Antiviral Used among Non-Severe COVID-19 Cases in Relation to Time till Viral Clearance: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of the Study Population
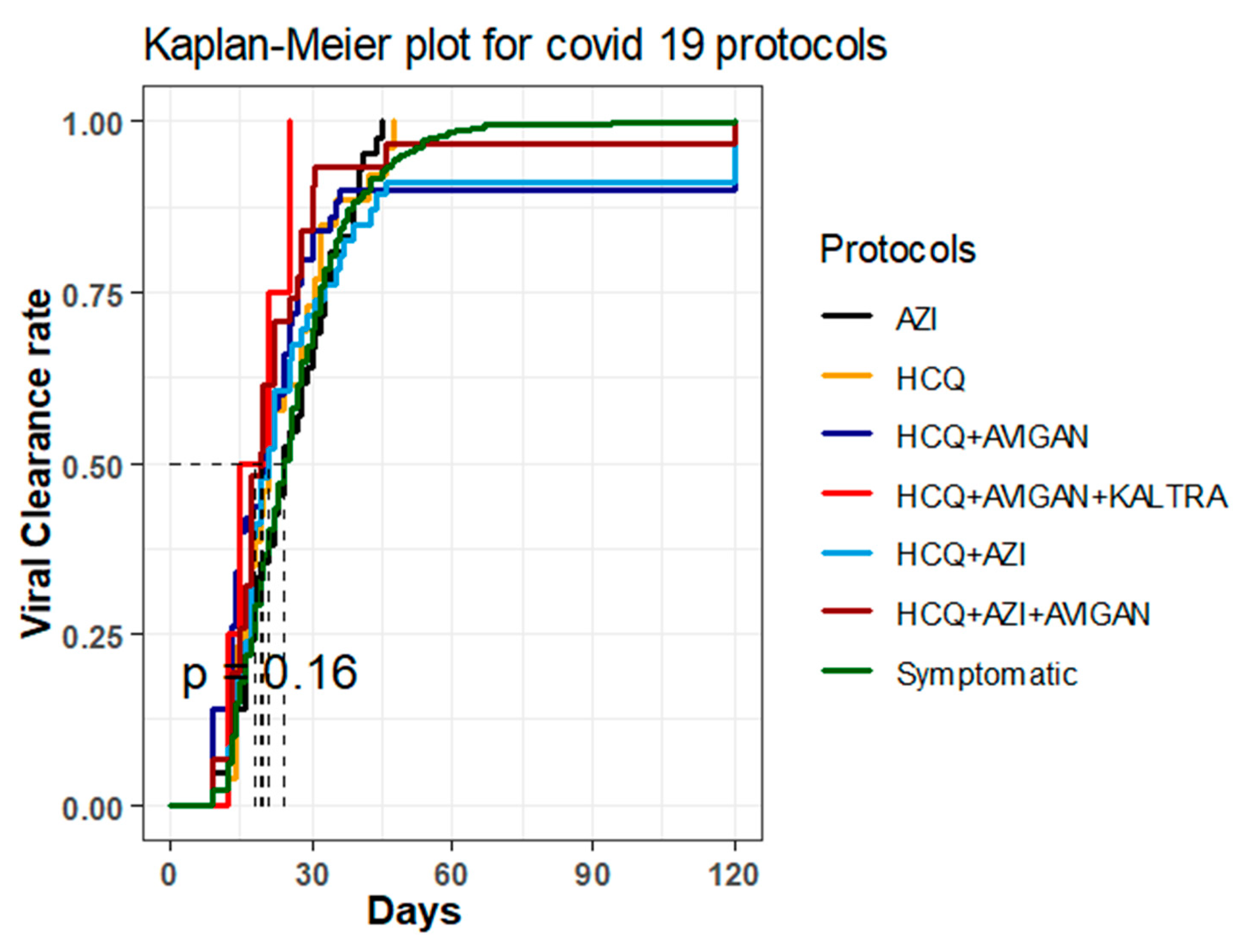
2.2. Time until Viral Clearance among Different Treatment Groups
2.3. The Effect of Age and Gender on Viral Clearance among Different Treatment Groups
3. Discussion
4. Materials and Methods
4.1. Institutional Review Board IRB
4.2. Study Design and Study Population
4.3. Data Management and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H. Drug Treatment Options for the 2019-New Coronavirus (2019-NCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wilde, A.H.; Jochmans, D.; Posthuma, C.C.; Zevenhoven-Dobbe, J.C.; van Nieuwkoop, S.; Bestebroer, T.M.; van den Hoogen, B.G.; Neyts, J.; Snijder, E.J. Screening of an FDA-Approved Compound Library Identifies Four Small-Molecule Inhibitors of Middle East Respiratory Syndrome Coronavirus Replication in Cell Culture. Antimicrob. Agents Chemother. 2014, 58, 4875–4884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.F.-W.; Yao, Y.; Yeung, M.-L.; Deng, W.; Bao, L.; Jia, L.; Li, F.; Xiao, C.; Gao, H.; Yu, P.; et al. Treatment with Lopinavir/Ritonavir or Interferon-Β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J. Infect. Dis. 2015, 212, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.M.; Cheng, V.C.C.; Hung, I.F.N.; Wong, M.M.L.; Chan, K.H.; Chan, K.S.; Kao, R.Y.T.; Poon, L.L.M.; Wong, C.L.P.; Guan, Y.; et al. Role of Lopinavir/Ritonavir in the Treatment of SARS: Initial Virological and Clinical Findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative Therapeutic Efficacy of Remdesivir and Combination Lopinavir, Ritonavir, and Interferon Beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Eng. Beijing China 2020, 6, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lu, Z.; Xu, T.; Chen, C.; Yang, G.; Zha, T.; Lu, J.; Xue, Y. Arbidol Monotherapy Is Superior to Lopinavir/Ritonavir in Treating COVID-19. J. Infect. 2020, 81, e21–e23. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Devaux, C.A.; Rolain, J.-M.; Colson, P.; Raoult, D. New Insights on the Antiviral Effects of Chloroquine against Coronavirus: What to Expect for COVID-19? Int. J. Antimicrob. Agents 2020, 55, 105938. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of Action of Hydroxychloroquine and Chloroquine: Implications for Rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Echeverría-Esnal, D.; Martin-Ontiyuelo, C.; Navarrete-Rouco, M.E.; De-Antonio Cuscó, M.; Ferrández, O.; Horcajada, J.P.; Grau, S. Azithromycin in the Treatment of COVID-19: A Review. Expert Rev. Anti Infect. Ther. 2021, 19, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.H.M.; Berwanger, O.; Fonseca, H.A.; Corrêa, T.D.; Ferraz, L.R.; Lapa, M.G.; Zampieri, F.G.; Veiga, V.C.; Azevedo, L.C.P.; Rosa, R.G.; et al. Azithromycin in Addition to Standard of Care versus Standard of Care Alone in the Treatment of Patients Admitted to the Hospital with Severe COVID-19 in Brazil (COALITION II): A Randomised Clinical Trial. Lancet 2020, 396, 959–967. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Z.; Lin, W.; Cai, W.; Wen, C.; Guan, Y.; Mo, X.; Wang, J.; Wang, Y.; Peng, P.; et al. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med 2020, 1, 105–113.e4. [Google Scholar] [CrossRef] [PubMed]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alhmeed, N.; Zaidi, A.R.Z.; Tobaiqy, M. Efficacy and Safety of Lopinavir/Ritonavir for Treatment of COVID-19: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2020, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Totura, A.L.; Bavari, S. Broad-Spectrum Coronavirus Antiviral Drug Discovery. Expert Opin. Drug Discov. 2019, 14, 397–412. [Google Scholar] [CrossRef] [Green Version]
- Corman, V.M.; Albarrak, A.M.; Omrani, A.S.; Albarrak, M.M.; Farah, M.E.; Almasri, M.; Muth, D.; Sieberg, A.; Meyer, B.; Assiri, A.M.; et al. Viral Shedding and Antibody Response in 37 Patients with Middle East Respiratory Syndrome Coronavirus Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.J.L. Persistent SARS-2 Infections Contribute to Long COVID-19. Med. Hypotheses 2021, 149, 110538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Ding, J.-G.; Li, J.; Hong, L.; Yu, X.-Q.; Ye, E.-L.; Sun, G.-Q.; Zhang, X.-X.; Chen, L.; Sun, Q.-F. Viral Kinetics and Factors Associated with Rapid Viral Clearance during Lopinavir/Ritonavir-Based Combination Therapy in Non-Severe COVID-19 Patients. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5788–5796. [Google Scholar] [CrossRef]
- Canini, L.; Perelson, A.S. Viral Kinetic Modeling: State of the Art. J. Pharmacokinet. Pharmacodyn. 2014, 41, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Ko, J.H.; Kim, Y.; Kim, Y.J.; Kim, J.M.; Chung, Y.S.; Kim, H.M.; Han, M.G.; Kim, S.Y.; Chin, B.S. Viral Load Kinetics of SARS-CoV-2 Infection in First Two Patients in Korea. J. Korean Med. Sci. 2020, 35, e86. [Google Scholar] [CrossRef]
- Gastine, S.; Pang, J.; Boshier, F.A.T.; Carter, S.J.; Lonsdale, D.O.; Cortina-Borja, M.; Hung, I.F.N.; Breuer, J.; Kloprogge, F.; Standing, J.F. Systematic Review and Patient-Level Meta-Analysis of SARS-CoV-2 Viral Dynamics to Model Response to Antiviral Therapies. Clin. Pharmacol. Ther. 2021, 110, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Huang, J.; Yin, P.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Kocayiğit, H.; Özmen Süner, K.; Tomak, Y.; Demir, G.; Yaylacı, S.; Dheir, H.; Güçlü, E.; Erdem, A.F. Observational Study of the Effects of Favipiravir vs Lopinavir/Ritonavir on Clinical Outcomes in Critically Ill Patients with COVID-19. J. Clin. Pharm. Ther. 2021, 46, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, C.; Zhu, Q.; Chen, X.; Chen, G.; Sun, W.; Xiao, Z.; Du, W.; Yao, J.; Li, G.; et al. Favipiravir in the Treatment of Patients with SARS-CoV-2 RNA Recurrent Positive after Discharge: A Multicenter, Open-Label, Randomized Trial. Int. Immunopharmacol. 2021, 97, 107702. [Google Scholar] [CrossRef] [PubMed]
- Guner, R.; Hasanoglu, I.; Kayaaslan, B.; Aypak, A.; Akinci, E.; Bodur, H.; Eser, F.; Kaya Kalem, A.; Kucuksahin, O.; Ates, I.; et al. Comparing ICU Admission Rates of Mild/Moderate COVID-19 Patients Treated with Hydroxychloroquine, Favipiravir, and Hydroxychloroquine plus Favipiravir. J. Infect. Public Health 2021, 14, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Pontali, E.; Volpi, S.; Antonucci, G.; Castellaneta, M.; Buzzi, D.; Tricerri, F.; Angelelli, A.; Caorsi, R.; Feasi, M.; Calautti, F.; et al. Safety and Efficacy of Early High-Dose IV Anakinra in Severe COVID-19 Lung Disease. J. Allergy Clin. Immunol. 2020, 146, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Gagliardini, R.; Cozzi-Lepri, A.; Mariano, A.; Taglietti, F.; Vergori, A.; Abdeddaim, A.; Di Gennaro, F.; Mazzotta, V.; Amendola, A.; D’Offizi, G.; et al. No Efficacy of the Combination of Lopinavir/Ritonavir Plus Hydroxychloroquine Versus Standard of Care in Patients Hospitalized With COVID-19: A Non-Randomized Comparison. Front. Pharmacol. 2021, 12, 621676. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of Hydroxychloroquine in Patients with COVID-19: Results of a Randomized Clinical Trial. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; et al. Clinical and Microbiological Effect of a Combination of Hydroxychloroquine and Azithromycin in 80 COVID-19 Patients with at Least a Six-Day Follow up: A Pilot Observational Study. Travel Med. Infect. Dis. 2020, 34, 101663. [Google Scholar] [CrossRef]
- Million, M.; Lagier, J.-C.; Gautret, P.; Colson, P.; Fournier, P.-E.; Amrane, S.; Hocquart, M.; Mailhe, M.; Esteves-Vieira, V.; Doudier, B.; et al. Early Treatment of COVID-19 Patients with Hydroxychloroquine and Azithromycin: A Retrospective Analysis of 1061 Cases in Marseille, France. Travel Med. Infect. Dis. 2020, 35, 101738. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.; et al. Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients with COVID-19 in New York State. JAMA 2020, 323, 2493–2502. [Google Scholar] [CrossRef]
- Gbinigie, K.; Frie, K. Should Azithromycin Be Used to Treat COVID-19? A Rapid Review. BJGP Open 2020, 4, bjgpopen20X101094. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L. Sex Influences Immune Responses to Viruses, and Efficacy of Prophylaxis and Treatments for Viral Diseases. BioEssays News Rev. Mol. Cell. Dev. Biol. 2012, 34, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Salciccia, S.; Eisenberg, M.L.; Maggi, M.; Lai, S.; Mastroianni, C.M.; Pasculli, P.; Ciardi, M.R.; Canale, V.; Ferro, M.; Busetto, G.M.; et al. Modeling the Contribution of Male Testosterone Levels to the Duration of Positive COVID Testing among Hospitalized Male COVID-19 Patients. Diagnostics 2021, 11, 581. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, F.; Bao, W.; Xue, Y.; Han, L.; Zhang, X.; Zhang, P.; Ji, Y.; Yin, D.; Bao, A.; et al. Clinical Features in Coronavirus Disease 2019 (COVID-19) Patients with Early Clearance and Prolonged Shedding of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA. Ann. Transl. Med. 2021, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Dodds, M.; Bentley, D.; Yeo, K.; Rayner, C. Dosing Will Be a Key Success Factor in Repurposing Antivirals for COVID-19. Br. J. Clin. Pharmacol. 2021, 87, 3451–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, D.; Liu, X.-Y.; Zhu, Y.-N.; Huang, L.; Dan, B.-T.; Zhang, G.-J.; Gao, Y.-H. Factors Associated with Prolonged Viral Shedding and Impact of Lopinavir/Ritonavir Treatment in Hospitalised Non-Critically Ill Patients with SARS-CoV-2 Infection. Eur. Respir. J. 2020, 56, 2000799. [Google Scholar] [CrossRef] [PubMed]
- Badu, K.; Oyebola, K.; Zahouli, J.Z.B.; Fagbamigbe, A.F.; de Souza, D.K.; Dukhi, N.; Amankwaa, E.F.; Tolba, M.F.; Sylverken, A.A.; Mosi, L.; et al. SARS-CoV-2 Viral Shedding and Transmission Dynamics: Implications of WHO COVID-19 Discharge Guidelines. Front. Med. 2021, 8, 648660. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Iwanami, S.; Oda, T.; Fujita, Y.; Kuba, K.; Miyazaki, T.; Ejima, K.; Iwami, S. Incomplete Antiviral Treatment May Induce Longer Durations of Viral Shedding during SARS-CoV-2 Infection. Life Sci. Alliance 2021, 4, e202101049. [Google Scholar] [CrossRef] [PubMed]
- National Guidelines for Clinical Management and Treatment of COVID-19. 2020. Available online: https://www.dha.gov.ae/en/HealthRegulation/Documents/COVID%20National%20Guidelines%20F-NAL%2018%20March.pdf (accessed on 1 March 2022).
- Clinical Managment of COVID-19—Intterim Guidance. 2020. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiD8On8jID3AhVDXRoKHcrIBOgQFnoECAsQAQ&url=https%3A%2F%2Fapps.who.int%2Firis%2Fbitstream%2Fhandle%2F10665%2F332196%2FWHO-2019-nCoV-clinical-2020.5-eng.pdf&usg=AOvVaw3KMolfl2CddTXJJH9o7N4J (accessed on 1 March 2022).

| AZI a (n = 81) | HCQ b (n = 40) | HCQ + AVIGAN c (n = 59) | HCQ + AVIGAN + KALETRA d (n = 4) | HCQ + AZI (n = 60) | HCQ + AZI + AVIGAN (n = 41) | Symptomatic Only (n = 1446) | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | Mean ± SD | 35.9 ± 7.9 | 40.0 ± 8.9 | 40.6 ± 9.4 | 37.2 ± 6.9 | 40.0 ± 10.0 | 39.9 ± 11.4 | 35.6 ± 9.0 | <0.001 * |
| Gender | female | 12 (5.0%) | 8 (3.4%) | 11 (4.6%) | 0 (0.0%) | 10 (4.2%) | 8 (3.4%) | 189 (79.4%) | 0.47 |
| male | 69 (4.6%) | 32 (2.1%) | 49 (3.3%) | 4 (0.3%) | 51 (3.4%) | 33 (2.2%) | 1257 (84.1%) |
| HR (95% CI) | HR (95% CI) | ||
|---|---|---|---|
| Age (years) | - | 1.00 (0.99–1.01, p = 0.931) | 1.00 (0.99–1.01, p = 0.719) |
| Gender | Female | - | - |
| Male | 1.09 (0.91–1.30, p = 0.337) | 1.11 (0.93–1.32, p = 0.270) | |
| Protocol | AZI a | - | - |
| HCQ b | 1.14 (0.70–1.86, p = 0.598) | 1.14 (0.70–1.87, p = 0.588) | |
| HCQ + AVIGAN c | 0.96 (0.63–1.46, p = 0.855) | 0.97 (0.64–1.48, p = 0.897) | |
| HCQ + AVIGAN + KALETRA d | 2.48 (0.89–6.92, p = 0.083) | 2.42 (0.87–6.77, p = 0.092) | |
| HCQ + AZI | 0.84 (0.55–1.28, p = 0.413) | 0.83 (0.54–1.27, p = 0.397) | |
| HCQ + AZI + AVIGAN | 1.36 (0.85–2.16, p = 0.200) | 1.37 (0.86–2.19, p = 0.184) | |
| Symptomatic only | 0.95 (0.70–1.30, p = 0.754) | 0.94 (0.69–1.29, p = 0.708) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafez, W.; Saleh, H.; Al Baha, Z.; Tariq, M.; Hamdan, S.; Ahmed, S. Antiviral Used among Non-Severe COVID-19 Cases in Relation to Time till Viral Clearance: A Retrospective Cohort Study. Antibiotics 2022, 11, 498. https://doi.org/10.3390/antibiotics11040498
Hafez W, Saleh H, Al Baha Z, Tariq M, Hamdan S, Ahmed S. Antiviral Used among Non-Severe COVID-19 Cases in Relation to Time till Viral Clearance: A Retrospective Cohort Study. Antibiotics. 2022; 11(4):498. https://doi.org/10.3390/antibiotics11040498
Chicago/Turabian StyleHafez, Wael, Husam Saleh, Ziad Al Baha, Mishal Tariq, Samah Hamdan, and Shougyat Ahmed. 2022. "Antiviral Used among Non-Severe COVID-19 Cases in Relation to Time till Viral Clearance: A Retrospective Cohort Study" Antibiotics 11, no. 4: 498. https://doi.org/10.3390/antibiotics11040498
APA StyleHafez, W., Saleh, H., Al Baha, Z., Tariq, M., Hamdan, S., & Ahmed, S. (2022). Antiviral Used among Non-Severe COVID-19 Cases in Relation to Time till Viral Clearance: A Retrospective Cohort Study. Antibiotics, 11(4), 498. https://doi.org/10.3390/antibiotics11040498







