Heat Stress-Mediated Activation of Immune–Inflammatory Pathways
Abstract
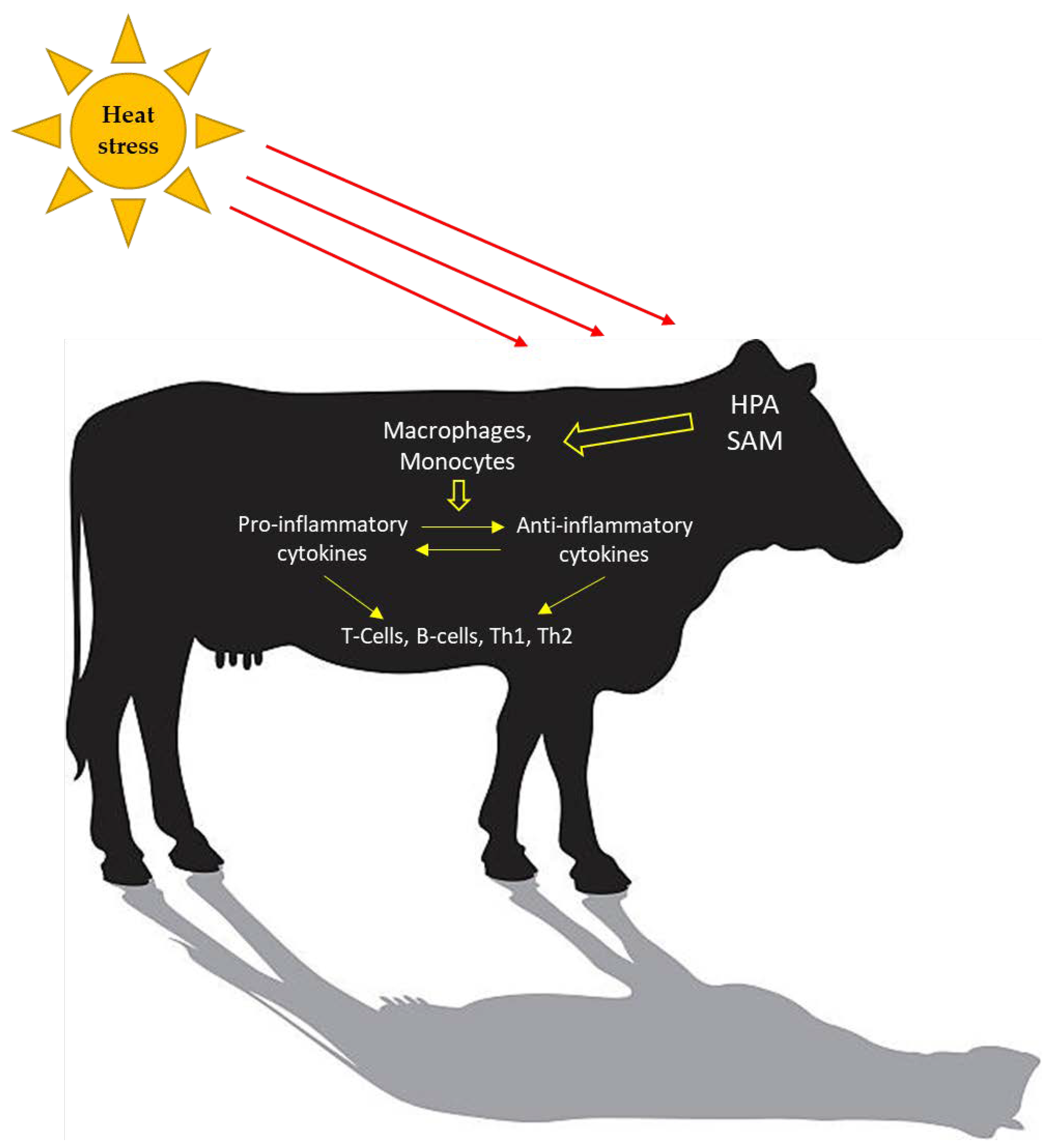
:1. Introduction
2. How Does Heat Stress Affect the Structure and Function of the Intestinal Mucosa?
2.1. Structural Aspects
2.2. Functional Aspects
3. What Components of the Immune System Are Activated during Heat Stress?
3.1. Heat Shock Proteins
3.2. Toll-Like Receptors
3.3. Reactive Oxygen Species
4. Nutritional Interventions to Avoid or Lessen the Effects of Heat Stress
4.1. Dietary Amino Acids
| Animal Model | Heat Stress Protocol 1 | Days of Sampling 2 | Nutritional Interventions | Intestinal Morphology | Intestinal Barrier Function | Ref. 14 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 3 | Product | Tissue | Item 10 | Change 11 | Tissue | AJ or TJ Protein 13 | Change | ||||
| Pigs | 35 ± 1.0 °C—12 h/day | 30 | EAA | L-arginine (1% of diet) | Jejunum | VH, V:C | ↑ | Jejunum | ZO-1 (mRNA) | = | [22] |
| CD | = | OCLD (mRNA) | ↑ | ||||||||
| Rat | 40 °C—3 h/day | 3 | EAA | L-arginine (250 mg/kg BW) | Jejunum | VH | ↑ | Jejunum | ZO-1, CLDN1 (mRNA) | ↑ | [101] |
| CD | ↓ | ||||||||||
| Rats | 40 °C—3 h/day | 3 | EAA | L-arginine (0.5% of diet) | Jejunum | VH, V:C | ↑ | Jejunum | ZO-1, OCLD, CLDN6, E-Cadherin (mRNA) | ↑ | [104] |
| CD | = | ZO-1, OCLD, CLDN6, E-Cadherin | ↑ | ||||||||
| Rats | 45 °C—25 min/day | 4 h | Prebiotic | Yeast culture 4 | SI12 | ZO-1, OCLD, CLDN, JAM-A | ↑ | [108] | |||
| Rats | 45 °C—25 min/day | 4 h | Prebiotic | Yeast culture | VH, MT | ↑ | [109] | ||||
| Broilers | 38 ± 1.0 °C—8 h/day | 5 | Prebiotic | GOS 5 (1% of diet) | Jejunum | E-Cadherin | ↓ | [49] | |||
| CLDN1, CLDN5, ZO-1 | = | ||||||||||
| Ileum | E-Cadherin, CLDN1, CLDN5, ZO-1 | = | |||||||||
| GOS (2.5% of diet) | Jejunum | E-cadherin, CLDN5, ZO-1 | ↓ | ||||||||
| CLDN1 | = | ||||||||||
| Ileum | E-Cadherin, CLDN 1, CLDN5, ZO-1 | = | |||||||||
| Broilers | 33 °C—10 h/day | 20 | Probiotic | Probiotic A 6 | Jejunum | VH | ↑ | OCLD | ↑ | [19] | |
| CD, V:C | = | ZO-1 | = | ||||||||
| Broilers | 35 ± 2 °C—24 h/day | 21 | Prebiotic | MOS 7 | Ileum | VH | ↓ | [110] | |||
| VW, VSA | = | ||||||||||
| CD | ↑ | ||||||||||
| Probiotic | Probiotic B 8 | VH | ↓ | ||||||||
| VW, CD, VSA | ↑ | ||||||||||
| Pre + Pro | Combination 9 | VH | ↓ | ||||||||
| VW, VSA | = | ||||||||||
| CD | ↑ | ||||||||||
| Broilers | 35 ± 2 °C—24 h/day | 42 | Prebiotic | MOS | VH, CD, VSA | = | [110] | ||||
| VW | ↑ | ||||||||||
| Probiotic | Probiotic B | VH, VW, CD, VSA | = | ||||||||
| Pre + Pro | Combination | VH, CD, VSA | ↑ | ||||||||
| VW | = | ||||||||||
| Rats | 40 °C—2 h/day | 3 | Antioxidant | Ferulic acid (50 mg/kg diet) | Jejunum | E-cadherin, OCLD, ZO-1 | ↑ | [44] | |||
4.2. Probiotic and Prebiotics
4.3. Antioxidants
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fajardo, L.F. Pathological effects of hyperthermia in normal tissues. Cancer Res. 1984, 44, 4826–4836. [Google Scholar]
- Lambert, G.P.; Gisolfi, C.V.; Berg, D.J.; Moseley, P.L.; Oberley, L.W.; Kregel, K.C. Selected Contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. 2002, 92, 1750–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Buettner, G.R.; Oberley, L.W.; Xu, L.; Matthes, R.D.; Gisolfi, C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol.—Heart Circ. Physiol. 2001, 280, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, J.W.; Hale, B.J.; Seibert, J.T.; Romoser, M.R.; Adur, M.K.; Keating, A.F.; Baumgard, L.H. Physiological mechanisms through which heat stress compromises reproduction in pigs. Mol. Reprod. Dev. 2017, 84, 934–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayorga, E.J.; Renaudeau, D.; Ramirez, B.C.; Ross, J.W.; Baumgard, L.H. Heat stress adaptations in pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bloemhof, S.; Van Der Waaij, E.H.; Merks, J.W.M.; Knol, E.F. Sow line differences in heat stress tolerance expressed in reproductive performance traits. J. Anim. Sci. 2008, 86, 3330–3337. [Google Scholar] [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Fouad, A.M.; Chen, W.; Ruan, D.; Wang, S.; Xia, W.G.; Zheng, C.T. Impact of heat stress on meat, egg quality, immunity and fertility in poultry and nutritional factors that overcome these effects: A review. Int. J. Poult. Sci. 2016, 15, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Tao, S.; Dahl, G.E. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological traits as affected by heat stress in sheep-A review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Oliver, S.R.R.; Phillips, N.A.A.; Novosad, V.L.L.; Bakos, M.P.P.; Talbert, E.E.E.; Clanton, T.L.L. Hyperthermia induces injury to the intestinal mucosa in the mouse: Evidence for an oxidative stress mechanism. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2012, 302, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef] [Green Version]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Koch, F.; Thom, U.; Albrecht, E.; Weikard, R.; Nolte, W.; Kuhla, B.; Kuehn, C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. USA 2019, 116, 10333–10338. [Google Scholar] [CrossRef] [Green Version]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, S431–S444. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; Tersteeg-Zijderveld, M.H.G.; Koolmees, P.A.; Fink-Gremmels, J. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian Pathol. 2015, 44, 19–22. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Chang, Q.; Guo, G.; Lan, R. Effects of chitosan oligosaccharides on intestinal oxidative stress and inflammation response in heat stressed rats. Exp. Anim. 2020, 70, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Xiong, Y.; Wu, Q.; Wang, M.; Liu, S.; Jiang, Z.; Wang, L. Effects of dietary supplementation with l-arginine on the intestinal barrier function in finishing pigs with heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.P.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 2013, 91, 5183–5193. [Google Scholar] [CrossRef] [PubMed]
- Wickramasuriya, S.S.; Kim, E.; Cho, H.M.; Shin, T.K.; Kim, B.; Lee, M.; Seo, S.; Heo, J.M.; Choi, H. Differential effects of dietary methionine isomers on broilers challenged with acute heat stress. J. Poult. Sci. 2019, 56, 195–203. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef]
- Yu, J.; Liu, F.; Yin, P.; Zhao, H.; Luan, W.; Hou, X.; Zhong, Y.; Jia, D.; Zan, J.; Ma, W.; et al. Involvement of oxidative stress and mitogen-activated protein kinase signaling pathways in heat stress-induced injury in the rat small intestine. Stress 2013, 16, 99–113. [Google Scholar] [CrossRef]
- Cheng, K.; Song, Z.; Li, S.; Yan, E.; Zhang, H.; Zhang, L.; Wang, C.; Wang, T. Effects of resveratrol on intestinal oxidative status and inflammation in heat-stressed rats. J. Therm. Biol. 2019, 85, 102415. [Google Scholar] [CrossRef]
- Liu, F.; Yin, J.; Du, M.; Yan, P.; Xu, J.; Zhu, X.; Yu, J. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling. J. Anim. Sci. 2009, 87, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhu, X.; Luan, W.; Xu, J. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp. Biochem. Physiol. Part A 2010, 156, 119–128. [Google Scholar] [CrossRef]
- Burrin, D.G.; Stoll, B.; Jiang, R.; Chang, X.; Hartmann, B.; Holst, J.J.; Greeley, G.H.; Reeds, P.J. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: How much is enough? Am. J. Clin. Nutr. 2000, 71, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.S.; Begum, S.M.K.N.; Rahman, M.M.; Mazumder, R.N.; Parvez, M.; Gazi, M.A.; Hasan, M.M.; Fahim, S.M.; Das, S.; Mahfuz, M.; et al. Alterations in the histological features of the intestinal mucosa in malnourished adults of Bangladesh. Sci. Rep. 2021, 11, 2355. [Google Scholar] [CrossRef]
- Nilaweera, K.N.; Speakman, J.R. Regulation of intestinal growth in response to variations in energy supply and demand. Obes. Rev. 2018, 19, 61–72. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Yan, M.; Xie, H.; Li, S.; Yu, Q.; He, S.; He, J. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016, 7, 1329–1338. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Lu, A.; Zhong, Y.; Hou, X.; Wang, N.; Jia, D.; Zan, J.; Zhao, H.; Xu, J.; et al. Reduction of intestinal mucosal immune function in heat-stressed rats and bacterial translocation. Int. J. Hyperth. 2012, 28, 756–765. [Google Scholar] [CrossRef]
- Darwich, A.S.; Aslam, U.; Ashcroft, D.M.; Rostami-Hodjegan, A. Meta-analysis of the turnover of intestinal epithelia in preclinical animal species and humans. Drug Metab. Dispos. 2014, 42, 2016–2022. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Huang, D.; Zhu, M.; Gao, C.; Yan, H.; Li, X.; Wang, X. Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress. J. Cell. Physiol. 2020, 235, 5613–5627. [Google Scholar] [CrossRef]
- Van Der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Sanz Fernandez, M.V.; Pearce, S.C.; Gabler, N.K.; Patience, J.F.; Wilson, M.E.; Socha, M.T.; Torrison, J.L.; Rhoads, R.P.; Baumgard, L.H. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal 2014, 8, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Moseley, P.L.; Gapen, C.; Wallen, E.S.; Walter, M.E.; Peterson, M.W. Thermal stress induces epithelial permeability. Am. J. Physiol.—Cell Physiol. 1994, 267, 425–434. [Google Scholar] [CrossRef]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Gabler, N.K.; Koltes, D.; Schaumberger, S.; Murugesan, G.R.; Reisinger, N. Diurnal heat stress reduces pig intestinal integrity and increases endotoxin translocation. Transl. Anim. Sci. 2018, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goo, D.; Kim, J.H.; Park, G.H.; Reyes, J.B.D.; Kil, D.Y. Effect of heat stress and stocking density on growth performance, breast meat quality, and intestinal barrier function in broiler chickens. Animals 2019, 9, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, V.; Rubach, J.K.; Sanders, D.J.; Pham, T.; Koltes, D.A.; Gabler, N.K.; Poss, M.J. Evaluation of the protective effects of zinc butyrate in IPEC-J2 cells and grower pigs under heat stress. Transl. Anim. Sci. 2019, 3, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Liu, F.; Xu, L.; Yin, P.; Li, D.; Mei, C.; Jiang, L.; Ma, Y.; Xu, J. Protective effects of ferulic acid against heat stress-induced intestinal epithelial barrier dysfunction in vitro and in vivo. PLoS ONE 2016, 11, e0145236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.C.; He, S.H.; Zheng, P.Y. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. J. Gastroenterol. Hepatol. 2007, 22, 1823–1831. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Nasu, Y.; Ido, A.; Tanoue, S.; Hashimoto, S.; Sasaki, F.; Kanmura, S.; Setoyama, H.; Numata, M.; Funakawa, K.; Moriuchi, A.; et al. Hepatocyte growth factor stimulates the migration of gastric epithelial cells by altering the subcellular localization of the tight junction protein ZO-1. J. Gastroenterol. 2013, 48, 193–202. [Google Scholar] [CrossRef]
- Dokladny, K.; Ye, D.; Kennedy, J.C.; Moseley, P.L.; Ma, T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: Regulatory role of heat shock factor-1. Am. J. Pathol. 2008, 172, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.G. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 2000, 59, 55–63. [Google Scholar] [CrossRef]
- Yi, H.; Hu, W.; Chen, S.; Lu, Z.; Wang, Y. Cathelicidin-WA Improves Intestinal Epithelial Barrier Function and Enhances Host Defense against Enterohemorrhagic Escherichia coli O157:H7 Infection. J. Immunol. 2017, 198, 1696–1705. [Google Scholar] [CrossRef] [Green Version]
- Strong, R.A.; Silva, E.B.; Cheng, H.W.; Eicher, S.D. Acute brief heat stress in late gestation alters neonatal calf innate immune functions1. J. Dairy Sci. 2015, 98, 7771–7783. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef]
- Swanson, R.M.; Tait, R.G.; Galles, B.M.; Duffy, E.M.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Heat stress-induced deficits in growth, metabolic efficiency, and cardiovascular function coincided with chronic systemic inflammation and hypercatecholaminemia in ractopamine-supplemented feedlot lambs. J. Anim. Sci. 2019, 98, skaa168. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, Y.; Li, J.; Bao, W.; Li, G.; Gao, Y.; Gu, X. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: A proteomic approach. Int. J. Mol. Sci. 2016, 17, 393. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Wang, C.; Hao, Y.; Gu, X.; Wang, H. Chronic heat stress induces acute phase responses and serum metabolome changes in finishing pigs. Animals 2019, 9, 395. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Foster, D.N.; Shurson, G.C. Effects of feeding diets containing bacitracin methylene disalicylate to heat-stressed finishing pigs. J. Anim. Sci. 2011, 89, 1830–1843. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Bailey, H.R.; Kennedy, A.M.; Löffler, F.E.; Ríus, A.G. Cooling and dietary crude protein affected milk production on heat-stressed dairy cows. Livest. Sci. 2020, 240, 104111. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Seidler, Y.; Bailey, H.R.; Whitacre, L.; Bargo, F.; Lüersen, K.; Rimbach, G.; Pighetti, G.M.; Ipharraguerre, I.R.; Ríus, A.G. A postbiotic from Aspergillus oryzae attenuates the impact of heat stress in ectothermic and endothermic organisms. Sci. Rep. 2021, 11, 6407. [Google Scholar] [CrossRef]
- Lim, C.L.; Wilson, G.; Brown, L.; Coombes, J.S.; Mackinnon, L.T. Pre-existing inflammatory state compromises heat tolerance in rats exposed to heat stress. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R186–R194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritossa, F. Discovery of the heat shock response. Cell Stress Chaperones 1996, 1, 97. [Google Scholar] [CrossRef] [Green Version]
- De Maio, A.; Gabriella Santoro, M.; Tanguay, R.M.; Hightower, L.E. Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: A new view of biology, a new society, and a new journal. Cell Stress Chaperones 2012, 17, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Chauhan, N.R.; Chowdhury, D.; Singh, A.; Meena, R.C.; Chakrabarti, A.; Singh, S.B. Heat stress modulated gastrointestinal barrier dysfunction: Role of tight junctions and heat shock proteins. Scand. J. Gastroenterol. 2017, 52, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [Green Version]
- Archana, P.R.; Aleena, J.; Pragna, P.; Vidya, M.K.; Abdul Niyas, P.A.; Bagath, M.; Krishnan, G.; Manimaran, A.; Beena, V.; Kurien, E.K.; et al. Role of Heat Shock Proteins in Livestock Adaptation to Heat Stress. J. Dairy Vet. Anim. Res. 2017, 5, 00127. [Google Scholar] [CrossRef]
- Hao, Y.; Feng, Y.; Li, J.; Gu, X. Role of MAPKs in HSP70’s Protection against Heat Stress-Induced Injury in Rat Small Intestine. Biomed Res. Int. 2018, 2018, 1571406. [Google Scholar] [CrossRef] [Green Version]
- Zhen, F.S.; Du, H.L.; Xu, H.P.; Luo, Q.B.; Zhang, X.Q. Tissue and allelic-specific expression of hsp70 gene in chickens: Basal and heat-stress-induced mRNA level quantified with real-time reverse transcriptase polymerase chain reaction. Br. Poult. Sci. 2006, 47, 449–455. [Google Scholar] [CrossRef]
- Manzerra, P.; Rush, S.J.; Brown, I.R. Tissue-Specific differences in heat shock protein hsc70 and hsp70 in the control and and hyperthermic rabbit. J. Cell. Physiol. 1997, 170, 130–137. [Google Scholar] [CrossRef]
- Leandro, N.S.M.; Gonzales, E.; Ferro, J.A.; Ferro, M.I.T.; Givisiez, P.E.N.; Macari, M. Expression of heat shock protein in broiler embryo tissues after acute cold or heat stress. Mol. Reprod. Dev. 2004, 67, 172–177. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Kang, D.; Park, J.; Choi, H.W.; Shim, K. Acute heat stress induces the differential expression of heat shock proteins in different sections of the small intestine of chickens based on exposure duration. Animals 2020, 10, 1234. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef]
- Asea, A.; Kraeft, S.K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 stimulates cytokine production through a CD 14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000, 6, 435–442. [Google Scholar] [CrossRef]
- Swaroop, S.; Mahadevan, A.; Shankar, S.K.; Adlakha, Y.K.; Basu, A. HSP60 critically regulates endogenous IL-1β production in activated microglia by stimulating NLRP3 inflammasome pathway. J. Neuroinflamm. 2018, 15, 177. [Google Scholar] [CrossRef]
- Martine, P.; Rébé, C. Heat shock proteins and inflammasomes. Int. J. Mol. Sci. 2019, 20, 4508. [Google Scholar] [CrossRef] [Green Version]
- Orhan, C.; Akdemir, F.; Sahin, N.; Tuzcu, M.; Komorowski, J.R.; Hayirli, A.; Sahin, K. Chromium histidinate protects against heat stress by modulating the expression of hepatic nuclear transcription factors in quail. Br. Poult. Sci. 2012, 53, 828–835. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Hatada, E.N.; Krappmann, D.; Scheidereit, C. NF-κB and the innate immune response. Curr. Opin. Immunol. 2000, 12, 52–58. [Google Scholar] [CrossRef]
- Sallustio, F.; Curci, C.; Stasi, A.; De Palma, G.; Divella, C.; Gramignoli, R.; Castellano, G.; Gallone, A.; Gesualdo, L. Role of toll-like receptors in actuating stem/progenitor cell repair mechanisms: Different functions in different cells. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Dangi, S.S.; Gupta, M.; Singh, J.; Thakur, N.; Naskar, S.; Nanda, P.K.; Mohanty, N.; Das, A.K.; Bandopadhayay, S.; et al. Expression of TLR genes in Black Bengal goat (Capra hircus) during different seasons. Small Rumin. Res. 2015, 124, 17–23. [Google Scholar] [CrossRef]
- Vandana, G.D.; Bagath, M.; Sejian, V.; Krishnan, G.; Beena, V.; Bhatta, R. Summer season induced heat stress impact on the expression patterns of different toll-like receptor genes in Malabari goats. Biol. Rhythm Res. 2019, 50, 466–482. [Google Scholar] [CrossRef]
- Dehbi, M.; Uzzaman, T.; Baturcam, E.; Eldali, A.; Ventura, W.; Bouchama, A. Toll-Like Receptor 4 and High-Mobility Group Box 1 Are Critical Mediators of Tissue Injury and Survival in a Mouse Model for Heatstroke. PLoS ONE 2012, 7, e44100. [Google Scholar] [CrossRef]
- Ju, X.-H.; Xu, H.-J.; Yong, Y.-H.; An, L.-L.; Jiao, P.-R.; Liao, M. Heat stress upregulation of Toll-like receptors 2/4 and acute inflammatory cytokines in peripheral blood mononuclear cell (PBMC) of Bama miniature pigs: An in vivo and in vitro study. Animal 2014, 8, 1462–1468. [Google Scholar] [CrossRef] [Green Version]
- Sophia, I.; Sejian, V.; Bagath, M.; Bhatta, R. Quantitative expression of hepatic toll-like receptors 1–10 mRNA in Osmanabadi goats during different climatic stresses. Small Rumin. Res. 2016, 141, 11–16. [Google Scholar] [CrossRef]
- Eslamizad, M.; Albrecht, D.; Kuhla, B. The effect of chronic, mild heat stress on metabolic changes of nutrition and adaptations in rumen papillae of lactating dairy cows. J. Dairy Sci. 2020, 103, 8601–8614. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol.—Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Liu, Q.; Zhang, S.; Peng, M.; Zhou, J.; Chen, L.; Fang, F. Effects of duration of thermal stress on growth performance, serum oxidative stress indices, the expression and localization of ABCG2 and mitochondria ROS production of skeletal muscle, small intestine and immune organs in broilers. J. Therm. Biol. 2019, 85, 102420. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Xie, W.; Gu, Z.; Xu, Q.; Su, L. Oxidative stress regulates mitogen-activated protein kinases and c-Jun activation involved in heat stress and lipopolysaccharide-induced intestinal epithelial cell apoptosis. Mol. Med. Rep. 2017, 16, 2579–2587. [Google Scholar] [CrossRef] [Green Version]
- Land, W. Allograft injury mediated by reactive oxygen species: From conserved proteins of Drosophila to acute and chronic rejection of human transplants. Part II: Role of reactive oxygen species in the induction of the heat shock response as a regulator of innate. Transplant. Rev. 2003, 17, 31–44. [Google Scholar] [CrossRef]
- Wang, L.; Xue, B.; Wang, K.; Li, S.; Li, Z. Effect of heat stress on endotoxin flux across mesenteric-drained and portal-drained viscera of dairy goat. J. Anim. Physiol. Anim. Nutr. 2011, 95, 468–477. [Google Scholar] [CrossRef]
- Wang, W.W.; Qiao, S.Y.; Li, D.F. Amino acids and gut function. Amino Acids 2009, 37, 105–110. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, W.; Wu, L.Y. Dietary L-arginine supplement alleviates hepatic heat stress and improves feed conversion ratio of Pekin ducks exposed to high environmental temperature. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1124–1131. [Google Scholar] [CrossRef]
- Pate, R.T.; Luchini, D.; Murphy, M.R.; Cardoso, F.C. Effects of rumen-protected methionine on lactation performance and physiological variables during a heat stress challenge in lactating Holstein cows. J. Dairy Sci. 2020, 103, 2800–2813. [Google Scholar] [CrossRef]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino acids as mediators of metabolic cross talk between host and pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef]
- Zhu, H.L.; Liu, Y.L.; Xie, X.L.; Huang, J.J.; Hou, Y.Q. Effect of l-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun. 2013, 19, 242–252. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Helou, H.; Mogilner, J.; Lurie, M.; Bernsteyn, A.; Coran, A.G.; Shiloni, E. Oral arginine improves intestinal recovery following ischemia-reperfusion injury in rat. Pediatr. Surg. Int. 2005, 21, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Huang, L.; Yin, P.; Liu, F.; Liu, Y.; Zhang, Z.; Lin, J.; Zou, W.; Li, C. l-Arginine alleviates heat stress-induced intestinal epithelial barrier damage by promoting expression of tight junction proteins via the AMPK pathway. Mol. Biol. Rep. 2019, 46, 6435–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, K.A.; Soares, A.D.N.; Wanner, S.P.; dos Santos, R.G.C.; Fernandes, S.O.A.; Martins, F.S.; Nicoli, J.R.; Coimbra, C.C.; Cardoso, V.N. L-arginine supplementation prevents increases in intestinal permeability and bacterial translocation in male swiss mice subjected to physical exercise under environmental heat stress. J. Nutr. 2014, 144, 218–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, K.; Guan, S.; Li, T.; Huang, R.; Wu, G.; Ruan, Z.; Yin, Y. Dietary L -arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br. J. Nutr. 2011, 105, 703–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Yin, P.; Liu, F.; Liu, Y.Y.; Liu, Y.Y.; Xia, Z. Protective effects of L-arginine on the intestinal epithelial barrier under heat stress conditions in rats and IEC-6 cell line. J. Anim. Physiol. Anim. Nutr. 2020, 104, 385–396. [Google Scholar] [CrossRef]
- Rowart, P.; Wu, J.; Caplan, M.J.; Jouret, F. Implications of AMPK in the formation of epithelial tight junctions. Int. J. Mol. Sci. 2018, 19, 2040. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Kalvandi, O.; Sadeghi, A.; Karimi, A. Methionine supplementation improves reproductive performance, antioxidant status, immunity and maternal antibody transmission in breeder Japanese quail under heat stress conditions. Arch. Anim. Breed. 2019, 62, 275–286. [Google Scholar] [CrossRef]
- Ducray, H.A.G.; Globa, L.; Pustovyy, O.; Morrison, E.; Vodyanoy, V.; Sorokulova, I. Yeast fermentate prebiotic improves intestinal barrier integrity during heat stress by modulation of the gut microbiota in rats. J. Appl. Microbiol. 2019, 127, 1192–1206. [Google Scholar] [CrossRef]
- Ducray, H.A.G.; Globa, L.; Pustovyy, O.; Reeves, S.; Robinson, L.; Vodyanoy, V.; Sorokulova, I. Mitigation of heat stress-related complications by a yeast fermentate product. J. Therm. Biol. 2016, 60, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Ijaz, A.; Sohail, A.; Shabbir, M.Z.; Rehman, H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhang, B.X.; Yao, K.Y.; Yoon, I.; Chung, Y.H.; Wang, J.K.; Liu, J.X. Effects of supplemental levels of Saccharomyces cerevisiae fermentation product on lactation performance in dairy cows under heat stress. Asian Australas. J. Anim. Sci. 2016, 29, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Al-Qaisi, M.; Horst, E.A.; Mayorga, E.J.; Goetz, B.M.; Abeyta, M.A.; Yoon, I.; Timms, L.L.; Appuhamy, J.A.; Baumgard, L.H. Effects of a Saccharomyces cerevisiae fermentation product on heat-stressed dairy cows. J. Dairy Sci. 2020, 103, 9634–9645. [Google Scholar] [CrossRef]
- Liu, J.; Ye, G.; Zhou, Y.; Liu, Y.; Zhao, L.; Liu, Y.; Chen, X.; Huang, D.; Liao, S.F.; Huang, K. Feeding glycerol-enriched yeast culture improves performance, energy status, and heat shock protein gene expression of lactating Holstein cows under heat stress. J. Anim. Sci. 2014, 92, 2494–2502. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Ye, G.; Gan, F.; Hamid, M.; Liao, S.; Huang, K. Protective effects of zymosan on heat stress-induced immunosuppression and apoptosis in dairy cows and peripheral blood mononuclear cells. Cell Stress Chaperones 2018, 23, 1069–1078. [Google Scholar] [CrossRef]
- Gan, F.; Ren, F.; Chen, X.; Lv, C.; Pan, C.; Ye, G.; Shi, J.; Shi, X.; Zhou, H.; Shituleni, S.A.; et al. Effects of selenium-enriched probiotics on heat shock protein mRNA levels in piglet under heat stress conditions. J. Agric. Food Chem. 2013, 61, 2385–2391. [Google Scholar] [CrossRef]
- Zhong, Y.; Cai, D.; Cai, W.; Geng, S.; Chen, L.; Han, T. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin. Nutr. 2009, 28, 575–580. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.T.; Schoterman, M.H.C.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell monolayers and B6C3F1 mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef] [Green Version]
- Varasteh, S.; Braber, S.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides exert a protective effect against heat stress in a Caco-2 cell model. J. Funct. Foods 2015, 16, 265–277. [Google Scholar] [CrossRef]
- Calamari, L.; Petrera, F.; Abeni, F.; Bertin, G. Metabolic and hematological profiles in heat stressed lactating dairy cows fed diets supplemented with different selenium sources and doses. Livest. Sci. 2011, 142, 128–137. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Celi, P.; Leury, B.J.; Clarke, I.J.; Dunshea, F.R. Dietary antioxidants at supranutritional doses improve oxidative status and reduce the negative effects of heat stress in sheep. J. Anim. Sci. 2014, 92, 3364–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibian, M.; Sadeghi, G.; Ghazi, S.; Moeini, M.M. Selenium as a feed supplement for heat-stressed poultry: A review. Biol. Trace Elem. Res. 2015, 165, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Celi, P.; Cottrell, J.J.; Chauhan, S.S.; Leury, B.J.; Dunshea, F.R. Effects of a short-term supranutritional selenium supplementation on redox balance, physiology and insulin-related metabolism in heat-stressed pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Ringuet, M.; Furness, J.B.; Dunshea, F.R. Betaine and antioxidants improve growth performance, breast muscle development and ameliorate thermoregulatory responses to cyclic heat exposure in broiler chickens. Animals 2018, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Cao, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; Shang, H.; Zhao, H. The protective effect of selenium from heat stress-induced porcine small intestinal epithelial cell line (IPEC-J2) injury is associated with regulation expression of selenoproteins. Br. J. Nutr. 2019, 122, 1081–1090. [Google Scholar] [CrossRef]
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Song, J.; Wang, N.; Liu, Q. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Int. J. Hyperth. 2018, 35, 112–121. [Google Scholar] [CrossRef]

| Animal Model | Heat Stress Protocol 1 | Days of Sampling 2 | Tissue | Villus Height | Crypt Depth | V:C 5 | Ref. 6 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Change 3 | % 4 | Change | % | Change | % | |||||
| Broilers | 33 °C–10 h/day–20 days | 20 | Jejunum | ↓ | 18.5 | ↑ | 10.0 | ↓ | 23.3 | [19] |
| Broilers | 39 ± 1 °C–8 h/day–4 days | 4 | Duodenum | ↓ | 18.4 | = | - | ↓ | 50.5 | [20] |
| Jejunum | ↓ | 17.6 | ↑ | 17.0 | = | - | ||||
| Ileum | ↓ | 20.2 | = | - | ↓ | 40.0 | ||||
| Broilers | 37 ± 2 °C–8 h/day–15 days | 15 | Jejunum | ↓ | 27.7 | ↑ | 28.2 | ↓ | 43.1 | [33] |
| Ileum | ↓ | 24.7 | ↑ | 28.8 | ↓ | 37.0 | ||||
| Broilers | 37 ± 1 °C —10 h/day–21 days | 21 | Jejunum | ↓ | 18.6 | ↑ | 38.2 | ↓ | 39.1 | [25] |
| Broilers | 33 ± 0.5 °C–3 h/day–1 day | 1 | Ileum | = | - | = | - | = | - | [24] |
| 7 † | Ileum | ↓ | 22.6 | ↑ | 14.5 | ↓ | 31.4 | |||
| Rats | 40 °C–2 h/day–10 days | 3 | Duodenum | ↓ | 21.9 | ↓ | 36.4 | NA7 | NA | [26] |
| Jejunum | ↓ | 33.1 | ↓ | 30.5 | NA | NA | ||||
| Ileum | ↓ | 36.1 | ↓ | 32.5 | NA | NA | ||||
| Rats | 40 ± 1 °C–1.5 h/day–3 days | 3 | Jejunum | ↓ | 22.2 | = | - | ↓ | 30.6 | [27] |
| Rats | 35 ± 1 °C–4 h/day–7 days | 7 | Duodenum | ↓ | 14.8 | = | - | = | - | [21] |
| Jejunum | ↓ | 28.9 | = | - | = | - | ||||
| Ileum | ↓ | 36.8 | = | - | ↓ | 21.0 | ||||
| Pigs | 40 °C–5 h/day–10 days | 1 | Duodenum | ↓ | 12.3 | = | - | = | - | |
| Jejunum | ↓ | 20.8 | ↓ | 17.4 | ↓ | 6.3 | [28] | |||
| Ileum | ↓ | 11.2 | = | - | = | - | ||||
| 3 | Duodenum | ↓ | 11.8 | ↓ | 23.1 | ↑ | 13.3 | |||
| Jejunum | ↓ | 18.8 | ↓ | 22.1 | = | - | ||||
| Ileum | ↓ | 10.4 | = | - | = | - | ||||
| Pigs | 40 °C–5 h/day–10 days | 1 | Duodenum | ↓ | 8.8 | = | - | NA | NA | [29] |
| Jejunum | ↓ | 21.3 | ↓ | 15.9 | NA | NA | ||||
| Ileum | = | - | = | - | NA | NA | ||||
| 3 | Duodenum | ↓ | 10.6 | = | - | NA | NA | |||
| Jejunum | ↓ | 22.2 | ↓ | 18.7 | NA | NA | ||||
| Ileum | ↓ | 9.7 | = | - | NA | NA | ||||
| Pigs | 35 ± 1 °C–24 h/day–7 days | 1 | Jejunum | ↓ | 14.6 | ↑ | 5.2 | ↓ | 17.6 | [23] |
| 3 | Jejunum | ↓ | 20.4 | ↑ | 4.5 | ↓ | 23.5 | |||
| 7 | Jejunum | ↓ | 22.9 | ↓ | 4.5 | ↓ | 17.6 | |||
| Pigs | 35 °C–12 h/day–30 days | 30 | Jejunum | ↓ | NA | = | - | ↓ | NA | [22] |
| HSP/TLR | Function | Aliases | Localization | Agents/Factors/Domains |
|---|---|---|---|---|
| HSP90 | Protects cells by preventing protein aggregation and enables protein stabilization and trafficking. It also facilitates the activation of numerous regulated proteins. | HSP90AA1, HSP90AB1, HSP90AA2P, HSP90B1 | Extracellular, Mitochondrion, Nucleus, Cytosol, Lysosome | HSF1 regulates the activation and release of HSP90 by binding the heat shock elements with the HSP90 promotors. |
| HSP70 | Helps induce protein folding and prevent protein aggregation. | HSPA4, HSPA1A, HSPA8, HSPA14, HSPA1B, HSPA5 | Extracellular, Nucleus, Cytosol | Gram-negative bacteria like E coli and their proteins. |
| HSP27 | Protect cells from oxidative stress by reducing the ROS through increased production of glutathione. | HSPB1 | Endoplasmic reticulum (ER), Cytoplasm | HSP20-like_chaperone, A-crystallin, Alpha-crystallin, ACD, HspB1. |
| TLR1 | Recognizes the pathogen-associated molecular patterns (PAMPs). | CD281 antigen | Plasma membrane, Golgi apparatus | Diacylated and triacylated lipopeptides. |
| TLR2 | Recognizes lipoteichoic acid (LTA). | CD282 antigen | Plasma membrane, Golgi apparatus | PAMPs. |
| TLR3 | Recognizes dsRNA. | CD283 antigen | Plasma membrane, Endosome, Lysosome | Viral dsRNA. |
| TLR4 | Recognizes lipopolysaccharide (LPS). | HToll, CD284 | Plasma membrane, Endosome | Triggered by the presence of Ni (2+). |
| TLR6 | Forms heterodimers with TLR2 and recognizes diacyl lipoproteins. | CD286 antigen | Plasma membrane, Golgi apparatus | Cooperates with LY96 and CD14 and acts via MYD88, TIRAP and TRAF6. |
| TLR7 | Recognizes the ssRNA of viruses and synthetic oligoribinucleotides such as imidazoquinoline and imiquimod. | IMD74 | Plasma membrane, Endosome, Lysosome | Uridine-containing single strand viral RNAs or guanosine analogs. |
| TLR8 | Recognizes various viral ssRNAs. | CD288 antigen | Plasma membrane, Endosome, Lysosome | GU-rich single-stranded RNA from SARS-CoV-2, SARS-CoV-1 and HIV-1 viruses. |
| TLR9 | Recognizes bacterial CpG-containing oligonucleotides (CpG ODNs). | CD274 molecule | Plasma membrane, ER, Endosome, Lysosome | Unmethylated cytidine-phosphate-guanosine (CpG) dinucleotides. |
| TLR10 | Enables transmembrane signalling receptor ability. | CD290 antigen | Plasma membrane | Acts via the MYD88 and TRAF6 proteins. |
| Animal Model | Heat Stress Protocol 1 | Days of Sampling 2 | Nutritional Interventions | Inflammation-Related Genes | Ref. 14 | |||
|---|---|---|---|---|---|---|---|---|
| Type 3 | Product | Tissue | mRNA Relative Expression | Change 13 | ||||
| Rat | 40 °C—3 h/day | 3 | EAA | L-arginine (250 mg/kg BW) | Jejunum | HSF1 | ↓ | [101] |
| HSP70, HSP90 | ↑ | |||||||
| Rats | 40 °C—3 h/day | 3 | EAA | L-arginine (0.5% diet) | Jejunum | NF-κΒ, IL-1β | ↓ | [104] |
| Cows | >74 THI—24 h/day | 60 | Prebiotic | Yeast culture 4 | PBL 11 | HSP70 | ↓ | [114] |
| Yeast culture 5 | HSP70 | ↓ | ||||||
| Cows | >72 THI—24 h/day | 28 | Prebiotic | Yeast extract 6 | Liver | HSP27, HSP90 | = | [115] |
| HSP70 | ↓ | |||||||
| Broilers | 38 ± 1.0 °C—8 h/day | 5 | Prebiotic | GOS7 (1% of diet) | Jejunum | IL-6 | = | [49] |
| HSP70, IL-8 | ↓ | |||||||
| HSF1, HSF3, HSP90 | = | |||||||
| Ileum | IL-6, IL-8, HSF1, HSF3, HSP70, HSP90 | = | ||||||
| GOS (2.5% of diet) | Jejunum | IL-6, IL-8, HSF3, HSP70, HSP90 | ↓ | |||||
| HSF1 | = | |||||||
| Ileum | IL-6, IL-8, HSF1, HSF3, HSP70, HSP90 | = | ||||||
| Pigs | 35 °C—8 h/day | 2 | Antioxidant | Se (0.3 ppm) and Vit E (50 IU/kg) | SI 12 | HSP70, HIF-1α, IL-8, TNF-α | = | [40] |
| Se (0.5 ppm) and Vit E (100 IU/kg) | SI | HSP70, HIF-1α, IL-8, TNF-α | = | |||||
| Se (1.0 ppm) and Vit E (200 IU/kg) | SI | HSP70, HIF-1α, IL-8, TNF-α | = | |||||
| Pigs | 25–38 °C—24h/day | 42 | Antioxidant | Se8 (0.46 mg/kg diet) | Liver | HSP70, HSP27 | ↓ | [116] |
| Kidney | HSP70, HSP27 | ↓ | ||||||
| Spleen | HSP70, HSP27 | ↓ | ||||||
| Probiotics | Probiotic mixture 9 (30 mL/kg diet) | Liver | HSP70, HSP27 | ↓ | ||||
| Kidney | HSP70, HSP27 | ↓ | ||||||
| Spleen | HSP70, HSP27 | ↓ | ||||||
| Mixture | Se + Probiotic 10 | Liver | HSP70, HSP27 | ↓ | ||||
| Kidney | HSP70, HSP27 | ↓ | ||||||
| Spleen | HSP70, HSP27 | ↓ | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantet, J.M.; Yu, Z.; Ríus, A.G. Heat Stress-Mediated Activation of Immune–Inflammatory Pathways. Antibiotics 2021, 10, 1285. https://doi.org/10.3390/antibiotics10111285
Cantet JM, Yu Z, Ríus AG. Heat Stress-Mediated Activation of Immune–Inflammatory Pathways. Antibiotics. 2021; 10(11):1285. https://doi.org/10.3390/antibiotics10111285
Chicago/Turabian StyleCantet, Juan M., Zhantao Yu, and Agustín G. Ríus. 2021. "Heat Stress-Mediated Activation of Immune–Inflammatory Pathways" Antibiotics 10, no. 11: 1285. https://doi.org/10.3390/antibiotics10111285
APA StyleCantet, J. M., Yu, Z., & Ríus, A. G. (2021). Heat Stress-Mediated Activation of Immune–Inflammatory Pathways. Antibiotics, 10(11), 1285. https://doi.org/10.3390/antibiotics10111285







