Rapid Microfluidic Immuno-Biosensor Detection System for the Point-of-Care Determination of High-Sensitivity Urinary C-Reactive Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
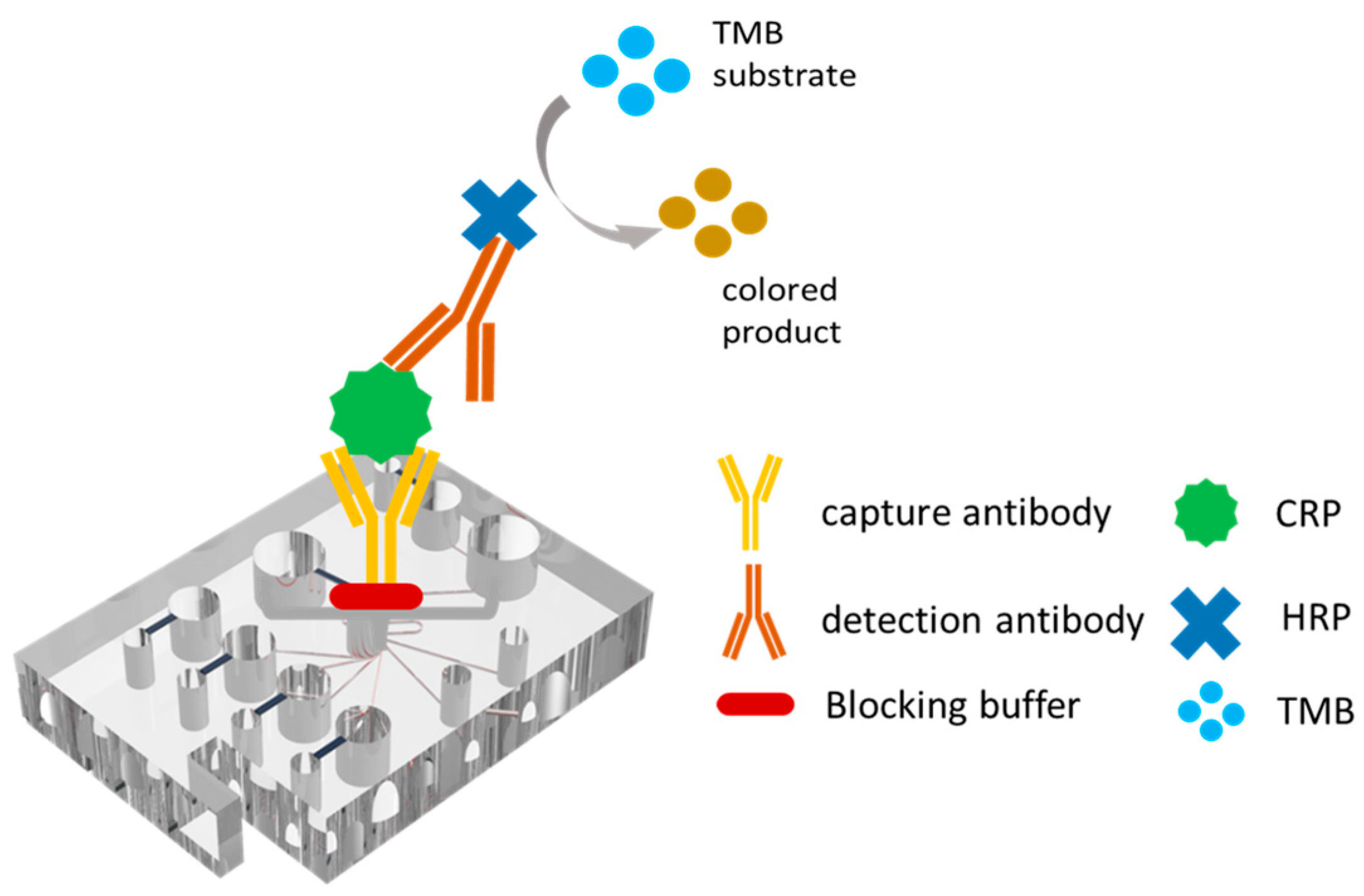
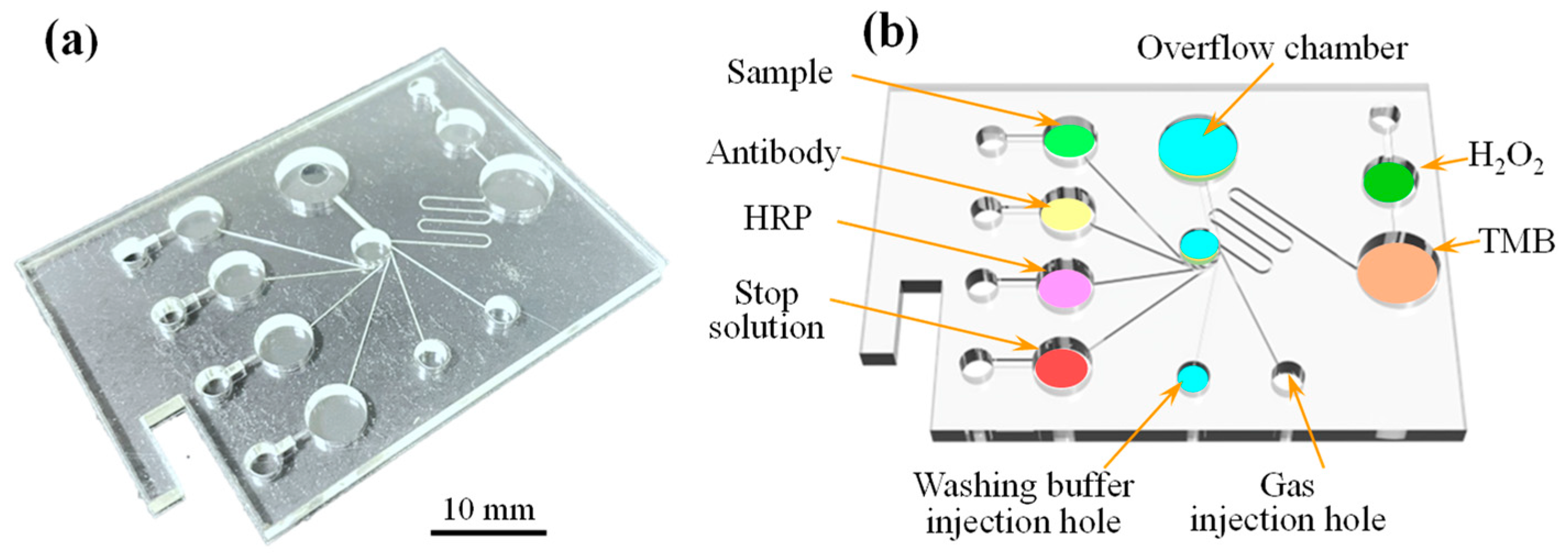
2.2. Manufacturing and Assembly of Microfluidic Spectrum-Chip
2.3. Micro-Spectrometer Detection Device
2.4. Experimental Details
3. Results and Discussion
3.1. Optimal Reaction Temperature on Microfluidic Spectrum-Chip
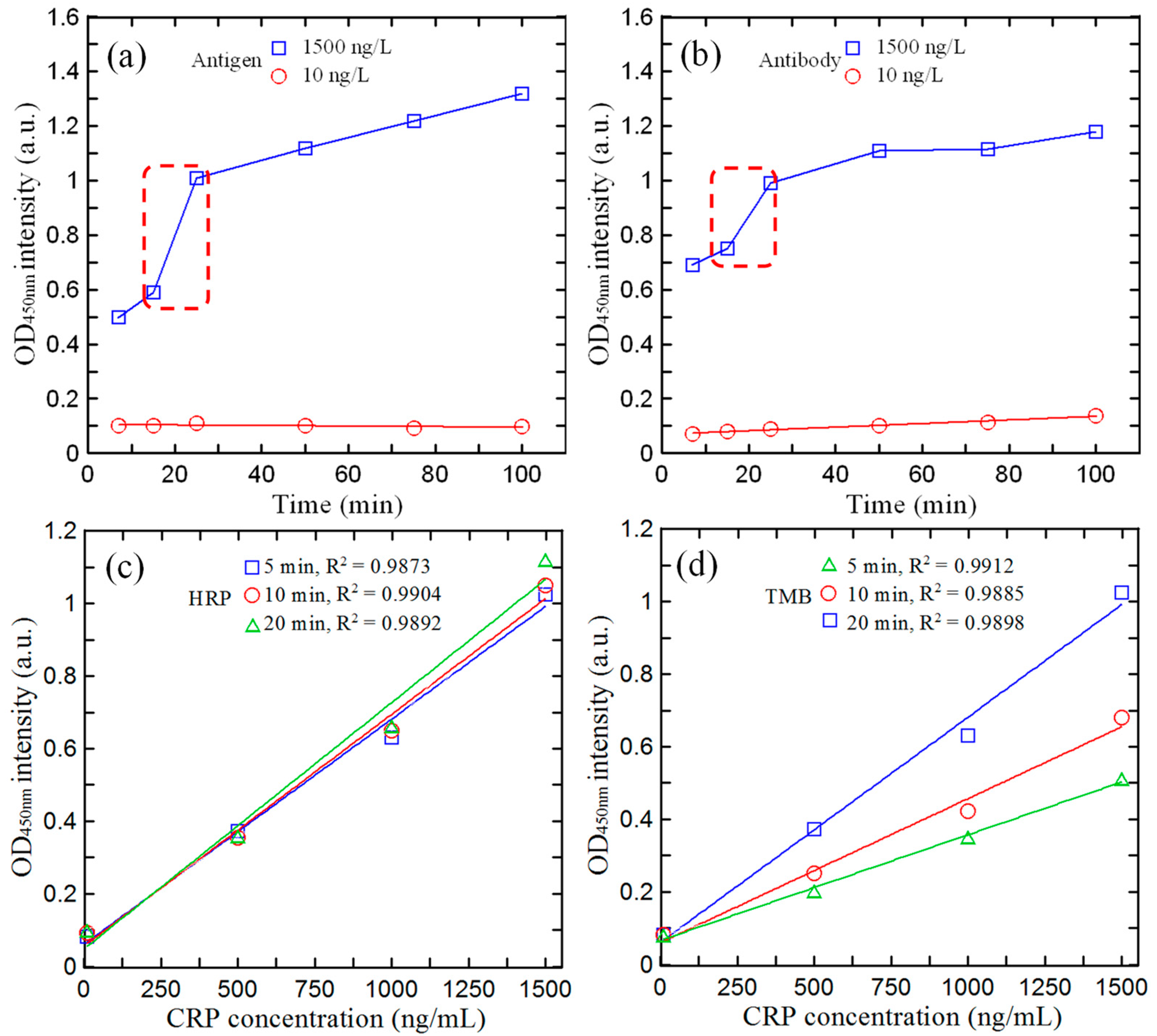
3.2. Optimal Reagent/Reaction Time on Microfluidic Spectrum-Chip
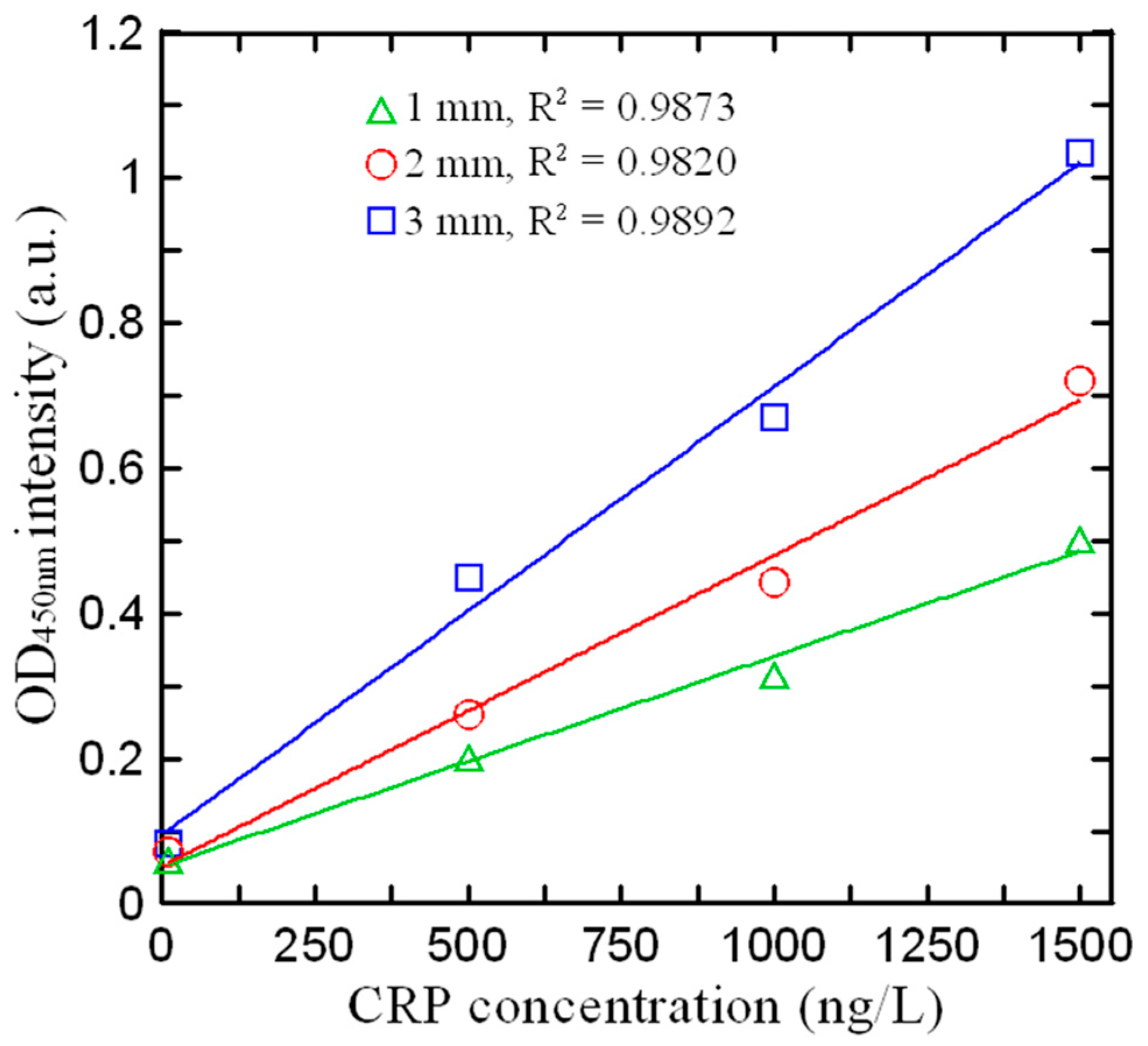
3.3. Effect of Detection Path Length on Absorbance Intensity
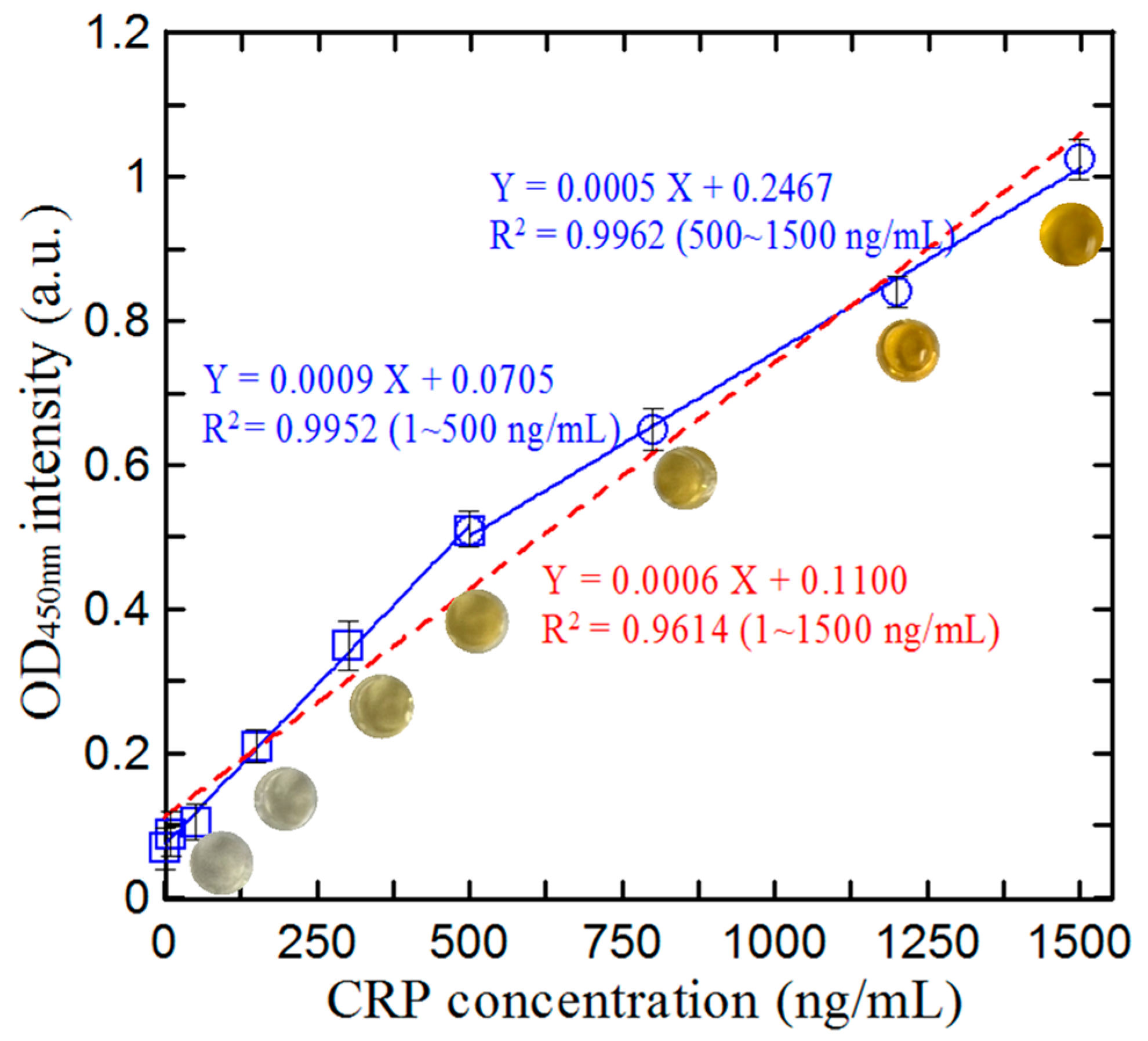
3.4. Calibration of Microfluidic ELISA Detection System
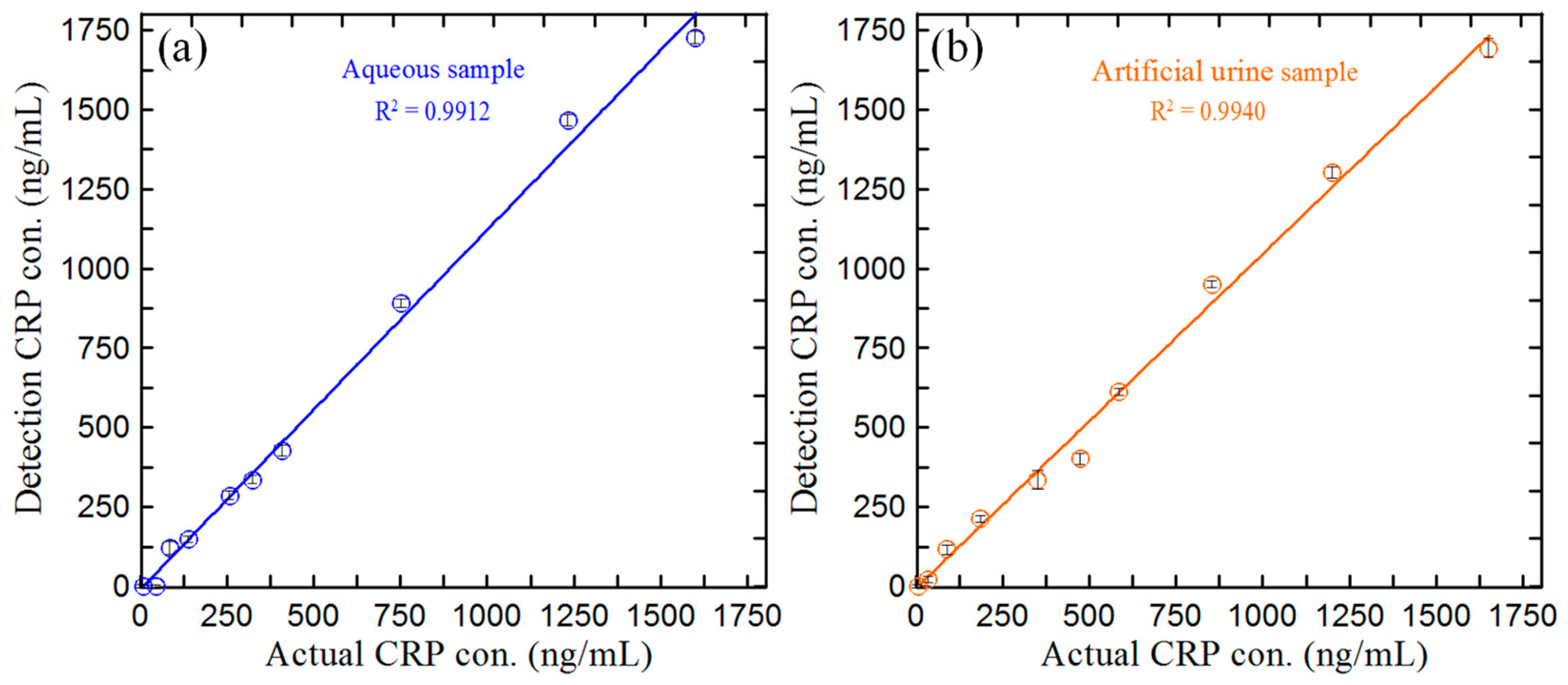
3.5. Detection of CRP Concentration in Blind Samples
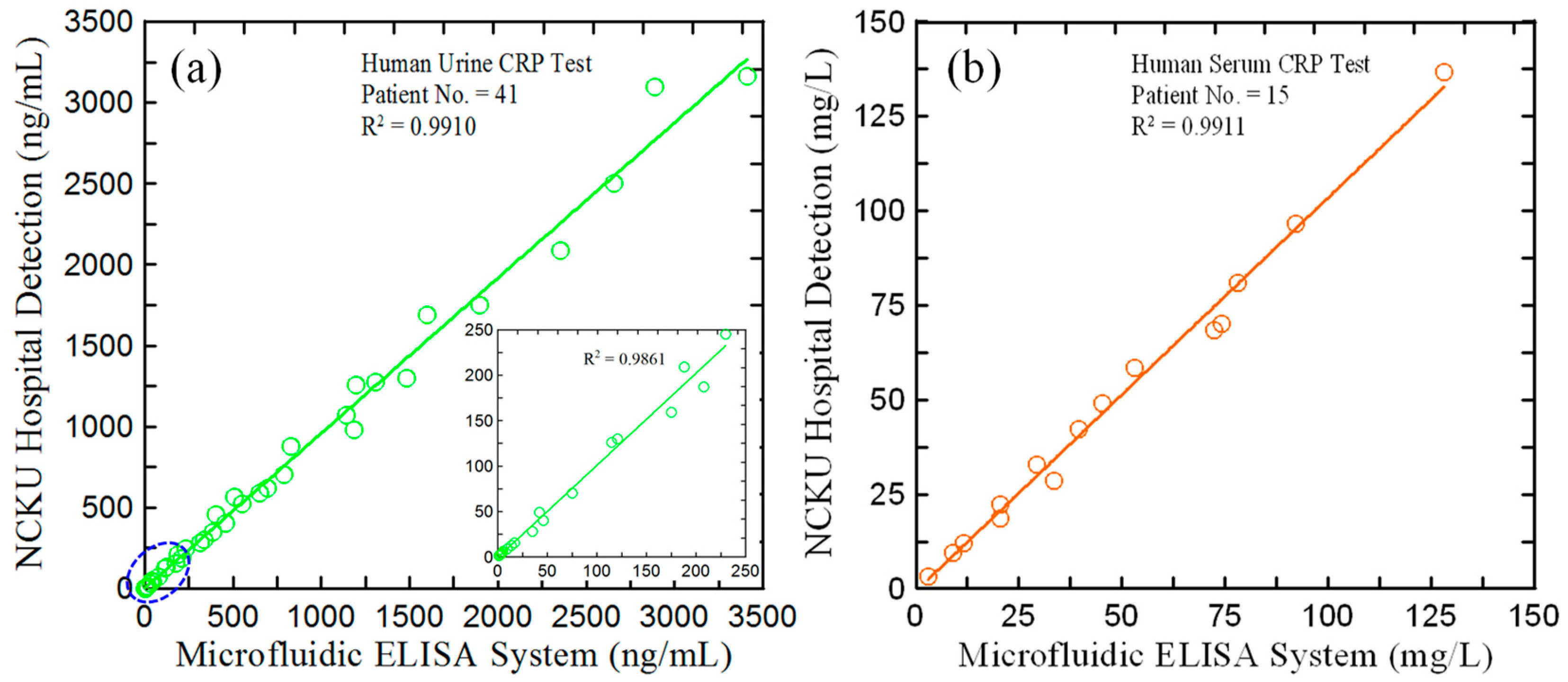
3.6. Application of Proposed Microfluidic ELISA Detection System to hs-CRP Determination in Real-World Urine Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 342848. [Google Scholar] [CrossRef] [PubMed]
- Henze, L.A.; Luong, T.T.; Boehme, B.; Masyout, J.; Schneider, M.P.; Brachs, S.; Lang, F.; Pieske, B.; Pasch, A.; Eckardt, K.U. Impact of C-reactive Protein on Osteo-/Chondrogenic Transdifferentiation and Calcification of Vascular Smooth Muscle Cells. Aging 2019, 11, 5445. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Schneider, E.; Luong, J.H. Surface Pplasmon Resonance-Based Immunoassay for human C-reactive Protein. Analyst 2015, 140, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Lee, J.H. Prediction of Cortical Defect Using C-reactive Protein and Urine Sodium to Potassium Ratio in Infants with Febrile Urinary Tract Infection. Yonsei Med. J. 2016, 57, 103. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Tseng, C.C.; Huang, K.H.; Chang, Y.C.; Fu, L.M. Microfluidic Sliding Paper-Based Device for Point-of-Care Determination of Albumin-to-Creatine Ratio in Human Urine. Biosensors 2022, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tang, M.Q.; Chen, J.; Zhu, Y.H.; Lei, C.B.; He, H.W.; Xu, X.H. A Sandwich ELISA-like Detection of C-reactive Protein in Blood by Citicoline-Bovine Serum Albumin Conjugate and Aptamer-Functionalized Gold Nanoparticles Nanozyme. Talanta 2020, 217, 121070. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Li, X.; Tian, Y.; Huang, Z.; Duan, Y. Core-Shell Au@ PdNPs Based Colorimetric Enhanced Lateral Flow Immunoassay for C-reactive Protein Detection. Sens. Actuators B Chem. 2023, 379, 133247. [Google Scholar] [CrossRef]
- Johannsen, B.; Baumgartner, D.; Karpíšek, M.; Stejskal, D.; Boillat-Blanco, N.; Knüsli, J.; Panning, M.; Paust, N.; Zengerle, R.; Mitsakakis, K. Patient Stratification for Antibiotic Prescriptions Based on the Bound-Free Phase Detection Immunoassay of C-Reactive Protein in Serum Samples. Biosensors 2023, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Li, Z.; Zhao, L.; Zhang, W.; Huang, L.; Meng, F.; Liu, G.L.; Hu, W. Ultrasensitive Detection of C-reactive Protein by a Novel Nanoplasmonic Immunoturbidimetry Assay. Biosensors 2022, 12, 958. [Google Scholar] [CrossRef]
- Pohanka, M. Diagnoses Based on C-reactive Protein Point-of-Care Tests. Biosensors 2022, 12, 344. [Google Scholar] [CrossRef]
- Fan, L.; Yan, W.; Chen, Q.; Tan, F.; Tang, Y.; Han, H.; Yu, R.; Xie, N.; Gao, S.; Chen, W. One-Component Dual-Readout Aggregation-Induced Emission Nanobeads for Qualitative and Quantitative Detection of C-Reactive Protein at the Point of Care. Anal. Chem. 2023, 96, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.J.; Muñoz-Torres, F.J.; Dye, B.A.; Leroux, B.G.; Ramírez-Vick, M.; Pérez, C.M. Longitudinal Association Between Periodontitis and Development of Diabetes. Diabetes Res. Clin. Pract. 2018, 141, 284–293. [Google Scholar] [CrossRef]
- Balayan, S.; Chauhan, N.; Rosario, W.; Jain, U. Biosensor Development for C-Reactive Protein Detection: A review. Appl. Surf. Sci. Adv. 2022, 12, 100343. [Google Scholar] [CrossRef]
- Bravin, C.; Amendola, V. Wide Range Detection of C-Reactive Protein with a Homogeneous Immunofluorimetric Assay Based on Cooperative Fluorescence Quenching Assisted by Gold Nanoparticles. Biosens. Bioelectron 2020, 169, 112591. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Zhang, C.; Meng, H.; Liu, B.; Wei, X. A Rapid Fluorescent Aptasensor for Point-of-Care Detection of C-Reactive Protein. Talanta 2022, 249, 123661. [Google Scholar] [CrossRef]
- Xu, A.; Li, J.; Zhang, S.; Pan, H. An Integrated Immunochromatographic Device for C-Reactive Protein Detection Using Hierarchical Dendritic Gold Nanostructure Films. Anal. Chim. Acta 2023, 1269, 341402. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Li, F.; Zhang, M. Based Chemiluminescence Enzyme-Linked Immunosorbent Assay Enhanced by Biotin-Streptavidin System for High-Sensitivity C-Reactive Protein Detection. Anal. Biochem. 2018, 559, 86–90. [Google Scholar] [CrossRef]
- Kokorina, A.A.; Ponomaryova, T.S.; Goryacheva, I.Y. Photoluminescence-Based Immunochemical Methods for Determination of C-Reactive Protein and Procalcitonin. Talanta 2021, 224, 121837. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Seong, T.Y.; Kim, W.M.; Kim, I.; Hwang, G.W.; Lee, W.S.; Lee, K.S. Enhanced Resolution of a Surface Plasmon Resonance Sensor Detecting C-Reactive PProtein via a Bimetallic Waveguide-Coupled Mode Approach. Sens. Actuators B Chem. 2018, 266, 311–317. [Google Scholar] [CrossRef]
- Gao, H.; Bai, Y.; He, B.; Tan, C.S. A Simple Label-Free Aptamer-Based Electrochemical Biosensor for the Sensitive Detection of C-Reactive Proteins. Biosensors 2022, 12, 1180. [Google Scholar] [CrossRef]
- Cui, M.; Che, Z.; Gong, Y.; Li, T.; Hu, W.; Wang, S. A Graphdiyne-Based Protein Molecularly Imprinted Biosensor for Highly Sensitive Human C-Reactive Protein Detection in Human Serum. Chem. Eng. J. 2022, 431, 133455. [Google Scholar] [CrossRef]
- Szot-Karpinska, K.; Kudła, P.; Orzeł, U.; Narajczyk, M.; Jönsson-Niedziółka, M.; Pałys, B.; Filipek, S.; Ebner, A.; Niedziółka-Jönsson, J. Investigation of Peptides for Molecular Recognition of C-Reactive Protein−Theoretical and Experimental Studies. Anal. Chem. 2023, 95, 14475–14483. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Min, J.; Song, Y.; Xu, C.; Li, J.; Moore, J.; Hu, E.; Parimon, T.; Wang, T.Y.; Dvoodi, E.; et al. A Wireless Patch for the Monitoring of C-Reactive Protein in Sweat. Nat. Biomed. Eng. 2023, 7, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.G.; He, J.W.; Jiang, L.Y.; Chen, D.N.; Wang, A.J.; Feng, J.J. Novel Sandwich-Typed Electrochemical Immunosensing of C-Reactive Protein Using Multiply Twinned AuPtRh Nanobead Chains and Nitrogen-Rich Porous Carbon Nanospheres Decorated with Au Nanoparticles. Sens. Actuators B Chem. 2022, 358, 131518. [Google Scholar] [CrossRef]
- An, Q.Q.; Feng, X.Z.; Zhan, T.; Cheng, Y.Y.; Han, G.C.; Chen, Z.; Kraatz, H.-B. A Simple Synthesis of a Core-Shell Structure PPy-Au Nanocomposite for Immunosensing of C-Reactive Protein. Talanta 2024, 267, 125158. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Lu, S.Y.; Chen, S.J.; Wang, J.M.; Fu, L.M.; Wu, Y.H. Microfluidic Aptasensor POC Device for Determination of Whole Blood Potassium. Anal. Chim. Acta 2022, 1203, 339722. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, L.; Bi, W.; Shen, H.; Li, D.; Yu, Z.; Gan, N. Ultrasensitive Microfluidic Immunosensor with Stir Bar Enrichment for Point-of-Care Test of Staphylococcus Aureus in Foods Triggered by DNAzyme-Assisted Click Reaction. Food Chem. 2022, 378, 132093. [Google Scholar] [CrossRef] [PubMed]
- Kraft, F.A.; Lehmann, S.; Di Maria, C.; Joksch, L.; Fitschen-Östern, S.; Fuchs, S.; Dell’Olio, F.; Gerken, M. Intensity-Based Camera Setup for Refractometric and Biomolecular Sensing with a Photonic Crystal Microfluidic Chip. Biosensors 2023, 13, 687. [Google Scholar] [CrossRef]
- Xue, K.; Lin, B.; Huang, S.; Cai, B.; Lai, W.; Zhan, T.; Liang, Y.; Zhang, C. A Dry Chemistry, Ultrasensitive Microfluidic Fiber Material-Based Immunosensor for Electrochemiluminescence Point-of-Care Testing of Luteinizing Hormone. Sens. Actuators B Chem. 2023, 393, 134259. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, R.; Huang, S.; Huang, Y.; Zhang, J.; Su, X.; Zhang, S.; Ge, S.; Zhang, J.; Xia, N. All-in-One Microfluidic Chip for 30-min Quantitative Point-of-Care-Testing of Nucleic Acids. Sens. Actuators B Chem. 2023, 390, 133939. [Google Scholar] [CrossRef]
- Peng, P.; Liu, C.; Li, Z.; Xue, Z.; Mao, P.; Hu, J.; Xu, F.; Yao, C.; You, M. Emerging ELISA Derived Technologies for in Vitro Diagnostics. TrAC Trends Anal. Chem. 2022, 152, 116605. [Google Scholar] [CrossRef]
- Yang, F.; Xu, L.; Dias, A.C.; Zhang, X. A Sensitive Sandwich ELISA Using a Modified Biotin-Streptavidin Amplified System for Histamine Detection in Fish, Prawn and Crab. Food Chem. 2021, 350, 129196. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Lamy de la Chapelle, M.; Marty, J.L. Recent Progresses in Optical Biosensors for Interleukin 6 Detection. Biosensors 2023, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Reis, N.M. Continuous Flow of Reagents Interferes with the Kinetics and Equilibrium of Antibody-Antigen Binding in Microfluidic Heterogeneous Immunoassays. Sens. Actuators B Chem. 2023, 395, 134454. [Google Scholar] [CrossRef]
- Duan, S.; Cai, T.; Zhu, J.; Yang, X.; Lim, E.G.; Huang, K.; Hoettges, K.; Zhang, Q.; Fu, H.; Guo, Q. Deep Larning-Assisted Ultra-Accurate Smartphone Testing of Paper-Based Colorimetric ELISA Assays. Anal. Chim. Acta 2023, 1248, 340868. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ko, C.H.; Wang, Y.N.; Fu, L.M.; Lee, S.Z. Rapid Detection of Artificial Sweeteners in Food Using Microfluidic Chromatography Detection System. Chem. Eng. J. 2021, 425, 131528. [Google Scholar] [CrossRef]
- Gao, R.; Chen, F.; Yang, D.; Zheng, L.; Jing, T.; Jia, H.; Chen, X.; Lu, Y.; Xu, S.; Zhang, D. Simultaneous SERS-Based Immunoassay of Dual Cardiac Markers on Pump-Free Hybrid Microfluidic Chip. Sens. Actuators B Chem. 2022, 369, 132378. [Google Scholar] [CrossRef]
- Xing, G.; Zhang, W.; Li, N.; Pu, Q.; Lin, J.M. Recent Progress on Microfluidic Biosensors for Rapid Detection of Pathogenic Bacteria. Chin. Chem. Lett. 2022, 33, 1743–1751. [Google Scholar] [CrossRef]
- Guan, T.; Shen, Y.; Jiang, Z.; Zhao, Y.; Liang, Z.; Liu, Y.; Shen, X.; Li, X.; Xu, Z.; Lei, H. An Ultrasensitive Microfluidic Chip-Based Immunoassay for Multiplex Determination of 11 PDE-5 Inhibitors in Adulterated Health Foods. Sens. Actuators B Chem. 2022, 358, 131450. [Google Scholar] [CrossRef]
- Mumtaz, Z.; Rashid, Z.; Ali, A.; Arif, A.; Ameen, F.; AlTami, M.S.; Yousaf, M.Z. Prospects of Microfluidic Technology in Nucleic Acid Detection Approaches. Biosensors 2023, 13, 584. [Google Scholar] [CrossRef]
- Chen, S.; Fan, F.; Zhang, Y.; Zeng, J.; Li, Y.; Xu, N.; Zhang, Y.; Meng, X.L.; Lin, J.M. Metabolites from Scutellarin Alleviating Deferoxamine-Induced Hypoxia Injury in BV2 Cells Cultured on Microfluidic Chip Combined with a Mass Spectrometer. Talanta 2023, 259, 124478. [Google Scholar] [CrossRef] [PubMed]
- Grigorev, G.V.; Lebedev, A.V.; Wang, X.; Qian, X.; Maksimov, G.V.; Lin, L. Advances in Microfluidics for Single Red Blood Cell Analysis. Biosensors 2023, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Prabhu, A.; Prasad, D.; kumar Garlapati, V.; Aminabhavi, T.M.; Mani, N.K.; Simal-Gandara, J. Paper-Based Microfluidic Devices for Food Adulterants: Cost-Effective Technological Monitoring Systems. Food Chem. 2022, 390, 133173. [Google Scholar] [CrossRef] [PubMed]
- Bakuova, N.; Toktarkan, S.; Dyussembinov, D.; Azhibek, D.; Rakhymzhanov, A.; Kostas, K.; Kulsharova, G. Design, Simulation, and Evaluation of Polymer-Based Microfluidic Devices via Computational Fluid Dynamics and Cell Culture “On-Chip”. Biosensors 2023, 13, 754. [Google Scholar] [CrossRef]
- Huang, R.; Quan, J.; Su, B.; Cai, C.; Cai, S.; Chen, Y.; Mou, Z.; Zhou, P.; Ma, D.; Cui, X. A Two-Step Competition Assay for Visual, Sensitive and Quantitative C-Reactive Protein Detection in Low-Cost Microfluidic Particle Accumulators. Sens. Actuators B Chem. 2022, 359, 131583. [Google Scholar] [CrossRef]
- Shang, Y.; Xiang, X.; Ye, Q.; Wu, Q.; Zhang, J.; Lin, J.M. Advances in Nanomaterial-Based Microfluidic Platforms for On-Site Detection of Foodborne Bacteria. TrAC Trends Anal. Chem. 2022, 147, 116509. [Google Scholar] [CrossRef]
- Chen, K.H.; Liu, C.C.; Lu, S.Y.; Chen, S.J.; Sheu, F.; Fu, L.M. Rapid Microfluidic Analysis Detection System for Sodium Dehydroacetate in Foods. Chem. Eng. J. 2022, 427, 131530. [Google Scholar] [CrossRef]
- Zhang, H.; Anoop, K.; Huang, C.; Sadr, R.; Gupte, R.; Dai, J.; Han, A. A Circular Gradient-Width Crossflow Microfluidic Platform for High-Efficiency Blood Plasma Separation. Sens. Actuators B Chem. 2022, 354, 131180. [Google Scholar] [CrossRef]
- Li, F.; You, M.; Li, S.; Hu, J.; Liu, C.; Gong, Y.; Yang, H.; Xu, F. Paper-Based Point-of-Care Immunoassays: Recent Advances and Emerging Trends. Biotechnol. Adv. 2020, 39, 107442. [Google Scholar] [CrossRef]
- Flora, F.C.; Relvas, S.B.; Silva, F.A.; Freire, M.G.; Chu, V.; Conde, J.P. Combined Use of Ionic Liquid-Based Aqueous Biphasic Systems and Microfluidic Devices for the Detection of Prostate-Specific Antigen. Biosensors 2023, 13, 334. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Yang, J.; Van Nguyen, H.; Poo, H.; Seo, T.S. Development of a High-Throughput Centrifugal Microsystem for Enzyme-Linked Immunosorbent Assay to Detect SARS-CoV-2. Chem. Eng. J. 2023, 472, 144808. [Google Scholar] [CrossRef]
- Hao, R.; Liu, L.; Yuan, J.; Wu, L.; Lei, S. Recent Advances in Field Effect Transistor Biosensors: Designing Strategies and Applications for Sensitive Assay. Biosensors 2023, 13, 426. [Google Scholar] [CrossRef]
- Wu, T.H.; Tsai, Y.C.; Kuo, F.C.; Lee, M.S.; Hu, C.C.; Lee, G.B. A Microfluidic Platform for Detection and Quantification of Two Biomarkers for Rheumatoid Arthritis. Sens. Actuators B Chem. 2023, 383, 133587. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, J.; Guo, J. Alphalisa Immunoassay Enabled Centrifugal Microfluidic System for “One-Step” Detection of Pepsinogen in Whole Blood. Sens. Actuators B Chem. 2023, 376, 133048. [Google Scholar] [CrossRef]
- Wu, K.; He, X.; Wang, J.; Pan, T.; He, R.; Kong, F.; Cao, Z.; Ju, F.; Huang, Z.; Nie, L. Recent Progress of Microfluidic Chips in Immunoassay. Front. Bioeng. Biotechnol. 2022, 10, 1112327. [Google Scholar] [CrossRef]
- Hou, Y.; Zheng, Y.; Zheng, X.; Sun, Y.; Yi, X.; Wu, Z.; Lin, J.M. Multidimensional Controllable Fabrication of Tumor Spheroids Based on a Microfluidic Device. Lab Chip 2023, 23, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Zhao, B.; Wu, W.; Zhao, M.; Bi, Y.; Hu, Q. Distance-Based Microfluidic Assays for Instrument-Free Visual Point-of-Care Testing. TrAC Trends Anal. Chem. 2023, 162, 117029. [Google Scholar] [CrossRef]
- Sanjayan, C.G.; Ravikumar, C.H.; Balakrishna, R.G. Perovskite QD Based Paper Microfluidic Device for Simultaneous Detection of Lung Cancer Biomarkers–Carcinoembryonic Antigen and Neuron Specific Enolase. Chem. Eng. J. 2023, 646, 142581. [Google Scholar]
- Zong, C.; Zhang, D.; Jiang, F.; Yang, H.; Liu, S.; Li, P. Metal-Enhanced Chemiluminescence Detection of C-Reaction Protein Based on Silver Nanoparticle Hybrid Probes. Talanta 2019, 199, 164–169. [Google Scholar] [CrossRef]
- Tsai, M.Z.; Hsiung, C.T.; Chen, Y.; Huang, C.S.; Hsu, H.Y.; Hsieh, P.Y. Real-Time CRP Detection from Whole Blood Using Micropost-Embedded Microfluidic Chip Incorporated with Label-Free Biosensor. Analyst 2018, 143, 503–510. [Google Scholar] [CrossRef]
- Boonkaew, S.; Szot-Karpińska, K.; Niedziółka-Jönsson, J.; Pałys, B.; Jönsson-Niedziółka, M. Point-of-Care Testing for C-Reactive Protein in a Sequential Microfluidic Device. Sens. Actuators B Chem. 2023, 397, 134659. [Google Scholar] [CrossRef]


| Method | Material | Analysis Time | Device Price | Sample Type | Detection Range | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| ELISA | citicoline-BSA conjugate, AuNPs-aptamer nanozyme | >3 h | High | Rat blood | 0.1–200 ng/mL | 8 pg/mL | [6] |
| Immuno-turbidimetric | BSA, PEG-6000-CRP conjugate | 45 min | High | Human serum | 1–500 ng/mL | 0.54 ng/mL | [9] |
| LFIA | CRP capture antibody, polyclonal goat anti-mouse IgG | 30 min | High | Human serum | 0.1–500 ng/mL | 0.1 ng/mL | [12] |
| Fluorescent | Goat antibody to human CRP | -- | High | Serum | 0.4–52 mg/L | 0.4 mg/L | [14] |
| Chemiluminescence | Monoclonal mouse anti-CRP antibodies, Poly-HRP streptavidin | 30 min | High | Human serum | 10–100 ng/mL | 0.49 ng/mL | [17] |
| Electrochemical | Anti-CRP antibody | -- | High | Human serum | 0.1–1000 ng/mL | 1 pg/mL | [24] |
| Metal-enhanced chemiluminescence | Ab1 and detection antibodies (Ab2) of CRP | 60 min | High | Human serum | 0.7–7000 ng/mL | 0.05 ng/mL | [59] |
| Lab-on-Chip immunoassays | guided-mode resonance sensor intergrated microfluidic chip, CRP antibody | >2 h | Low | Rat blood | 0.64–5000 ng/mL | 3.2 ng/mL | [60] |
| Lab-on-Chip electrochemical | P3-CRP peptide | 60 min | High | Human serum | 0.01~105 ng/mL | 47 pg/mL | [61] |
| Proposed method | Polyclonal Anti-CRP Antibody, Mouse Anti-Human CRP Capture Antibody | <50 min | Low | Human serum and urine | 1–1500 ng/mL | 0.1 ng/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-J.; Lu, S.-Y.; Tseng, C.-C.; Huang, K.-H.; Chen, T.-L.; Fu, L.-M. Rapid Microfluidic Immuno-Biosensor Detection System for the Point-of-Care Determination of High-Sensitivity Urinary C-Reactive Protein. Biosensors 2024, 14, 283. https://doi.org/10.3390/bios14060283
Chen S-J, Lu S-Y, Tseng C-C, Huang K-H, Chen T-L, Fu L-M. Rapid Microfluidic Immuno-Biosensor Detection System for the Point-of-Care Determination of High-Sensitivity Urinary C-Reactive Protein. Biosensors. 2024; 14(6):283. https://doi.org/10.3390/bios14060283
Chicago/Turabian StyleChen, Szu-Jui, Song-Yu Lu, Chin-Chung Tseng, Kuan-Hsun Huang, To-Lin Chen, and Lung-Ming Fu. 2024. "Rapid Microfluidic Immuno-Biosensor Detection System for the Point-of-Care Determination of High-Sensitivity Urinary C-Reactive Protein" Biosensors 14, no. 6: 283. https://doi.org/10.3390/bios14060283
APA StyleChen, S. -J., Lu, S. -Y., Tseng, C. -C., Huang, K. -H., Chen, T. -L., & Fu, L. -M. (2024). Rapid Microfluidic Immuno-Biosensor Detection System for the Point-of-Care Determination of High-Sensitivity Urinary C-Reactive Protein. Biosensors, 14(6), 283. https://doi.org/10.3390/bios14060283






