Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Plants
2.2. Extraction and Isolation
2.3. Characterisation of Secondary Metabolites Extracted from R. yaundensis and S. triloba
2.4. Acanthamoeba castellanii Cultures
2.5. Amoebicidal Assays
2.6. Henrietta Lacks Cervical Adenocarcinoma (HeLa) Cell Lines Cultivation
2.7. Adhesion Assays
2.8. Encystation Assays
2.9. Excystation Assays
2.10. In Vitro Cytotoxicity Assays
2.11. Amoeba-Mediated Host Cell Death
2.12. Statistical Analyses
3. Results
3.1. Plant-Based Natural Compounds Isolated from R. yaundensis and S. triloba
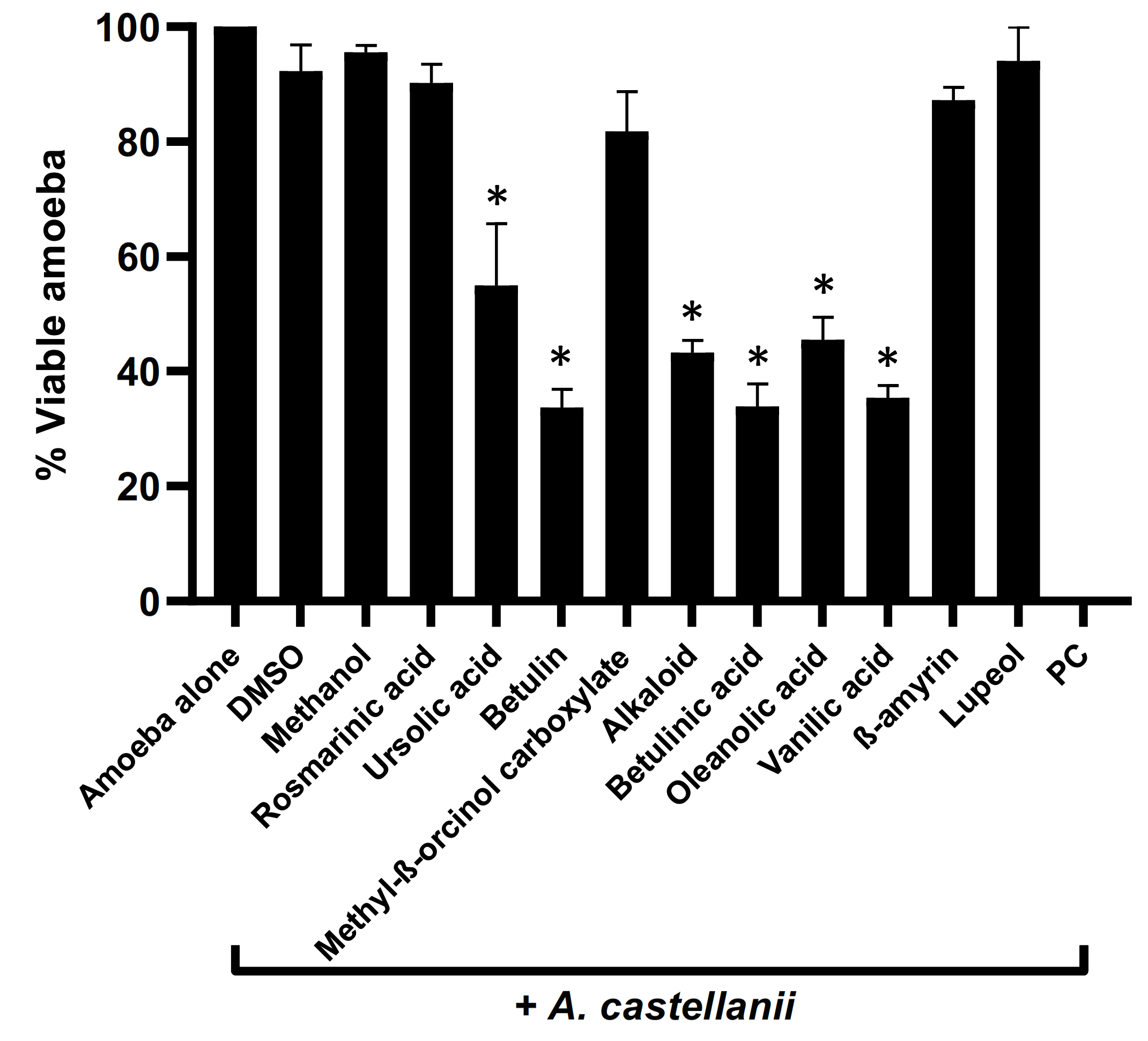
3.2. Plant-Based Natural Compounds Presented Effective Amoebicidal Activity against A. castellanii
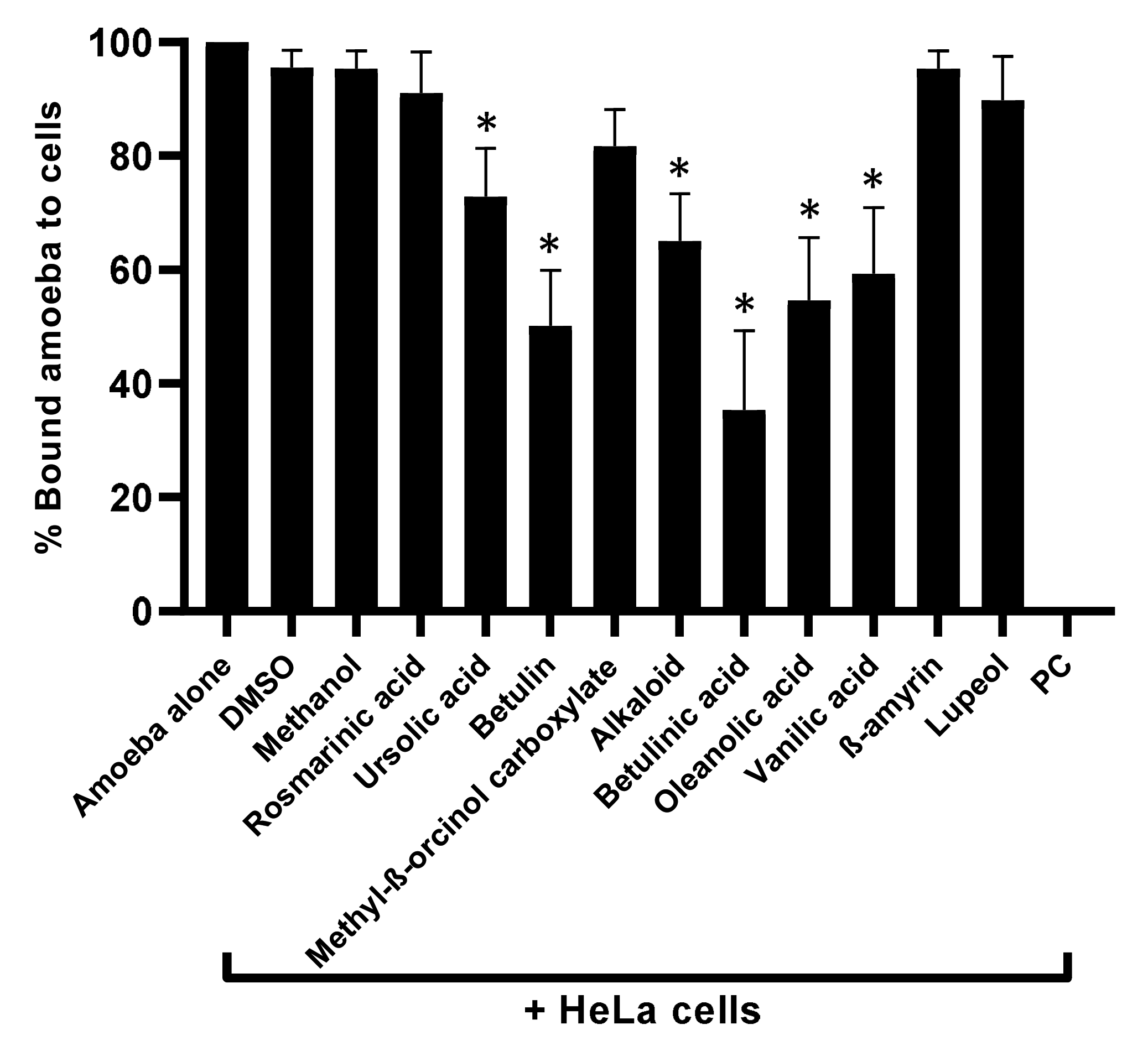
3.3. Natural Compounds Tested Blocked Amoebae Binding to Human Cells
3.4. Plant-Based Secondary Metabolites Considerably Inhibited Amoebae Encystation and Excystation
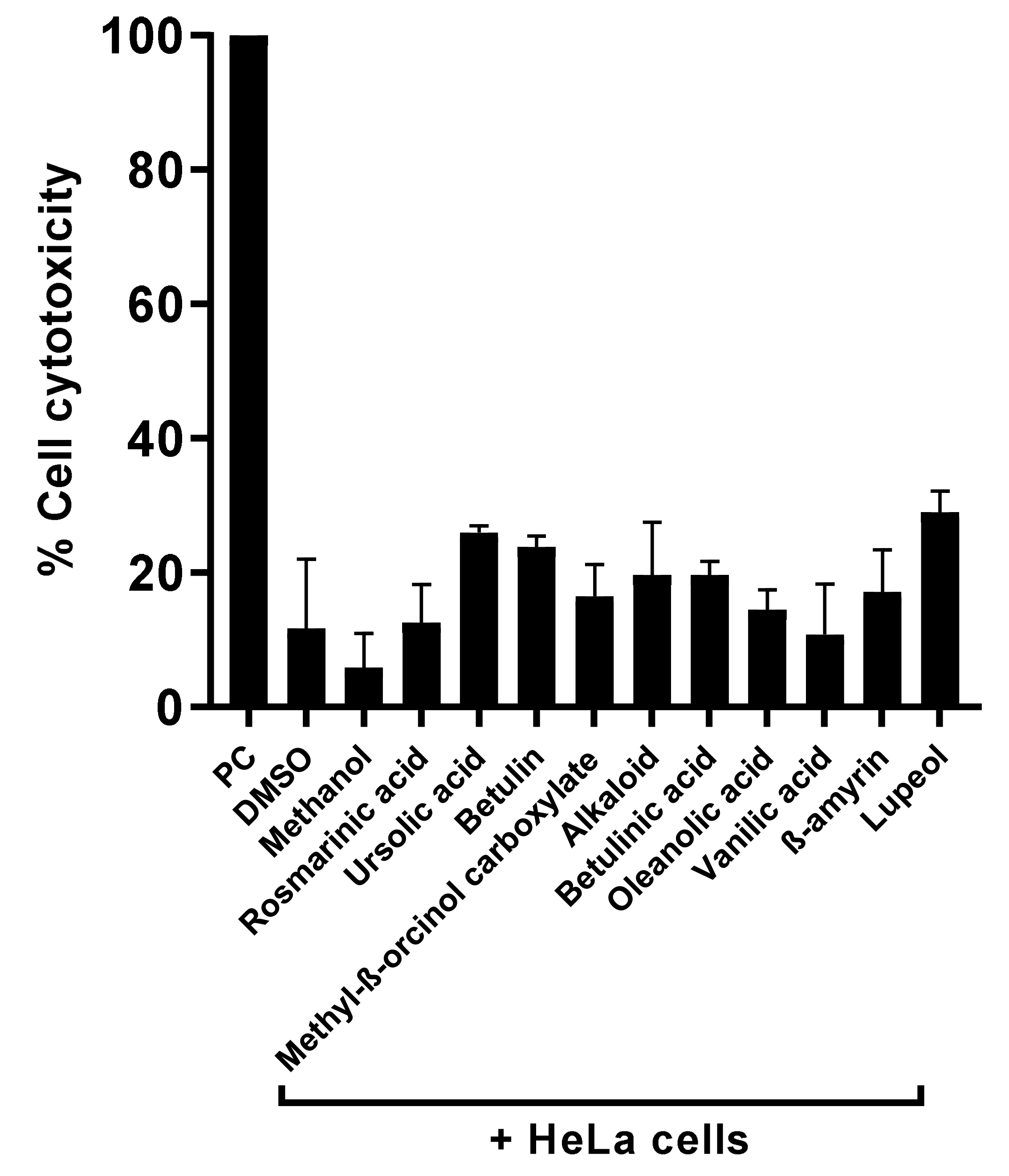
3.5. Natural Compounds Tested Showed Marginal Cytotoxic Properties against Human Cell Lines and Reduced Amoebae-Mediated Host Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo-Morales, J.; Martín-Navarro, C.M.; López-Arencibia, A.; Arnalich-Montiel, F.; Piñero, J.E.; Valladares, B. Acanthamoeba keratitis: An emerging disease gathering importance worldwide? Trends Parasitol. 2013, 29, 181–187. [Google Scholar] [CrossRef] [PubMed]
- De Lacerda, A.G.; Lira, M. Acanthamoeba keratitis: A review of biology, pathophysiology and epidemiology. Ophthalmic Physiol. Opt. 2021, 41, 116–135. [Google Scholar]
- Maciver, S.K.; Piñero, J.E.; Lorenzo-Morales, J. Is Naegleria fowleri an emerging parasite? Trends Parasitol. 2020, 36, 19–28. [Google Scholar]
- Aksozek, A.; McClellan, K.; Howard, K.; Niederkorn, J.Y.; Alizadeh, H. Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J. Parasitol. 2002, 88, 621–623. [Google Scholar] [CrossRef]
- Turner, N.A.; Russell, A.D.; Furr, J.R.; Lloyd, D. Resistance, biguanide sorption and biguanide-induced pentose leakage during encystment of Acanthamoeba castellanii. J. Appl. Microbiol. 2004, 96, 1287–1295. [Google Scholar]
- Varacalli, G.; Di Zazzo, A.; Mori, T.; Dohlman, T.H.; Spelta, S.; Coassin, M.; Bonini, S. Challenges in Acanthamoeba Keratitis: A Review. J. Clin. Med. 2021, 10, 942. [Google Scholar] [CrossRef]
- Abjani, F.; Khan, N.A.; Jung, S.Y.; Siddiqui, R. Status of the effectiveness of contact lens disinfectants in Malaysia against keratitis-causing pathogens. Exp. Parasitol. 2017, 183, 187–193. [Google Scholar] [CrossRef]
- Sangkanu, S.; Mitsuwan, W.; Mahboob, T.; Mahabusarakam, W.; Chewchanwuttiwong, S.; Siphakdi, P.; Jimoh, T.O.; Wilairatana, P.; Dolma, K.G.; de Lourdes Pereira, M.; et al. Phytochemical, anti-Acanthamoeba, and anti-adhesion properties of Garcinia mangostana flower as preventive contact lens solution. Acta Trop. 2022, 226, 106266. [Google Scholar] [CrossRef]
- Sifaoui, I.; Yanes, E.C.; Reyes-Batlle, M.; Rodríguez-Expósito, R.L.; Bazzocchi, I.L.; Jiménez, I.A.; Piñero, J.E.; Lorenzo-Morales, J.; Weaver, L.K. High oxygen concentrations inhibit Acanthamoeba spp. Parasitol. Res. 2021, 120, 3001–3005. [Google Scholar] [CrossRef]
- Mungroo, M.R.; Tong, T.; Khan, N.A.; Anuar, T.S.; Maciver, S.K.; Siddiqui, R. Development of anti-acanthamoebic approaches. Int. Microbiol. 2021, 24, 363–371. [Google Scholar] [CrossRef]
- Mungroo, M.R.; Khan, N.A.; Maciver, S.; Siddiqui, R. Opportunistic free-living amoebal pathogens. Pathog. Glob. Health 2021, 1–15. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Current strategies to treat Acanthamoeba keratitis: A patent overview. Pharm. Pat. Anal. 2020, 9, 135–137. [Google Scholar]
- Lackner, P.; Beer, R.; Broessner, G.; Helbok, R.; Pfausler, B.; Brenneis, C.; Auer, H.; Walochnik, J.; Schmutzhard, E. Acute granulomatous Acanthamoeba encephalitis in an immunocompetent patient. Neurocrit. Care 2010, 12, 91–94. [Google Scholar] [CrossRef]
- Padzik, M.; Hendiger, E.B.; Chomicz, L.; Grodzik, M.; Szmidt, M.; Grobelny, J.; Lorenzo-Morales, J. Tannic acid-modified silver nanoparticles as a novel therapeutic agent against Acanthamoeba. Parasitol. Res. 2018, 117, 3519–3525. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Chin, Y.W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. Am. Assoc. Pharm. Sci. J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [Green Version]
- Anyanwu, M.U.; Okoye, R.C. Antimicrobial activity of Nigerian medicinal plants. J. Intercult. Ethnopharmacol. 2017, 6, 240. [Google Scholar]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Majeed, L.R.; Nisar, B.; Nisar, H.; Bhat, A.A.; Ganai, B.A. Phytomedicines: Diversity, extraction, and conservation strategies. In Phytomedicine; Academic Press: Cambridge, MA, USA, 2021; pp. 1–33. [Google Scholar]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef]
- Costa, J.F.O.; Barbosa-Filho, J.M.; de Azevedo Maia, G.L.; Guimarães, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; de Carvalho, L.C.P.; Soares, M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Akin, M.; Sahin, B.; Saki, N. Hydroxypropyl methyl cellulose-Satureja hortensis L. ethanol extract mixtures as antimicrobial coating for sutures, identification of phenolic acids by using LC-MS-MS and TLC techniques. J. Liq. Chromatogr. Relat. Technol. 2022, 1–8. [Google Scholar] [CrossRef]
- Khatoon, B.; Zikr Ur Rehman, S.; Yousuf, S.; Lateef, M.; Essombo, M.F.A.; Kamdem Waffo, A.F.; Ali, M.S. New bioactive monoterpene indole alkaloid from Rinorea yaundensis. Engl. Nat. Prod. Res. 2020, 1–10. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Rodríguez-Talavera, I.; Sifaoui, I.; Rodríguez-Expósito, R.L.; Rocha-Cabrera, P.; Piñero, J.E.; Lorenzo-Morales, J. In vitro amoebicidal effects of arabinogalactan-based ophthalmic solution. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 9–16. [Google Scholar] [CrossRef]
- Saeed, B.Q.; Hussain, K.; Akbar, N.; Khan, H.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Nanovesicles containing curcumin hold promise in the development of new formulations of anti-Acanthamoebic agents. Mol. Biochem. Parasitol. 2021, 247, 111430. [Google Scholar] [CrossRef]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Siddiqui, R. Brain-eating amoebae: Silver nanoparticle conjugation enhanced efficacy of anti-amoebic drugs against Naegleria fowleri. ACS Chem. Neurosci. 2017, 8, 2626–2630. [Google Scholar] [CrossRef]
- Anwar, A.; Ting, E.L.S.; Anwar, A.; ul Ain, N.; Faizi, S.; Shah, M.R.; Khan, N.A.; Siddiqui, R. Antiamoebic activity of plant-based natural products and their conjugated silver nanoparticles against Acanthamoeba castellanii (ATCC 50492). AMB Express 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Aqeel, Y.; Siddiqui, R.; Iftikhar, H.; Khan, N.A. The effect of different environmental conditions on the encystation of Acanthamoeba castellanii belonging to the T4 genotype. Exp. Parasitol. 2013, 135, 30–35. [Google Scholar] [CrossRef]
- Lakhundi, S.; Khan, N.A.; Siddiqui, R. Inefficacy of marketed contact lens disinfection solutions against keratitis-causing Acanthamoeba castellanii belonging to the T4 genotype. Exp. Parasitol. 2014, 141, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Shing, B.; Singh, S.; Podust, L.M.; McKerrow, J.H.; Debnath, A. The antifungal drug isavuconazole is both amebicidal and cysticidal against Acanthamoeba castellanii. Antimicrob. Agents Chemother. 2020, 64, e02223-19. [Google Scholar] [CrossRef] [PubMed]
- Martín-Navarro, C.M.; López-Arencibia, A.; Sifaoui, I.; Reyes-Batlle, M.; Fouque, E.; Osuna, A.; Valladares, B.; Piñero, J.E.; Héchard, Y.; Maciver, S.K.; et al. Amoebicidal activity of caffeine and maslinic acid by the induction of programmed cell death in Acanthamoeba. Antimicrob. Agents Chemother. 2017, 61, e02660-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Khan, N.A. Antibacterial activities of selected pure compounds isolated from gut bacteria of animals living in polluted environments. Antibiotics 2020, 9, 190. [Google Scholar] [CrossRef]
- Soopramanien, M.; Khan, N.A.; Abdalla, S.A.O.; Sagathevan, K.; Siddiqui, R. Scorpion and Frog Organ Lysates are Potential Source of Antitumour Activity. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 3011. [Google Scholar] [CrossRef]
- Carnt, N.; Stapleton, F. Strategies for the prevention of contact lens-related Acanthamoeba keratitis: A review. Ophthalmic Physiol. Opt. 2016, 36, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Maycock, N.J.; Jayaswal, R. Update on Acanthamoeba keratitis: Diagnosis, treatment, and outcomes. Cornea 2016, 35, 713–720. [Google Scholar] [CrossRef]
- Pradhan, N. Etymologia: Acanthamoeba. Emerg. Infect. Dis. 2020, 26, 1855. [Google Scholar] [CrossRef]
- Lau, H.L.; De Lima Corvino, D.F.; Guerra, F.M., Jr.; Malik, A.M.; Lichtenberger, P.N.; Gultekin, S.H.; Ritter, J.M.; Roy, S.; Ali, I.K.M.; Cope, J.R.; et al. Granulomatous amoebic encephalitis caused by Acanthamoeba in a patient with AIDS: A challenging diagnosis. Acta Clin. Belg. 2021, 76, 127–131. [Google Scholar] [CrossRef]
- Peters, W.; Pasvol, G. Atlas of Tropical Medicine and Parasitology: Text with CD-ROM; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Norouzi, M.; Saberi, R.; Niyyati, M.; Lorenzo-Morales, J.; Mirjalali, H.; Fatemi, M.; Javanmard, E.; Karamati, S.A. Molecular Identification of Pathogenic Free-Living Amoeba from Household Biofilm Samples in Iran: A Risk Factor for Acanthamoeba Keratitis. Microorganisms 2021, 9, 2098. [Google Scholar] [CrossRef]
- Wink, M. Annual Plant Reviews, Functions and Biotechnology of Plant Secondary Metabolites; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 39. [Google Scholar]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- El-Sayed, N.; Khalifa, T.; Ibrahim, M.; Mabry, T. Constituents from Salvia triloba. Fitoterapia 2001, 72, 850–853. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Rárová, L.; Janovská, L.; Šaman, D.; Wimmer, Z. Enhancing effect of cystamine in its amides with betulinic acid as antimicrobial and antitumor agent in vitro. Steroids 2019, 148, 91–98. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Cunha, A.B.; Batista, R.; Castro, M.Á.; David, J.M. Chemical strategies towards the synthesis of betulinic acid and its more potent antiprotozoal analogues. Molecules 2021, 26, 1081. [Google Scholar] [CrossRef]
- Polat, Z.A.; Vural, A.; Ozan, F.; Tepe, B.; Özcelik, S.; Cetin, A. In vitro evaluation of the amoebicidal activity of garlic (Allium sativum) extract on Acanthamoeba castellanii and its cytotoxic potential on corneal cells. J. Ocul. Pharmacol. Ther. 2008, 24, 8–14. [Google Scholar] [CrossRef]
- Vunda, S.L.L.; Sauter, I.P.; Cibulski, S.P.; Roehe, P.M.; Bordignon, S.A.L.; Rott, M.B.; Apel, M.A.; von Poser, G.L. Chemical composition and amoebicidal activity of Croton pallidulus, Croton ericoides, and Croton isabelli (Euphorbiaceae) essential oils. Parasitol. Res. 2012, 111, 961–966. [Google Scholar] [CrossRef]
- Degerli, S.; Tepe, B.; Celiksoz, A.; Berk, S.; Malatyali, E. In vitro amoebicidal activity of Origanum syriacum and Origanum laevigatum on Acanthamoeba castellanii cysts and trophozoites. Exp. Parasitol. 2012, 131, 20–24. [Google Scholar] [CrossRef]
- Shing, B.; Balen, M.; McKerrow, J.H.; Debnath, A. Acanthamoeba Keratitis: An update on amebicidal and cysticidal drug screening methodologies and potential treatment with azole drugs. Expert Rev. Anti-Infect. Ther. 2021, 19, 1427–1441. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Al Maqtari, Q.A.A.; Al Maqtari, M.A. In vitro antibacterial activity of different Yemeni leaves extracts of Lawsonia inermis against some bacterial pathogens. Int. J. Res. Stud. Biosci. 2014, 2, 52–57. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, R.; Akbar, N.; Khatoon, B.; Kawish, M.; Ali, M.S.; Shah, M.R.; Khan, N.A. Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii. Antibiotics 2022, 11, 248. https://doi.org/10.3390/antibiotics11020248
Siddiqui R, Akbar N, Khatoon B, Kawish M, Ali MS, Shah MR, Khan NA. Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii. Antibiotics. 2022; 11(2):248. https://doi.org/10.3390/antibiotics11020248
Chicago/Turabian StyleSiddiqui, Ruqaiyyah, Noor Akbar, Bushra Khatoon, Muhammad Kawish, Muhammad Shaiq Ali, Muhammad Raza Shah, and Naveed Ahmed Khan. 2022. "Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii" Antibiotics 11, no. 2: 248. https://doi.org/10.3390/antibiotics11020248
APA StyleSiddiqui, R., Akbar, N., Khatoon, B., Kawish, M., Ali, M. S., Shah, M. R., & Khan, N. A. (2022). Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii. Antibiotics, 11(2), 248. https://doi.org/10.3390/antibiotics11020248







