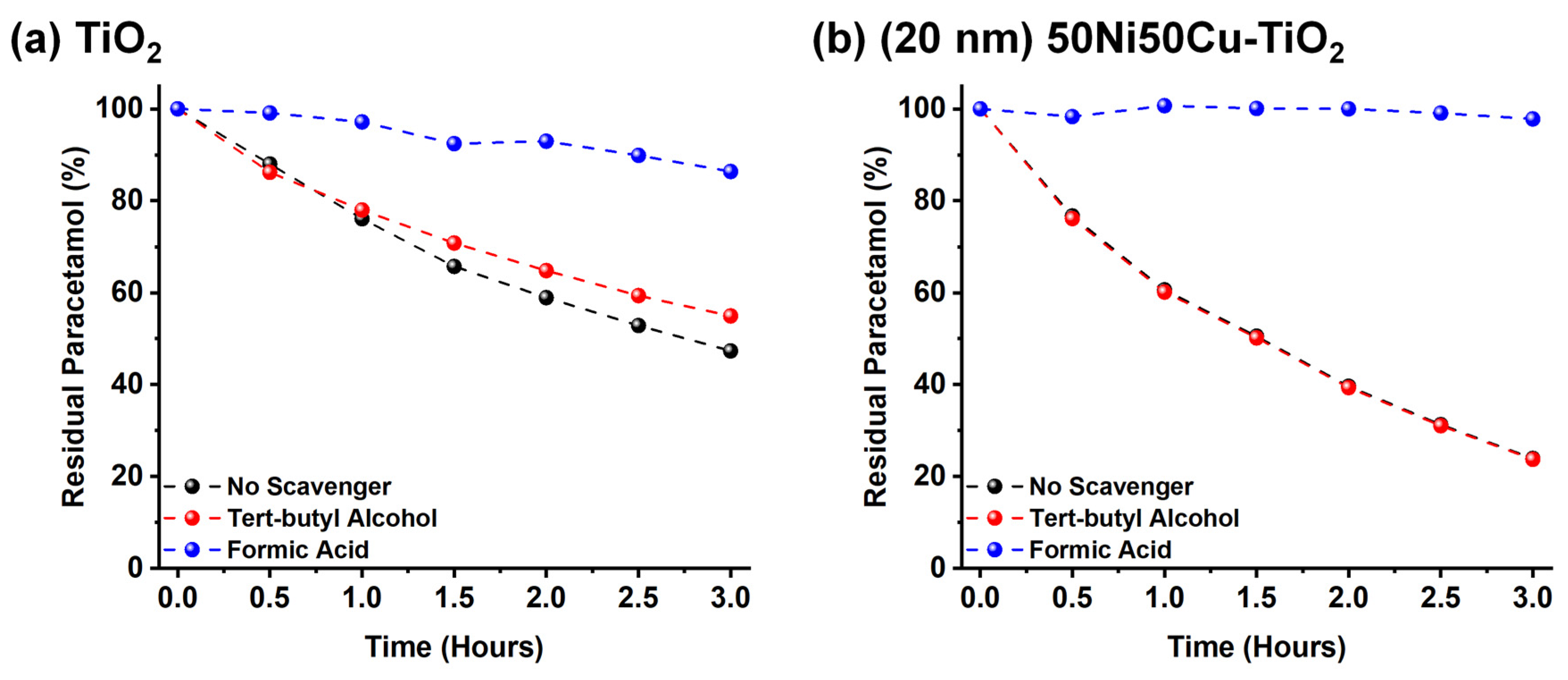
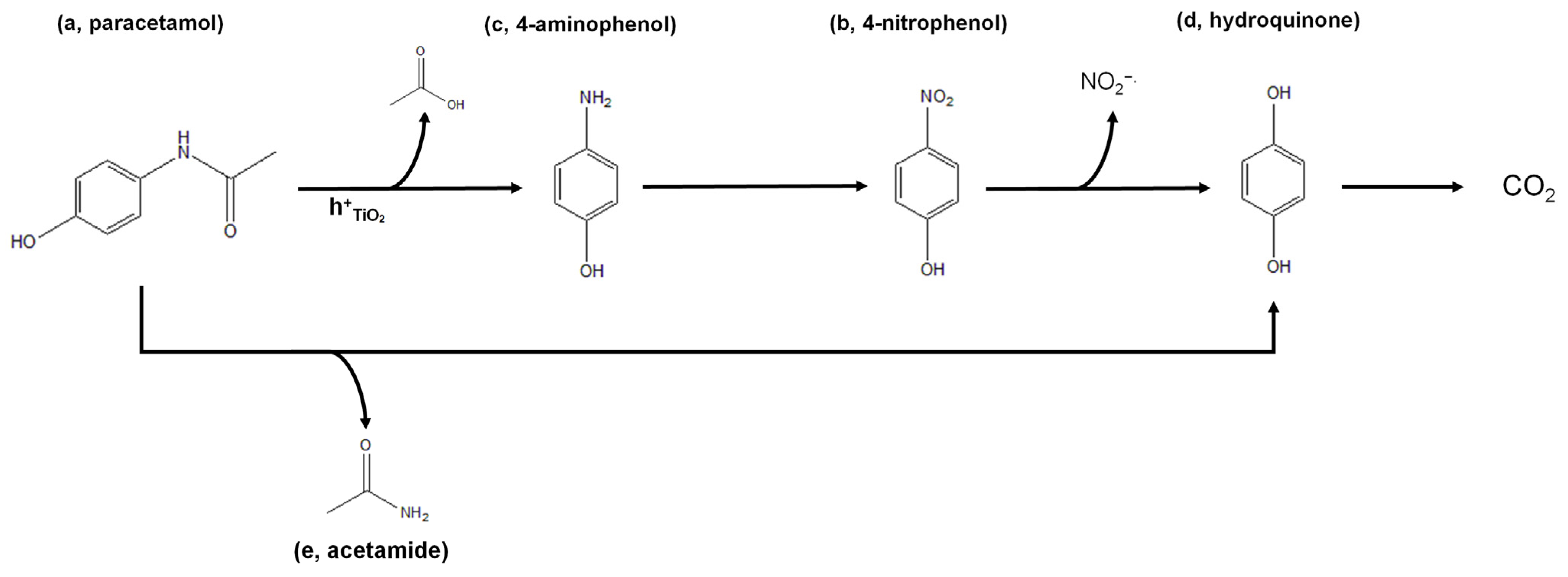
| Irradiation Time (hours) | COD (O2 mg L−1) | Degraded Paracetamol | Mineralization Degree |
|---|---|---|---|
| 0 | 22.4 mg L−1 | 0% | 0% |
| 3 | 14.4 mg L−1 | 78% | 36% |
| 24 | 4.7 mg L−1 | 98.8% | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, M.; Zava, M.; Grande, T.; Prina, V.; Monticelli, D.; Roncoroni, G.; Rampazzi, L.; Hildebrand, H.; Altomare, M.; Schmuki, P.; et al. Enhanced Photocatalytic Paracetamol Degradation by NiCu-Modified TiO2 Nanotubes: Mechanistic Insights and Performance Evaluation. Nanomaterials 2024, 14, 1577. https://doi.org/10.3390/nano14191577
Pinna M, Zava M, Grande T, Prina V, Monticelli D, Roncoroni G, Rampazzi L, Hildebrand H, Altomare M, Schmuki P, et al. Enhanced Photocatalytic Paracetamol Degradation by NiCu-Modified TiO2 Nanotubes: Mechanistic Insights and Performance Evaluation. Nanomaterials. 2024; 14(19):1577. https://doi.org/10.3390/nano14191577
Chicago/Turabian StylePinna, Marco, Martina Zava, Tommaso Grande, Veronica Prina, Damiano Monticelli, Gianluca Roncoroni, Laura Rampazzi, Helga Hildebrand, Marco Altomare, Patrik Schmuki, and et al. 2024. "Enhanced Photocatalytic Paracetamol Degradation by NiCu-Modified TiO2 Nanotubes: Mechanistic Insights and Performance Evaluation" Nanomaterials 14, no. 19: 1577. https://doi.org/10.3390/nano14191577






