A Novel Bifidobacterium longum Subsp. longum T1 Strain from Cow’s Milk: Homeostatic and Antibacterial Activity against ESBL-Producing Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. Morphological Characteristics of Pure Cultures of BLLT1, BLLT2, and BLLT3 Isolates of Bifidobacteria
2.2. Genomic Fingerprinting of BLLT1, BLLT2, and BLLT3 Isolates
2.3. Tolerance of BLLT1 to Gastric and Intestinal Stresses
2.4. Adhesion of BLLT1 to Enterocytes
2.5. BLLT1 Antibacterial Activity
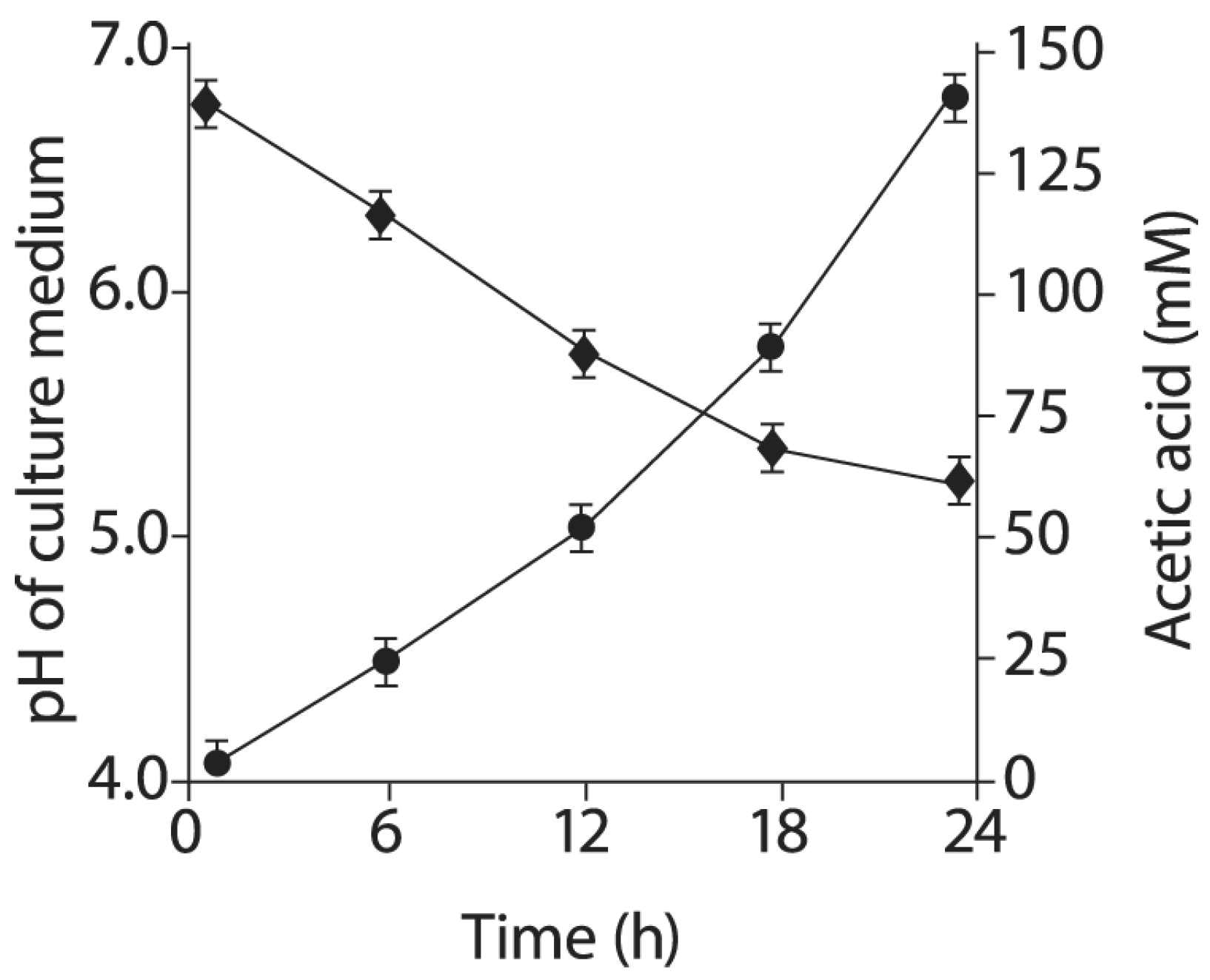
2.6. Acetic Acid Production by BLLT1 Strain and pH Reduction of the Nutrient Medium
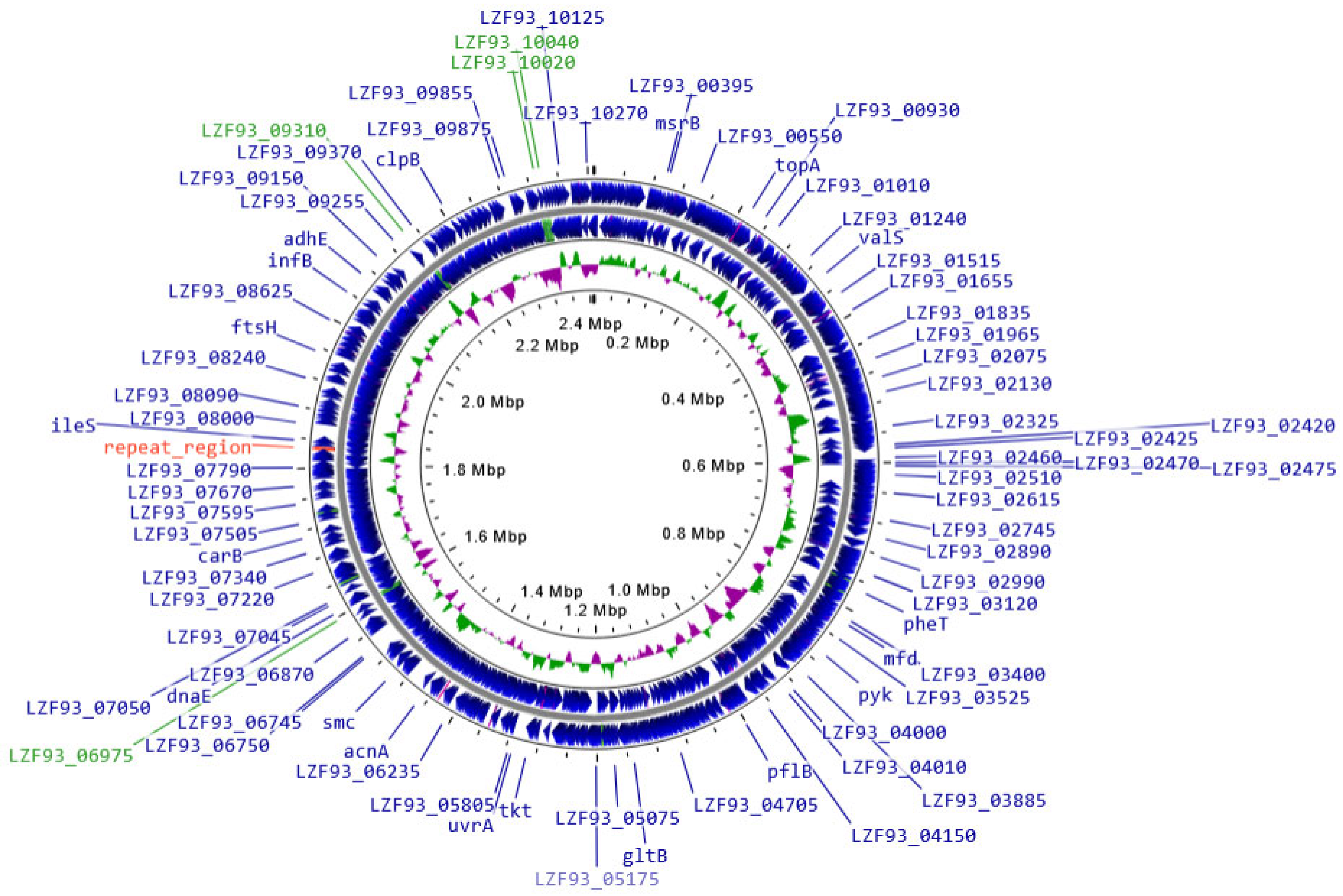
2.7. BLLT1 Whole Genome Analysis
2.8. BLLT1 Inhibits TLR4 mRNA Expression Induced by ESBL-E Pathogens in Enterocytes
2.9. Protection of Intestinal Barrier in HT-29 Enterocyte Monolayers by BLLT1
2.10. Inhibition of Intestinal Paracellular Permeability in HT-29 Enterocyte Monolayers by BLLT1
2.11. Inhibition of Zonulin Secretion in the Enterocyte Monolayers HT-29 by BLLT1
2.12. BLLT1 Stimulates IAP mRNA Expression in Enterocytes
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Assessment of the Morphology of Bifidobacteria BLLT1, BLLT2, and BLLT3 Isolates
4.3. Genomic Fingerprinting of B. longum Isolates
4.4. Determination of BLLT1 Strain Tolerance to Gastric and Intestinal Stresses
4.5. Enterocytes and Growth Conditions
4.5.1. Human Enterocytes
4.5.2. Porcine Enterocytes
4.5.3. Chicken Enterocytes
4.5.4. Bovine Enterocytes
4.6. BLLT1 Adhesion
4.7. Determination of BLLT1 Antagonistic Activity
4.8. Acetic Acid Determination in Culture Liquid of BLLT1
4.9. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction
4.10. Co-Culture of HT-29 Enterocytes with BLLT1 and ESBL-E Strains to Quantify the Levels of TLR4 and IAP mRNA Expression
4.11. TEER Measurements
4.12. Paracellular Permeability Evaluation
4.13. Zonulin Quantification
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coque, T.M.; Novais, A.; Carattoli, A.; Poirel, L.; Pitout, J.; Peixe, L.; Baquero, F.; Cantón, R.; Nordmann, P. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 2008, 14, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.-H.; Blanco, J.; Leflon-Guibout, V.; Demarty, R.; Alonso, M.P.; Caniça, M.M.; Park, Y.-J.; Lavigne, J.-P.; Pitout, J.; Johnson, J.R. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2008, 61, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Kiratisin, P.; Apisarnthanarak, A.; Laesripa, C.; Saifon, P. Molecular characterization and epidemiology of extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-M family is endemic. Antimicrob. Agents Chemother. 2008, 52, 2818–2824. [Google Scholar] [CrossRef]
- Carattoli, A. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef]
- Onnberg, A.; Mölling, P.; Zimmermann, J.; Söderquist, B. Molecular and phenotypic characterization of Escherichia coli and Klebsiella pneumoniae producing extended-spectrum β-lactamases with focus on CTX-M in a low-endemic area in Sweden. APMIS 2011, 119, 287–295. [Google Scholar] [CrossRef]
- Klemm, E.J.; Wong, V.K.; Dougan, G. Emergence of dominant multidrug-resistant bacterial clades: Lessons from history and whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2018, 115, 12872–12877. [Google Scholar] [CrossRef]
- Tchesnokova, V.L.; Rechkina, E.; Chan, D.; Haile, H.G.; Larson, L.; Ferrier, K.; Schroeder, D.W.; Solyanik, T.; Shibuya, S.; Hansen, K.; et al. Pandemic Uropathogenic Fluoroquinolone-resistant Escherichia coli Have Enhanced Ability to Persist in the Gut and Cause Bacteriuria in Healthy Women. Clin. Infect. Dis. 2020, 70, 937–939. [Google Scholar] [CrossRef]
- Machado, E.; Costa, P.; Carvalho, A.; on behalf of the Sarel Investigators. Occurrence of Healthcare-Associated Infections (HAIs) by Escherichia coli and Klebsiella spp. Producing Extended-Spectrum β-lactamases (ESBL) and/or Carbapenemases in Portuguese Long-Term Care Facilities. Pathogens 2022, 11, 1019. [Google Scholar] [CrossRef]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef]
- Leverstein-van Hall, M.A.; Dierikx, C.M.; Cohen Stuart, J.; Voets, G.M.; van den Munckhof, M.P.; van Essen-Zandbergen, A.; Platteel, T.; Fluit, A.C.; van de Sande-Bruinsma, N.; Scharinga, J.; et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 2011, 17, 873–880. [Google Scholar] [CrossRef]
- Dierikx, C.M.; van der Goot, J.A.; Smith, H.E.; Kant, A.; Mevius, D.J. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: A descriptive study. PLoS ONE 2013, 8, e79005. [Google Scholar] [CrossRef] [PubMed]
- Agersø, Y.; Jensen, J.D.; Hasman, H.; Pedersen, K. Spread of extended spectrum cephalosporinase-producing Escherichia coli clones and plasmids from parent animals to broilers and to broiler meat in a production without use of cephalosporins. Foodborne Pathog. Dis. 2014, 11, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Börjesson, S.; Landén, A.; Bengtsson, B. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J. Antimicrob. Chemother. 2014, 69, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Jakobi, G.; Stephan, R.; Hächler, H.; Nüesch-Inderbinen, M. Replicon typing of plasmids carrying bla CTX-M-1 in Enterobacteriaceae of animal, environmental and human origin. Front. Microbiol. 2014, 5, 555. [Google Scholar] [CrossRef]
- Zurfluh, K.; Wang, J.; Klumpp, J.; Nüesch-Inderbinen, M.; Fanning, S.; Stephan, R. Vertical transmission of highly similar bla CTX-M-1-harboring IncI1 plasmids in Escherichia coli with different MLST types in the poultry production pyramid. Front. Microbiol. 2014, 5, 519. [Google Scholar] [CrossRef]
- Dahms, C.; Hübner, N.-O.; Kossow, A.; Mellmann, A.; Dittmann, K.; Kramer, A. Occurrence of ESBL-Producing Escherichia coli in Livestock and Farm Workers in Mecklenburg-Western Pomerania, Germany. PLoS ONE 2015, 10, e0143326. [Google Scholar] [CrossRef]
- Ghodousi, A.; Bonura, C.; Di Noto, A.M.; Mammina, C. Extended-Spectrum ß-Lactamase, AmpC-Producing, and Fluoroquinolone-Resistant Escherichia coli in Retail Broiler Chicken Meat, Italy. Foodborne Pathog. Dis. 2015, 12, 619–625. [Google Scholar] [CrossRef]
- Maciuca, I.E.; Williams, N.J.; Tuchilus, C.; Dorneanu, O.; Guguianu, E.; Carp-Carare, C.; Rimbu, C.; Timofte, D. High Prevalence of Escherichia coli-Producing CTX-M-15 Extended-Spectrum Beta-Lactamases in Poultry and Human Clinical Isolates in Romania. Microb. Drug Resist. 2015, 21, 651–662. [Google Scholar] [CrossRef]
- Huijbers, P.M.C.; Graat, E.A.M.; van Hoek, A.H.A.M.; Veenman, C.; de Jong, M.C.M.; van Duijkeren, E. Transmission dynamics of extended-spectrum β-lactamase and AmpC β-lactamase-producing Escherichia coli in a broiler flock without antibiotic use. Prev. Vet. Med. 2016, 131, 12–19. [Google Scholar] [CrossRef]
- Saliu, E.-M.; Vahjen, W.; Zentek, J. Types and prevalence of extended-spectrum beta-lactamase producing Enterobacteriaceae in poultry. Anim. Health Res. Rev. 2017, 18, 46–57. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Q.; Zhang, S.; Zhang, J.; Yang, G.; Wang, J.; Xue, L.; Chen, M. Characterization of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae from Retail Food in China. Front. Microbiol. 2018, 9, 1709. [Google Scholar] [CrossRef] [PubMed]
- Projahn, M.; Daehre, K.; Semmler, T.; Guenther, S.; Roesler, U.; Friese, A. Environmental adaptation and vertical dissemination of ESBL-/pAmpC-producing Escherichia coli in an integrated broiler production chain in the absence of an antibiotic treatment. Microb. Biotechnol. 2018, 11, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Freire Martín, I.; AbuOun, M.; Reichel, R.; La Ragione, R.M.; Woodward, M.J. Sequence analysis of a CTX-M-1 IncI1 plasmid found in Salmonella 4,5,12:i:-, Escherichia coli and Klebsiella pneumoniae on a UK pig farm. J. Antimicrob. Chemother. 2014, 69, 2098–2101. [Google Scholar] [CrossRef]
- Hammad, A.M.; Hoffmann, M.; Gonzalez-Escalona, N.; Abbas, N.H.; Yao, K.; Koenig, S.; Allué-Guardia, A.; Eppinger, M. Genomic features of colistin resistant Escherichia coli ST69 strain harboring mcr-1 on IncHI2 plasmid from raw milk cheese in Egypt. Infect. Genet. Evol. 2019, 73, 126–131. [Google Scholar] [CrossRef]
- Dantas Palmeira, J.; Ferreira, H.M.N. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in cattle production—A threat around the world. Heliyon 2020, 6, e03206. [Google Scholar] [CrossRef]
- Waade, J.; Seibt, U.; Honscha, W.; Rachidi, F.; Starke, A.; Speck, S.; Truyen, U. Multidrug-resistant enterobacteria in newborn dairy calves in Germany. PLoS ONE 2021, 16, e0248291. [Google Scholar] [CrossRef]
- Founou, L.L.; Founou, R.C.; Allam, M.; Ismail, A.; Essack, S.Y. Genome Analysis of ESBL-Producing Escherichia coli Isolated from Pigs. Pathogens 2022, 11, 776. [Google Scholar] [CrossRef]
- Heikkilä, A.-M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-specific production losses in bovine mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef]
- Eisenberger, D.; Carl, A.; Balsliemke, J.; Kämpf, P.; Nickel, S.; Schulze, G.; Valenza, G. Molecular Characterization of Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolates from Milk Samples of Dairy Cows with Mastitis in Bavaria, Germany. Microb. Drug Resist. 2018, 24, 505–510. [Google Scholar] [CrossRef]
- Su, Y.; Yu, C.-Y.; Tsai, Y.; Wang, S.-H.; Lee, C.; Chu, C. Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Escherichia coli from the milk of cows with clinical mastitis in Southern Taiwan. J. Microbiol. Immunol. Infect. 2016, 49, 892–901. [Google Scholar] [CrossRef]
- Freitag, C.; Michael, G.B.; Kadlec, K.; Hassel, M.; Schwarz, S. Detection of plasmid-borne extended-spectrum β-lactamase (ESBL) genes in Escherichia coli isolates from bovine mastitis. Vet. Microbiol. 2017, 200, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Filioussis, G.; Kachrimanidou, M.; Christodoulopoulos, G.; Kyritsi, M.; Hadjichristodoulou, C.; Adamopoulou, M.; Tzivara, A.; Kritas, S.K.; Grinberg, A. Short communication: Bovine mastitis caused by a multidrug-resistant, mcr-1-positive (colistin-resistant), extended-spectrum β-lactamase-producing Escherichia coli clone on a Greek dairy farm. J. Dairy Sci. 2020, 103, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Goulart, D.B.; Mellata, M. Escherichia coli Mastitis in Dairy Cattle: Etiology, Diagnosis, and Treatment Challenges. Front. Microbiol. 2022, 13, 928346. [Google Scholar] [CrossRef] [PubMed]
- El-Mohandes, S.S.; Eid, R.H.; Allam, A.M.; Abou-Zeina, H.A.A.; Elbayoumy, M.K. Phenotyping and genotyping studies on extended-spectrum β-lactamase-producing Escherichia coli isolates from mastitic cows on dairy farms in Egypt. Vet. World 2022, 15, 890–897. [Google Scholar] [CrossRef]
- Blanco, M.; Alonso, M.P.; Nicolas-Chanoine, M.-H.; Dahbi, G.; Mora, A.; Blanco, J.E.; López, C.; Cortés, P.; Llagostera, M.; Leflon-Guibout, V.; et al. Molecular epidemiology of Escherichia coli producing extended-spectrum {beta}-lactamases in Lugo (Spain): Dissemination of clone O25b:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2009, 63, 1135–1141. [Google Scholar] [CrossRef]
- Zhong, Y.-M.; Liu, W.-E.; Liang, X.-H.; Li, Y.-M.; Jian, Z.-J.; Hawkey, P.M. Emergence and spread of O16-ST131 and O25b-ST131 clones among faecal CTX-M-producing Escherichia coli in healthy individuals in Hunan Province, China. J. Antimicrob. Chemother. 2015, 70, 2223–2227. [Google Scholar] [CrossRef]
- Gonçalves, D.; Cecílio, P.; Ferreira, H. Nursing homes and long-term care facilities: Reservoirs of CTX-M-15-producing Escherichia coli O25b-ST131 in Portugal. J. Glob. Antimicrob. Resist. 2016, 7, 69–71. [Google Scholar] [CrossRef]
- Koutsianos, D.; Athanasiou, L.; Mossialos, D.; Koutoulis, K.C. Colibacillosis in poultry: A disease overview and the new perspectives for its control and prevention. J. Hell. Vet. Med. Soc. 2021, 71, 2425. [Google Scholar] [CrossRef]
- Fisher, J.F.; Meroueh, S.O.; Mobashery, S. Bacterial resistance to beta-lactam antibiotics: Compelling opportunism, compelling opportunity. Chem. Rev. 2005, 105, 395–424. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef]
- Paterson, D.L.; Mulazimoglu, L.; Casellas, J.M.; Ko, W.C.; Goossens, H.; Von Gottberg, A.; Mohapatra, S.; Trenholme, G.M.; Klugman, K.P.; McCormack, J.G.; et al. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin. Infect. Dis. 2000, 30, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Hujer, K.M.; Hujer, A.M.; Yeiser, B.; Bonomo, M.D.; Rice, L.B.; Bonomo, R.A. International Klebsiella Study Group Extended-spectrum beta-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: Dominance and widespread prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob. Agents Chemother. 2003, 47, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Blaak, H.; Hamidjaja, R.A.; van Hoek, A.H.A.M.; de Heer, L.; de Roda Husman, A.M.; Schets, F.M. Detection of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli on flies at poultry farms. Appl. Environ. Microbiol. 2014, 80, 239–246. [Google Scholar] [CrossRef]
- Balbuena-Alonso, M.G.; Camps, M.; Cortés-Cortés, G.; Carreón-León, E.A.; Lozano-Zarain, P.; Rocha-Gracia, R.D.C. Strain belonging to an emerging, virulent sublineage of ST131 Escherichia coli isolated in fresh spinach, suggesting that ST131 may be transmissible through agricultural products. Front. Cell. Infect. Microbiol. 2023, 13, 1237725. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Koçak, M.; Büyükkaragöz, B.; Çelebi Tayfur, A.; Çaltik, A.; Köksoy, A.Y.; Çizmeci, Z.; Günbey, S. Causative pathogens and antibiotic resistance in children hospitalized for urinary tract infection. Pediatr. Int. 2016, 58, 467–471. [Google Scholar] [CrossRef]
- Russo, T.A.; Johnson, J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003, 5, 449–456. [Google Scholar] [CrossRef]
- Yun, K.W.; Lee, M.-K.; Kim, W.; Lim, I.S. Uropathogenic Escherichia coli ST131 in urinary tract infections in children. Korean J. Pediatr. 2017, 60, 221–226. [Google Scholar] [CrossRef]
- Tumbarello, M.; Sanguinetti, M.; Montuori, E.; Trecarichi, E.M.; Posteraro, B.; Fiori, B.; Citton, R.; D’Inzeo, T.; Fadda, G.; Cauda, R.; et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: Importance of inadequate initial antimicrobial treatment. Antimicrob. Agents Chemother. 2007, 51, 1987–1994. [Google Scholar] [CrossRef]
- Kolman, K.B. Cystitis and Pyelonephritis: Diagnosis, Treatment, and Prevention. Prim. Care 2019, 46, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, E.; Patel, J.B.; Bilker, W.B.; Edelstein, P.H.; Fishman, N.O. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: Risk factors for infection and impact of resistance on outcomes. Clin. Infect. Dis. 2001, 32, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Briggs, G.G.; McKeown, A.; Bustillo, G. Urinary tract infections during pregnancy. Ann. Pharmacother. 2004, 38, 1692–1701. [Google Scholar] [CrossRef]
- Lane, M.C.; Mobley, H.L.T. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007, 72, 19–25. [Google Scholar] [CrossRef]
- Suff, N.; Karda, R.; Diaz, J.A.; Ng, J.; Baruteau, J.; Perocheau, D.; Tangney, M.; Taylor, P.W.; Peebles, D.; Buckley, S.M.K.; et al. Ascending Vaginal Infection Using Bioluminescent Bacteria Evokes Intrauterine Inflammation, Preterm Birth, and Neonatal Brain Injury in Pregnant Mice. Am. J. Pathol. 2018, 188, 2164–2176. [Google Scholar] [CrossRef]
- Shah, N.M.; Charani, E.; Ming, D.; Cheah, F.-C.; Johnson, M.R. Antimicrobial stewardship and targeted therapies in the changing landscape of maternal sepsis. J. Intensive Med. 2024, 4, 46–61. [Google Scholar] [CrossRef]
- Al-Assil, B.; Mahfoud, M.; Hamzeh, A.R. Resistance trends and risk factors of extended spectrum β-lactamases in Escherichia coli infections in Aleppo, Syria. Am. J. Infect. Control 2013, 41, 597–600. [Google Scholar] [CrossRef]
- Reid, R.; Al-Bayati, M.; Samarasinghe, S. Genotypic Identification of Extended-Spectrum β-Lactamase (ESBL)—Producing Enterobacteriaceae from Urinary Tract Infections in the Leicestershire Area, United Kingdom: A One Health Prospective. J. Infect. Dis. Diagn. 2018, 3, 1000122. [Google Scholar] [CrossRef]
- Zeynudin, A.; Pritsch, M.; Schubert, S.; Messerer, M.; Liegl, G.; Hoelscher, M.; Belachew, T.; Wieser, A. Prevalence and antibiotic susceptibility pattern of CTX-M type extended-spectrum β-lactamases among clinical isolates of gram-negative bacilli in Jimma, Ethiopia. BMC Infect. Dis. 2018, 18, 524. [Google Scholar] [CrossRef]
- Uddin, F.; Imam, S.H.; Khan, S.; Khan, T.A.; Ahmed, Z.; Sohail, M.; Elnaggar, A.Y.; Fallatah, A.M.; El-Bahy, Z.M. NDM Production as a Dominant Feature in Carbapenem-Resistant Enterobacteriaceae Isolates from a Tertiary Care Hospital. Antibiotics 2021, 11, 48. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, Y.; Yu, J.; Li, S.; Zhang, Y.; Wang, H.; Lai, X.; Liu, D.; Mao, L.; Luo, Y.; et al. Bacterial characteristics of carbapenem-resistant Enterobacteriaceae (CRE) colonized strains and their correlation with subsequent infection. BMC Infect. Dis. 2021, 21, 638. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.; Kock, M.M.; Coetzee, J.; Hoosien, E.; Peirano, G.; Strydom, K.-A.; Ehlers, M.M.; Mbelle, N.M.; Shashkina, E.; Haslam, D.B.; et al. Klebsiella pneumoniae ST307 with blaOXA-181, South Africa, 2014–2016. Emerg. Infect. Dis. 2019, 25, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gutiérrez, B.; Pérez-Galera, S.; Salamanca, E.; de Cueto, M.; Calbo, E.; Almirante, B.; Viale, P.; Oliver, A.; Pintado, V.; Gasch, O.; et al. A Multinational, Preregistered Cohort Study of β-Lactam/β-Lactamase Inhibitor Combinations for Treatment of Bloodstream Infections Due to Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2016, 60, 4159–4169. [Google Scholar] [CrossRef]
- Muhammed, M.; Flokas, M.E.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Comparison Between Carbapenems and β-Lactam/β-Lactamase Inhibitors in the Treatment for Bloodstream Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2017, 4, ofx099. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients with E. coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Rodríguez-Baño, J. Current options for the treatment of infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae in different groups of patients. Clin. Microbiol. Infect. 2019, 25, 932–942. [Google Scholar] [CrossRef]
- Abubakar, U.; Tangiisuran, B.; Elnaem, M.H.; Sulaiman, S.A.S.; Khan, F.U. Mortality and its predictors among hospitalized patients with infections due to extended spectrum beta-lactamase (ESBL) Enterobacteriaceae in Malaysia: A retrospective observational study. Futur. J. Pharm. Sci. 2022, 8, 17. [Google Scholar] [CrossRef]
- ElBaradei, A.; Maharem, D.A.; Kader, O.; Ghareeb, M.K.; Naga, I.S. Fecal carriage of ESBL-producing Escherichia coli in Egyptian patients admitted to the Medical Research Institute hospital, Alexandria University. AIMS Microbiol. 2020, 6, 422–433. [Google Scholar] [CrossRef]
- Giedraitienė, A.; Vitkauskienė, A.; Pavilonis, A.; Patamsytė, V.; Genel, N.; Decre, D.; Arlet, G. Prevalence of O25b-ST131 clone among Escherichia coli strains producing CTX-M-15, CTX-M-14 and CTX-M-92 β-lactamases. Infect. Dis. 2017, 49, 106–112. [Google Scholar] [CrossRef]
- Gonçalves, D.; Cecílio, P.; Faustino, A.; Iglesias, C.; Branca, F.; Estrada, A.; Ferreira, H. Intra- and Extra-Hospital Dissemination of IMP-22-Producing Klebsiella pneumoniae in Northern Portugal: The Breach of the Hospital Frontier Toward the Community. Front. Microbiol. 2021, 12, 777054. [Google Scholar] [CrossRef]
- Biehl, L.M.; Schmidt-Hieber, M.; Liss, B.; Cornely, O.A.; Vehreschild, M.J.G.T. Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients—Review of the literature from a clinical perspective. Crit. Rev. Microbiol. 2016, 42, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.; Sewid, A.H.; Hamouda, H.I.; Elharrif, M.G.; El-Demerdash, A.S.; Alharthi, A.; Hashim, N.; Hamad, A.A.; Selim, S.; Alkhalifah, D.H.M.; et al. Rhamnolipid-Coated Iron Oxide Nanoparticles as a Novel Multitarget Candidate against Major Foodborne E. coli Serotypes and Methicillin-Resistant S. aureus. Microbiol. Spectr. 2022, 10, e0025022. [Google Scholar] [CrossRef] [PubMed]
- Dawwam, G.E.; Al-Shemy, M.T.; El-Demerdash, A.S. Green synthesis of cellulose nanocrystal/ZnO bio-nanocomposites exerting antibacterial activity and downregulating virulence toxigenic genes of food-poisoning bacteria. Sci. Rep. 2022, 12, 16848. [Google Scholar] [CrossRef]
- Becker, E.; Projahn, M.; Burow, E.; Käsbohrer, A. Are There Effective Intervention Measures in Broiler Production against the ESBL/AmpC Producer Escherichia coli? Pathogens 2021, 10, 608. [Google Scholar] [CrossRef]
- Ishnaiwer, M.; Bezabih, Y.; Javaudin, F.; Sassi, M.; Bemer, P.; Batard, E.; Dion, M. In vitro and in vivo activity of new strains of Bacillus subtilis against ESBL-producing Escherichia coli: An experimental study. J. Appl. Microbiol. 2022, 132, 2270–2279. [Google Scholar] [CrossRef]
- Ceccarelli, D.; van Essen-Zandbergen, A.; Smid, B.; Veldman, K.T.; Boender, G.J.; Fischer, E.A.J.; Mevius, D.J.; van der Goot, J.A. Competitive Exclusion Reduces Transmission and Excretion of Extended-Spectrum-β-Lactamase-Producing Escherichia coli in Broilers. Appl. Environ. Microbiol. 2017, 83, e03439-16. [Google Scholar] [CrossRef]
- Dame-Korevaar, A.; Fischer, E.A.J.; van der Goot, J.; Velkers, F.; Ceccarelli, D.; Mevius, D.; Stegeman, A. Early life supply of competitive exclusion products reduces colonization of extended spectrum beta-lactamase-producing Escherichia coli in broilers. Poult. Sci. 2020, 99, 4052–4064. [Google Scholar] [CrossRef]
- Ebrahem, A.F.; El-Demerdash, A.S.; Orady, R.M.; Nabil, N.M. Modulatory Effect of Competitive Exclusion on the Transmission of ESBL E. coli in Chickens. Probiotics Antimicrob. Proteins 2024, 16, 1087–1098. [Google Scholar] [CrossRef]
- FAO; UNEP; WHO. One Health Joint Plan of Action, 2022–2026; World Organisation for Animal Health (WOAH) (Founded as OIE): Paris, France, 2022; ISBN 978-92-5-136957-9. [Google Scholar]
- Bergmann, K.R.; Liu, S.X.L.; Tian, R.; Kushnir, A.; Turner, J.R.; Li, H.-L.; Chou, P.M.; Weber, C.R.; De Plaen, I.G. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am. J. Pathol. 2013, 182, 1595–1606. [Google Scholar] [CrossRef]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.-Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Karlyshev, A.V.; Gould, S. Ligilactobacillus salivarius 2102–15 complete genome sequence data. Data Brief 2023, 50, 109564. [Google Scholar] [CrossRef]
- Lobry, J.R. Asymmetric substitution patterns in the two DNA strands of bacteria. Mol. Biol. Evol. 1996, 13, 660–665. [Google Scholar] [CrossRef]
- de Jong, A.; van Hijum, S.A.F.T.; Bijlsma, J.J.E.; Kok, J.; Kuipers, O.P. BAGEL: A web-based bacteriocin genome mining tool. Nucleic Acids Res. 2006, 34, W273–W279. [Google Scholar] [CrossRef]
- Wishart, D.S.; Han, S.; Saha, S.; Oler, E.; Peters, H.; Grant, J.R.; Stothard, P.; Gautam, V. PHASTEST: Faster than PHASTER, better than PHAST. Nucleic Acids Res. 2023, 51, W443–W450. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Taylor, T.D.; Ohno, H.; Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 2012, 3, 449–454. [Google Scholar] [CrossRef]
- Abe, F.; Ishibashi, N.; Shimamura, S. Effect of administration of bifidobacteria and lactic acid bacteria to newborn calves and piglets. J. Dairy Sci. 1995, 78, 2838–2846. [Google Scholar] [CrossRef]
- Laursen, M.F.; Pekmez, C.T.; Larsson, M.W.; Lind, M.V.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Bode, L.; Dragsted, L.O.; Michaelsen, K.F.; et al. Maternal milk microbiota and oligosaccharides contribute to the infant gut microbiota assembly. ISME Commun. 2021, 1, 21. [Google Scholar] [CrossRef]
- Masco, L.; Huys, G.; Gevers, D.; Verbrugghen, L.; Swings, J. Identification of Bifidobacterium species using rep-PCR fingerprinting. Syst. Appl. Microbiol. 2003, 26, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Jarocki, P.; Podleśny, M.; Komoń-Janczara, E.; Kucharska, J.; Glibowska, A.; Targoński, Z. Comparison of various molecular methods for rapid differentiation of intestinal bifidobacteria at the species, subspecies and strain level. BMC Microbiol. 2016, 16, 159. [Google Scholar] [CrossRef] [PubMed]
- Jena, R.; Choudhury, P.K.; Puniya, A.K.; Tomar, S.K. Efficacy of BOX-PCR fingerprinting for taxonomic discrimination of bifidobacterial species isolated from diverse sources. 3 Biotech 2021, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Bottacini, F.; Kato, K.; Mitsuyama, E.; Yoshida, K.; Horigome, A.; Xiao, J.-Z.; van Sinderen, D. Genomic diversity and distribution of Bifidobacterium longum subsp. longum across the human lifespan. Sci. Rep. 2018, 8, 85. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Dong, J.; Shi, J.; Guan, J.; Liu, D.; Liu, F.; Li, B.; Huo, G. Identification, Characterization, and Antioxidant Potential of Bifidobacterium longum subsp. longum Strains Isolated from Feces of Healthy Infants. Front. Microbiol. 2021, 12, 756519. [Google Scholar] [CrossRef]
- Kelly, W.J.; Cookson, A.L.; Altermann, E.; Lambie, S.C.; Perry, R.; Teh, K.H.; Otter, D.E.; Shapiro, N.; Woyke, T.; Leahy, S.C. Genomic analysis of three Bifidobacterium species isolated from the calf gastrointestinal tract. Sci. Rep. 2016, 6, 30768. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.; Gai, Z.; Han, M. Bifidobacterium longum subsp. longum BL21 ameliorates alcoholic liver disease in mice through enhancement of the hepatic antioxidant capacity and modulation of the gut microbiota. J. Appl. Microbiol. 2023, 134, lxad251. [Google Scholar] [CrossRef]
- Arboleya, S.; Stanton, C.; Ryan, C.A.; Dempsey, E.; Ross, P.R. Bosom Buddies: The Symbiotic Relationship between Infants and Bifidobacterium longum ssp. longum and ssp. infantis. Genetic and Probiotic Features. Annu. Rev. Food Sci. Technol. 2016, 7, 1–21. [Google Scholar] [CrossRef]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964–18969. [Google Scholar] [CrossRef]
- Meng, D.; Zhu, W.; Ganguli, K.; Shi, H.N.; Walker, W.A. Anti-inflammatory effects of Bifidobacterium longum subsp infantis secretions on fetal human enterocytes are mediated by TLR-4 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G744–G753. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Bueno, D.; Juan, D.; Latorre, J.; Shehata, A.; Eisenreich, W.; Tellez-Isaias, G. Strategies to attack pathogenic avian microorganisms: From probiotics to postbiotics. Ger. J. Vet. Res. 2024, 4, 95–118. [Google Scholar] [CrossRef]
- Turroni, F.; Foroni, E.; Serafini, F.; Viappiani, A.; Montanini, B.; Bottacini, F.; Ferrarini, A.; Bacchini, P.L.; Rota, C.; Delledonne, M.; et al. Ability of Bifidobacterium breve to grow on different types of milk: Exploring the metabolism of milk through genome analysis. Appl. Environ. Microbiol. 2011, 77, 7408–7417. [Google Scholar] [CrossRef]
- Martín, R.; Jiménez, E.; Heilig, H.; Fernández, L.; Marín, M.L.; Zoetendal, E.G.; Rodríguez, J.M. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl. Environ. Microbiol. 2009, 75, 965–969. [Google Scholar] [CrossRef]
- Ugolev, A.M. Membrane digestion. Gut 1972, 13, 735–747. [Google Scholar] [CrossRef]
- Di Gioia, D.; Aloisio, I.; Mazzola, G.; Biavati, B. Bifidobacteria: Their impact on gut microbiota composition and their applications as probiotics in infants. Appl. Microbiol. Biotechnol. 2014, 98, 563–577. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Chichlowski, M.; De Lartigue, G.; German, J.B.; Raybould, H.E.; Mills, D.A. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 321–327. [Google Scholar] [CrossRef]
- Arboleya, S.; Bottacini, F.; O’Connell-Motherway, M.; Ryan, C.A.; Ross, R.P.; van Sinderen, D.; Stanton, C. Gene-trait matching across the Bifidobacterium longum pan-genome reveals considerable diversity in carbohydrate catabolism among human infant strains. BMC Genom. 2018, 19, 33. [Google Scholar] [CrossRef]
- Garrido, D.; Ruiz-Moyano, S.; Kirmiz, N.; Davis, J.C.; Totten, S.M.; Lemay, D.G.; Ugalde, J.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596. Sci. Rep. 2016, 6, 35045. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Munoz-Munoz, J.; van Sinderen, D. Plant Glycan Metabolism by Bifidobacteria. Front. Microbiol. 2021, 12, 609418. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Akiyama, T.; Matsuda, K.; Gawad, A.; Makino, H.; Ishikawa, E.; Oishi, K.; Kushiro, A.; Fujimoto, J. Long-term colonization exceeding six years from early infancy of Bifidobacterium longum subsp. longum in human gut. BMC Microbiol. 2018, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Shimizu, K.; Nomoto, K.; Hamabata, T.; Ozawa, A.; Takeda, Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 2004, 72, 2240–2247. [Google Scholar] [CrossRef]
- Henrick, B.M.; Hutton, A.A.; Palumbo, M.C.; Casaburi, G.; Mitchell, R.D.; Underwood, M.A.; Smilowitz, J.T.; Frese, S.A. Elevated Fecal pH Indicates a Profound Change in the Breastfed Infant Gut Microbiome Due to Reduction of Bifidobacterium over the Past Century. mSphere 2018, 3, 10-1128. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Singh, N.; Martin, P.M.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Shi, H.; Robertson, K.D.; Munn, D.H.; Liu, K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1405–G1415. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A. Innate immunity: Impact on the adaptive immune response. Curr. Opin. Immunol. 1997, 9, 4–9. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016, 16, 35–50. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Kircheis, R.; Planz, O. Special Issue “The Role of Toll-Like Receptors (TLRs) in Infection and Inflammation 2.0”. Int. J. Mol. Sci. 2024, 25, 9709. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, X.-P.; Fan, J.; Liu, Q.; Anwar, K.N.; Frey, R.S.; Malik, A.B. LPS activation of Toll-like receptor 4 signals CD11b/CD18 expression in neutrophils. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L655-62. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Yang, Y.; Wandler, A.M.; Postlethwait, J.H.; Guillemin, K. Dynamic Evolution of the LPS-Detoxifying Enzyme Intestinal Alkaline Phosphatase in Zebrafish and Other Vertebrates. Front. Immunol. 2012, 3, 314. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.; Barton, G.M.; Flavell, R.A.; Medzhitov, R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 2002, 420, 329–333. [Google Scholar] [CrossRef]
- Bonham, K.S.; Orzalli, M.H.; Hayashi, K.; Wolf, A.I.; Glanemann, C.; Weninger, W.; Iwasaki, A.; Knipe, D.M.; Kagan, J.C. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 2014, 156, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.C.; Su, T.; Horng, T.; Chow, A.; Akira, S.; Medzhitov, R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 2008, 9, 361–368. [Google Scholar] [CrossRef]
- Yi, Y.-S. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef]
- Barker, J.H.; Weiss, J.P. Detecting lipopolysaccharide in the cytosol of mammalian cells: Lessons from MD-2/TLR4. J. Leukoc. Biol. 2019, 106, 127–132. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Gribar, S.C.; Anand, R.J.; Sodhi, C.P.; Hackam, D.J. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J. Leukoc. Biol. 2008, 83, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Sugi, Y.; Hosono, A.; Kaminogawa, S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J. Immunol. 2009, 183, 6522–6529. [Google Scholar] [CrossRef]
- Guzzo, C.; Ayer, A.; Basta, S.; Banfield, B.W.; Gee, K. IL-27 enhances LPS-induced proinflammatory cytokine production via upregulation of TLR4 expression and signaling in human monocytes. J. Immunol. 2012, 188, 864–873. [Google Scholar] [CrossRef]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Malo, M.S. A High Level of Intestinal Alkaline Phosphatase Is Protective Against Type 2 Diabetes Mellitus Irrespective of Obesity. eBioMedicine 2015, 2, 2016–2023. [Google Scholar] [CrossRef]
- Bates, J.M.; Akerlund, J.; Mittge, E.; Guillemin, K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007, 2, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.F.; Austen, W.G.; Zhang, X.; Munene, G.; Mostafa, G.; Biswas, S.; McCormack, M.; Eberlin, K.R.; Nguyen, J.T.; Tatlidede, H.S.; et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc. Natl. Acad. Sci. USA 2008, 105, 3551–3556. [Google Scholar] [CrossRef]
- Malo, M.S.; Alam, S.N.; Mostafa, G.; Zeller, S.J.; Johnson, P.V.; Mohammad, N.; Chen, K.T.; Moss, A.K.; Ramasamy, S.; Faruqui, A.; et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 2010, 59, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.N.; Yammine, H.; Moaven, O.; Ahmed, R.; Moss, A.K.; Biswas, B.; Muhammad, N.; Biswas, R.; Raychowdhury, A.; Kaliannan, K.; et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann. Surg. 2014, 259, 715–722. [Google Scholar] [CrossRef]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 2016, 202, 225–234. [Google Scholar] [CrossRef]
- Lallès, J.-P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 2019, 77, 710–724. [Google Scholar] [CrossRef]
- Santos, G.M.; Ismael, S.; Morais, J.; Araújo, J.R.; Faria, A.; Calhau, C.; Marques, C. Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function. Microorganisms 2022, 10, 746. [Google Scholar] [CrossRef]
- Tam, J.S.Y.; Coller, J.K.; Hughes, P.A.; Prestidge, C.A.; Bowen, J.M. Toll-like receptor 4 (TLR4) antagonists as potential therapeutics for intestinal inflammation. Indian J. Gastroenterol. 2021, 40, 5–21. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Ruiz, L.; Sanchez-Gallardo, R.; van Sinderen, D. Isolation of Chromosomal and Plasmid DNA from Bifidobacteria. Methods Mol. Biol. 2021, 2278, 21–29. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef]
- Huang, Y.; Adams, M.C. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int. J. Food Microbiol. 2004, 91, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Schierack, P.; Nordhoff, M.; Pollmann, M.; Weyrauch, K.D.; Amasheh, S.; Lodemann, U.; Jores, J.; Tachu, B.; Kleta, S.; Blikslager, A.; et al. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem. Cell Biol. 2006, 125, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Rath, N.C.; Liyanage, R.; Gupta, A.; Packialakshmi, B.; Lay, J.O. A method to culture chicken enterocytes and their characterization. Poult. Sci. 2018, 97, 4040–4047. [Google Scholar] [CrossRef]
- Katwal, P.; Thomas, M.; Uprety, T.; Hildreth, M.B.; Kaushik, R.S. Development and biochemical and immunological characterization of early passage and immortalized bovine intestinal epithelial cell lines from the ileum of a young calf. Cytotechnology 2019, 71, 127–148. [Google Scholar] [CrossRef]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Boyko, N.; Cresci, A. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501(®), Lactobacillus paracasei IMC 502(®) and SYNBIO(®) against pathogens. J. Appl. Microbiol. 2014, 117, 518–527. [Google Scholar] [CrossRef]
- Dubey, U.K.; Mistry, V.V. Growth characteristics of bifidobacteria in infant formulas. J. Dairy Sci. 1996, 79, 1146–1155. [Google Scholar] [CrossRef]
- Abramov, V.M.; Kosarev, I.V.; Machulin, A.V.; Deryusheva, E.I.; Priputnevich, T.V.; Panin, A.N.; Chikileva, I.O.; Abashina, T.N.; Manoyan, A.M.; Akhmetzyanova, A.A.; et al. Anti-Salmonella Defence and Intestinal Homeostatic Maintenance In Vitro of a Consortium Containing Limosilactobacillus fermentum 3872 and Ligilactobacillus salivarius 7247 Strains in Human, Porcine, and Chicken Enterocytes. Antibiotics 2024, 13, 30. [Google Scholar] [CrossRef]
- Lu, C.-C.; Kuo, H.-C.; Wang, F.-S.; Jou, M.-H.; Lee, K.-C.; Chuang, J.-H. Upregulation of TLRs and IL-6 as a marker in human colorectal cancer. Int. J. Mol. Sci. 2014, 16, 159–177. [Google Scholar] [CrossRef]
- Takeuchi, A.; Hisamatsu, K.; Okumura, N.; Sugimitsu, Y.; Yanase, E.; Ueno, Y.; Nagaoka, S. IIAEK Targets Intestinal Alkaline Phosphatase (IAP) to Improve Cholesterol Metabolism with a Specific Activation of IAP and Downregulation of ABCA1. Nutrients 2020, 12, 2859. [Google Scholar] [CrossRef]


| Gastric Stress * | Intestinal Stress * | ||||||
|---|---|---|---|---|---|---|---|
| 10 min | 30 min | 60 min | 5 h | ||||
| Experiment, ×107 CFU/mL | Control, ×107 CFU/mL | Experiment, ×107 CFU/mL | Control, ×107 CFU/mL | Experiment, ×107 CFU/mL | Control, ×107 CFU/mL | Experiment, ×107 CFU/mL | Control, ×108 CFU/mL |
| 2.85 ± 0.58 | 3.14 ± 0.52 | 2.92 ± 0.49 | 3.21 ± 0.55 | 2.84 ± 0.44 | 3.16 ± 0.47 | 2.95 ± 0.62 | 1.15 ± 0.51 |
| RD = 1.1 ± 0.2 Very good | RD = 1.1 ± 0.1 Very good | RD = 1.1 ± 0.3 Very good | RD = 3.9 ± 0.4 Very good | ||||
| Enterocytes | BLLT1 Adhesion Indicators | |
|---|---|---|
| Adhesion Activity (%) | Adhesion Index | |
| Human HT-29 | 100 | 34 ± 3 |
| Porcine IPEC-j2 | 100 | 25 ± 3 |
| Chicken CPCE | 100 | 29 ± 3 |
| Bovine BPCE | 100 | 36 ± 3 |
| Strain | SLBL Type | 0 h | 24 h | ||
|---|---|---|---|---|---|
| C 1 | JC 2 | C 1 | JC 2 | ||
| E. coli ATCC BAA 2326 | STX-M-15 | 1 × 106 | 2 × 106 | 9 × 108 | <102 |
| E. coli ATCC BAA 204 | SHV-2 | 2 × 105 | 3 × 105 | 8 × 108 | <102 |
| E. coli ATCC BAA 198 | TEM-26 | 3 × 105 | 3 × 105 | 7 × 108 | <102 |
| E. coli IIE Wo 8015 | OXA | 2 × 105 | 3 × 105 | 7 × 108 | <102 |
| E. coli IIE Wo 8034 | SHV | 1 × 106 | 1 × 106 | 6 × 108 | <102 |
| E. coli IIE Wo 8042 | STX-M | 4 × 105 | 4 × 105 | 8 × 108 | <102 |
| E. coli IIE Wo 8059 | TEM | 2 × 105 | 2 × 105 | 7 × 108 | <102 |
| E. coli IIE Wo 8123 | TEM | 3 × 105 | 3 × 105 | 8 × 108 | <102 |
| E. coli IIE Wo 8147 | STX-M | 2 × 105 | 2 × 105 | 7 × 108 | <102 |
| E. coli IIE WLM 8194 | STX-M | 5 × 105 | 5 × 105 | 8 × 108 | <102 |
| E. coli IIE NB 8215 | TEM | 3 × 105 | 3 × 105 | 8 × 108 | <102 |
| E. coli IIE NB 8224 | STX-M | 4 × 105 | 4 × 105 | 8 × 108 | <102 |
| E. coli IIE Co 8316 | STX-M | 2 × 105 | 3 × 105 | 9 × 108 | <102 |
| E. coli IIE Ca 8320 | STX-M | 5 × 105 | 5 × 105 | 8 × 108 | <102 |
| E. coli IIE Co 8356 | TEM | 3 × 105 | 3 × 105 | 8 × 108 | <102 |
| E. coli IIE Ca 8341 | TEM | 2 × 105 | 2 × 105 | 8 × 108 | <102 |
| E. coli IIE Pi 8420 | SHV | 4 × 105 | 3 × 105 | 8 × 108 | <102 |
| E. coli IIE WP 8436 | SHV | 1 × 105 | 1 × 105 | 9 × 108 | <102 |
| E. coli IIE LH 8508 | OXA | 2 × 105 | 3 × 105 | 7 × 108 | <102 |
| E. coli IIE Egg 8517 | OXA | 3 × 105 | 3 × 105 | 8 × 108 | <102 |
| E. coli IIE LH 8525 | STX-M | 4 × 105 | 4 × 105 | 8 × 108 | <102 |
| E. coli IIE Egg 8532 | STX-M | 1 × 105 | 1 × 105 | 9 × 108 | <102 |
| E. coli IIE BC 8644 | TEM | 6 × 105 | 6 × 105 | 8 × 108 | <102 |
| E. coli IIE BC 8650 | STX-M | 4 × 105 | 4 × 105 | 8 × 108 | <102 |
| E. coli IIE BC 8675 | OXA | 2 × 105 | 3 × 105 | 8 × 108 | <102 |
| E. coli IIE BC 8689 | SHV | 3 × 105 | 3 × 105 | 8 × 108 | <102 |
| E. coli Strains | TLR4 mRNA (Fold Change) | |||
|---|---|---|---|---|
| Control | BLLT1 | E. coli | BLLT1+ E. coli | |
| E. coli ATCC BAA 2326 | 0.9 ± 0.1 | 0.8 ± 0.1 * | 10.1 ± 0.5 ** | 1.0 ± 0.2 * |
| E. coli ATCC BAA 204 | 1.0 ± 0.2 | 0.7 ± 0.1 * | 10.3 ± 0.3 ** | 1.2 ± 0.2 * |
| E. coli ATCC BAA 198 | 0.8 ± 0.2 | 0.8 ± 0.1 * | 9.1 ± 0.4 ** | 1.0 ± 0.1 * |
| E. coli IIE Wo 8015 | 0.9 ± 0.3 | 0.8 ± 0.1 * | 10.5 ± 0.6 ** | 1.1 ± 0.2 * |
| E. coli IIE Wo 8034 | 0.9 ± 0.2 | 0.8 ± 0.1 * | 11.8 ± 0.8 ** | 1.0 ± 0.2 * |
| E. coli IIE Wo 8042 | 0.8 ± 0.2 | 0.8 ± 0.1 * | 10.4 ± 0.7 ** | 1.0 ± 0.1 * |
| E. coli IIE Wo 8059 | 0.9 ± 0.3 | 0.7 ± 0.1 * | 9.4 ± 0.5 ** | 1.2 ± 0.2 * |
| E. coli IIE Wo 8123 | 0.9 ± 0.2 | 0.7 ± 0.1 * | 11.0 ± 0.6 ** | 1.0 ± 0.2 * |
| E. coli IIE Wo 8147 | 0.8 ± 0.2 | 0.7 ± 0.1 * | 10.9 ± 0.9 ** | 1.0 ± 0.1 * |
| E. coli IIE WLM 8194 | 1.1 ± 0.3 | 0.9 ± 0.2 * | 9.5 ± 0.5 ** | 1.1 ± 0.2 * |
| E. coli IIE NB 8215 | 0.9 ± 0.1 | 0.8 ± 0.1 * | 11.3 ± 0.7 ** | 1.3 ± 0.1 * |
| E. coli IIE NB 8224 | 0.9 ± 0.2 | 0.7 ± 0.1 * | 10.1 ± 0.6 ** | 1.0 ± 0.2 * |
| E. coli IIE Co 8316 | 0.8 ± 0.1 | 0.7 ± 0.1 * | 9.0 ± 0.4 ** | 1.2 ± 0.1 * |
| E. coli IIE Ca 8320 | 1.0 ± 0.2 | 0.9 ± 0.2 * | 11.8 ± 0.5 ** | 1.3 ± 0.1 * |
| E. coli IIE Co 8356 | 0.8 ± 0.2 | 0.6 ± 0.1 * | 10.2 ±0.4 ** | 1.3 ± 0.2 * |
| E. coli IIE Ca 8341 | 1.1 ± 0.2 | 0.7 ± 0.2 * | 12.6 ±0.7 ** | 1.4 ± 0.2 * |
| E. coli IIE Pi 8420 | 0.9 ± 0.2 | 0.8 ± 0.1 * | 11.2 ±0 6 ** | 1.0 ± 0.1 * |
| E. coli IIE WP 8436 | 0.8 ± 0.1 | 0.7 ± 0.1 * | 10.7 ± 0.8 ** | 1.3 ± 0.2 * |
| E. coli IIE LH 8508 | 0.9 ± 0.1 | 0.7 ± 0.1 * | 8.2 ± 0.4 ** | 1.1 ± 0.2 * |
| E. coli IIE Egg 8517 | 0.9 ± 0.2 | 0.8 ± 0.1 * | 9.0 ± 0.5** | 1.0 ± 0.1 * |
| E. coli IIE LH 8525 | 0.8 ± 0.1 | 0.7 ± 0.1 * | 10.4 ± 0.6 ** | 1.2 ± 0.2 * |
| E. coli IIE Egg 8532 | 0.9 ± 0.1 | 0.7 ± 0.1 * | 11.6 ± 0.7 ** | 1.0 ± 0.2 * |
| E. coli IIE BC 8644 | 0.9 ± 0.2 | 0.8 ± 0.1 * | 10.1 ± 0.4 ** | 1.3 ± 0.2 * |
| E. coli IIE BC 8650 | 0.8 ± 0.2 | 0.7 ± 0.1 * | 9.3 ± 0.6 ** | 1.2 ± 0.1 * |
| E. coli IIE BC 8675 | 1.1 ± 0.1 | 0.9 ± 0.1 * | 12.0 ±0.7 ** | 1.4 ± 0.1 * |
| E. coli IIE BC 8689 | 1.0 ± 0.1 | 0.8 ± 0.1 * | 11.5 ± 0.6 ** | 1.3 ± 0.2 * |
| E. coli Strains | TEER Value (Ω × cm2) | |||
|---|---|---|---|---|
| Control | BLLT1 | E. coli | BLLT1+ E. coli | |
| E. coli ATCC BAA 2326 | 254 ± 9 | 267 ± 10 * | 106 ± 5 ** | 238 ± 8 * |
| E. coli ATCC BAA 204 | 257 ± 11 | 265 ± 8 * | 110 ± 6 ** | 242 ± 10 * |
| E. coli ATCC BAA 198 | 249 ± 8 | 260 ± 11 * | 104 ± 8 ** | 235 ± 9 * |
| E. coli IIE Wo 8015 | 252 ± 9 | 264 ± 10 * | 112 ± 5 ** | 232 ± 9 * |
| E. coli IIE Wo 8034 | 245 ± 12 | 258 ± 11 * | 105 ± 9 ** | 230 ± 10 * |
| E. coli IIE Wo 8042 | 247 ± 8 | 255 ± 10 * | 109 ± 7 ** | 229 ± 8 * |
| E. coli IIE Wo 8059 | 250 ± 10 | 268 ± 12 * | 111 ± 6 ** | 232 ± 11 * |
| E. coli IIE Wo 8123 | 245 ± 7 | 252 ± 10 * | 107 ± 5 ** | 234 ± 9 * |
| E. coli IIE Wo 8147 | 253 ± 11 | 274 ± 10 * | 112 ± 8 ** | 240 ± 11 * |
| E. coli IIE WLM 8194 | 256 ± 9 | 283 ± 11 * | 111 ± 9 ** | 238 ± 10 * |
| E. coli IIE NB 8215 | 248 ± 12 | 265 ± 10 * | 108 ± 6 ** | 226 ± 8 * |
| E. coli IIE NB 8224 | 250 ± 8 | 267 ± 10 * | 109 ± 7 ** | 235 ± 9 * |
| E. coli IIE Co 8316 | 257 ± 11 | 284 ± 9 * | 112 ± 5 ** | 240 ± 10 * |
| E. coli IIE Ca 8320 | 259 ± 10 | 272 ± 11 * | 108 ± 9 ** | 239 ± 8 * |
| E. coli IIE Co 8356 | 252 ± 8 | 279 ± 10 * | 106 ± 7 ** | 241 ± 10 * |
| E. coli IIE Ca 8341 | 254 ± 12 | 268 ± 11 * | 113 ± 6 ** | 235 ± 9 * |
| E. coli IIE Pi 8420 | 247 ± 11 | 269 ± 10 * | 104 ± 9 ** | 232 ± 10 * |
| E. coli IIE WP 8436 | 255 ± 9 | 284 ± 12 * | 111 ± 7 ** | 238 ± 11 * |
| E. coli IIE LH 8508 | 243 ± 11 | 269 ± 10 * | 105 ± 8 ** | 229 ± 12 * |
| E. coli IIE Egg 8517 | 252 ± 8 | 271 ± 9 * | 104 ± 5 ** | 233 ± 8 * |
| E. coli IIE LH 8525 | 258 ± 10 | 275 ± 10 * | 103 ± 6 ** | 237 ± 10 * |
| E. coli IIE Egg 8532 | 240 ± 7 | 267 ± 11 * | 99 ± 5 ** | 234 ± 11 * |
| E. coli IIE BC 8644 | 248 ± 12 | 273 ± 10 * | 105 ± 9 ** | 236 ± 10 * |
| E. coli IIE BC 8650 | 253 ± 8 | 285 ± 11 * | 111 ± 8 ** | 232 ± 11 * |
| E. coli IIE BC 8675 | 259 ± 8 | 274 ± 10 * | 108 ± 9 ** | 241 ± 10 * |
| E. coli IIE BC 8689 | 250 ± 10 | 282 ± 11 * | 104 ± 6 ** | 235 ± 9 * |
| E. coli Strains | Paracellular Permeability (%) | |||
|---|---|---|---|---|
| Control | BLLT1 | E. coli | BLLT1+ E. coli | |
| E. coli ATCC BAA 2326 | 3.2 ±0.6 | 2.0 ± 0.5 * | 46 ± 2 ** | 4.8 ± 0.9 * |
| E. coli ATCC BAA 204 | 3.6 ± 0.8 | 2.6 ± 0.7 * | 43 ± 2 ** | 4.9 ± 1.2 * |
| E. coli ATCC BAA 198 | 3.7 ± 0.7 | 2.3 ± 0.4 * | 47 ± 3 ** | 5.5 ± 0.6 * |
| E. coli IIE Wo 8015 | 3.3 ± 0.5 | 2.4 ± 0.6 * | 42 ± 4 ** | 4.8 ± 1.1 * |
| E. coli IIE Wo 8034 | 3.3 ± 0.4 | 2.2 ± 0.5 * | 48 ± 5 ** | 4.5 ± 1.0 * |
| E. coli IIE Wo 8042 | 3.9 ± 1.0 | 2.6 ± 0.6 * | 45 ± 2 ** | 4.8 ± 0.9 * |
| E. coli IIE Wo 8059 | 3.6 ± 0.6 | 2.3 ± 0.7 * | 47 ± 3 ** | 5.4 ± 0.5 * |
| E. coli IIE Wo 8123 | 3.4 ±0.8 | 2.0 ± 0.5 * | 49 ± 2 ** | 4.9 ± 1.1 * |
| E. coli IIE Wo 8147 | 3.5 ± 0.7 | 2.7 ± 0.8 * | 42 ± 3 ** | 3.3 ± 1.4 * |
| E. coli IIE WLM 8194 | 3.1 ± 0.4 | 2.8 ± 0.7 * | 47 ± 2 ** | 4.5 ± 0.7 * |
| E. coli IIE NB 8215 | 3.8 ± 1.0 | 2.4 ± 0.6 * | 43 ± 4 ** | 4.2 ± 1.2 * |
| E. coli IIE NB 8224 | 3.8 ± 0.9 | 2.6 ± 0.7 * | 46 ± 2 ** | 4.9 ± 0.8 * |
| E. coli IIE Co 8316 | 3.2 ± 0.7 | 2.5 ± 0.8 * | 44 ± 3 ** | 4.4 ± 1.1 * |
| E. coli IIE Ca 8320 | 3.5 ± 0.8 | 2.2 ± 0.6 * | 49 ± 2 ** | 3.9 ± 1.4 * |
| E. coli IIE Co 8356 | 3.2 ± 0.5 | 2.9 ± 0.9 * | 47 ± 3 ** | 4.3 ± 1.2 * |
| E. coli IIE Ca 8341 | 3.9 ± 0.9 | 2.4 ± 0.5 * | 51 ± 2 ** | 5.8 ± 0.9 * |
| E. coli IIE Pi 8420 | 3.1 ± 0.4 | 2.8 ± 0.8 * | 45 ± 3 ** | 4.5 ± 0.9 * |
| E. coli IIE WP 8436 | 3.6 ± 0.6 | 2.7 ± 0.9 * | 53 ± 2 ** | 4.8 ± 0.6 * |
| E. coli IIE LH 8508 | 3.8 ± 1.0 | 2.8 ± 0.7 * | 48 ± 3 ** | 5.2 ± 0.8 * |
| E. coli IIE Egg 8517 | 3.9 ± 0.9 | 2.7 ± 0.6 * | 46 ± 2 ** | 4.4 ± 1.1 * |
| E. coli IIE LH 8525 | 3.1 ± 0.7 | 2.5 ± 0.5 * | 49 ± 2 ** | 4.8 ± 1.2 * |
| E. coli IIE Egg 8532 | 3.7 ± 0.9 | 2.5 ± 0.8 * | 52 ± 2 ** | 5.6 ± 0.7 * |
| E. coli IIE BC 8644 | 3.6 ± 0.6 | 2.7 ± 0.7 * | 47 ± 3 ** | 4.8 ± 0.9 * |
| E. coli IIE BC 8650 | 3.8 ± 1.0 | 2.0 ± 0.4 * | 45 ± 3 ** | 4.9 ± 1.2 * |
| E. coli IIE BC 8675 | 3.2 ± 0.7 | 2.7 ± 0.8 * | 50 ± 2 ** | 5.3 ± 0.6 * |
| E. coli IIE BC 8689 | 3.0 ± 0.5 | 2.7 ± 0.9 * | 44 ± 3 ** | 4.5 ± 1.1 * |
| E. coli Strains | Zonulin (ng/mL) | |||
|---|---|---|---|---|
| Control | BLLT1 | E. coli | BLLT1+ E. coli | |
| E. coli ATCC BAA 2326 | 1.7 ± 0.4 | 1.4 ± 0.2 * | 15 ± 2 ** | 1.8 ± 0.3 * |
| E. coli ATCC BAA 204 | 1.6 ± 0.3 | 1.5 ± 0.2 * | 15 ± 2 ** | 1.7 ± 0.3 * |
| E. coli ATCC BAA 198 | 1.8 ± 0.2 | 1.4 ± 0.3 * | 14 ± 3 ** | 1.9 ± 0.4 * |
| E. coli IIE Wo 8015 | 1.7 ± 0.3 | 1.4 ± 0.2 * | 16 ± 2 ** | 1.9 ± 0.2 * |
| E. coli IIE Wo 8034 | 1.6 ± 0.2 | 1.5 ± 0.3 * | 15 ± 3 ** | 1.8 ± 0.3 * |
| E. coli IIE Wo 8042 | 1.6 ± 0.2 | 1.3 ± 0.2 * | 14 ± 2 ** | 1.8 ± 0.2 * |
| E. coli IIE Wo 8059 | 1.6 ± 0.3 | 1.4 ± 0.2 * | 15 ± 2 ** | 1.9 ± 0.3 * |
| E. coli IIE Wo 8123 | 1.7 ± 0.2 | 1.3 ± 0.2 * | 14 ± 3 ** | 1.8 ± 0.3 * |
| E. coli IIE Wo 8147 | 1.6 ± 0.2 | 1.5 ± 0.2 * | 15 ± 3 ** | 1.8 ± 0.2 * |
| E. coli IIE WLM 8194 | 1.6 ± 0.3 | 1.4 ± 0.2 * | 14 ± 2 ** | 1.9 ± 0.3 * |
| E. coli IIE NB 8215 | 1.5 ± 0.2 | 1.3 ± 0.4 * | 15 ± 3 ** | 1.7 ± 0.2 * |
| E. coli IIE NB 8224 | 1.7 ± 0.3 | 1.4 ± 0.2 * | 14 ± 2 ** | 1.9 ± 0.3 * |
| E. coli IIE Co 8316 | 1.6 ± 0.4 | 1.5 ± 0.2 * | 15 ± 3 ** | 1.9 ± 0.2 * |
| E. coli IIE Ca 8320 | 1.6 ± 0.3 | 1.5 ± 0.3 * | 14 ± 3 ** | 1.8 ± 0.2 * |
| E. coli IIE Co 8356 | 1.6 ± 0.3 | 1.4 ± 0.3 * | 15 ± 3 ** | 1.9 ± 0.2 * |
| E. coli IIE Ca 8341 | 1.7 ± 0.3 | 1.4 ± 0.2 * | 15 ± 2 ** | 1.9 ± 0.3 * |
| E. coli IIE Pi 8420 | 1.7 ± 0.2 | 1.5 ± 0.2 * | 15 ± 3 ** | 2.0 ± 0.3 * |
| E. coli IIE WP 8436 | 1.6 ± 0.3 | 1.4 ± 0.3 * | 15 ± 2 ** | 1.8 ± 0.3 * |
| E. coli IIE LH 8508 | 1.5 ± 0.3 | 1.4 ± 0.2 * | 14 ± 3 ** | 1.8 ± 0.2 * |
| E. coli IIE Egg 8517 | 1.6 ± 0.2 | 1.3 ± 0.2 * | 15 ± 2 ** | 1.8 ± 0.2 * |
| E. coli IIE LH 8525 | 1.7 ± 0.2 | 1.5 ± 0.3 * | 15 ± 2 ** | 1.9 ± 0.3 * |
| E. coli IIE Egg 8532 | 1.6 ± 0.2 | 1.4 ± 0.2 * | 15 ± 2 ** | 2.0 ± 0.3 * |
| E. coli IIE BC 8644 | 1.7 ± 0.3 | 1.4 ± 0.3 * | 16 ± 3 ** | 1.8 ± 0.3 * |
| E. coli IIE BC 8650 | 1.6 ± 0.3 | 1.3 ± 0.2 * | 15 ± 2 ** | 1.8 ± 0.2 * |
| E. coli IIE BC 8675 | 1.7 ± 0.2 | 1.4 ± 0.3 * | 16 ± 2 ** | 1.9 ± 0.2 * |
| E. coli IIE BC 8689 | 1.6 ± 0.2 | 1.5 ± 0.2 * | 14 ± 3 ** | 1.9 ± 0.3 * |
| E. coli Strains | IAP mRNA of Control (%) | |||
|---|---|---|---|---|
| Control | BLLT1 | E. coli | BLLT1+ E. coli | |
| E. coli ATCC BAA 2326 | 100 | 126 ± 5 * | 37 ± 4 ** | 120 ± 3 * |
| E. coli ATCC BAA 204 | 100 | 135 ± 3 * | 33 ± 4 ** | 127 ± 4 * |
| E. coli ATCC BAA 198 | 100 | 128 ± 4 * | 32 ± 5 ** | 121 ± 3 * |
| E. coli IIE Wo 8015 | 100 | 129 ± 5 * | 35 ± 3 ** | 122 ± 3 * |
| E. coli IIE Wo 8034 | 100 | 134 ± 3 * | 37 ± 3 ** | 128 ± 5 * |
| E. coli IIE Wo 8042 | 100 | 131 ± 3 * | 33 ± 4 ** | 124 ± 3 * |
| E. coli IIE Wo 8059 | 100 | 125 ± 2 * | 38 ± 4 ** | 119 ± 4 * |
| E. coli IIE Wo 8123 | 100 | 137 ± 3 * | 39 ± 3 ** | 125 ± 3 * |
| E. coli IIE Wo 8147 | 100 | 130 ± 4 * | 36 ± 4 ** | 124 ± 5 * |
| E. coli IIE WLM 8194 | 100 | 135 ± 4 * | 38 ± 3 ** | 126 ± 4 * |
| E. coli IIE NB 8215 | 100 | 132 ± 4 * | 37 ± 3 ** | 123 ± 3 * |
| E. coli IIE NB 8224 | 100 | 137 ± 4 * | 28 ± 2 * | 125 ± 4 * |
| E. coli IIE Co 8316 | 100 | 138 ± 2 * | 36 ± 3 ** | 130 ± 5 * |
| E. coli IIE Ca 8320 | 100 | 129 ± 3 * | 27 ± 2 ** | 121 ± 4 * |
| E. coli IIE Co 8356 | 100 | 133 ± 4 * | 24 ± 2 ** | 125 ± 3 * |
| E. coli IIE Ca 8341 | 100 | 127 ± 4 * | 39 ± 5 ** | 118 ± 3 * |
| E. coli IIE Pi 8420 | 100 | 130 ± 5 * | 31 ± 4 ** | 122 ± 4 * |
| E. coli IIE WP 8436 | 100 | 126 ± 4 * | 32 ± 5 ** | 119 ± 3 * |
| E. coli IIE LH 8508 | 100 | 134 ± 5 * | 38 ± 3 ** | 127 ± 4 * |
| E. coli IIE Egg 8517 | 100 | 137 ± 5 * | 34 ± 3 ** | 128 ± 5 * |
| E. coli IIE LH 8525 | 100 | 129 ± 3 * | 31 ± 4 ** | 120 ± 3 * |
| E. coli IIE Egg 8532 | 100 | 136 ± 4 * | 39 ± 5 ** | 127 ± 4 * |
| E. coli IIE BC 8644 | 100 | 125 ± 6 * | 37 ± 4 ** | 118 ± 3 * |
| E. coli IIE BC 8650 | 100 | 128 ± 3 * | 33 ± 5 ** | 119 ± 3 * |
| E. coli IIE BC 8675 | 100 | 133 ± 5 * | 38 ± 2 ** | 126 ± 4 * |
| E. coli IIE BC 8689 | 100 | 139 ± 4 * | 35 ± 3 ** | 130 ± 3 * |
| Bacteria | Strain ESBL 3 Type | Growth Conditions |
|---|---|---|
| B. longum subsp. longum | IIE 1 T1 | MRS a 37 °C anaerobically 24 h |
| B. longum subsp. longum | IIE T2 | The same |
| B. longum subsp. longum | IIE T3 | The same |
| E. coli | ATCC 2 BAA2326 STX-M-15 | LB b 37 °C aerobically 18 h |
| E. coli | ATCC BAA 204 SHV-2 | The same |
| E. coli | ATCC BAA 198 TEM-26 | The same |
| E. coli | IIE PW 4 8015 5 OXA | The same |
| E. coli | IIE PW 8034 6 SHV | The same |
| E. coli | IIE PW 8042 7 STX-M | The same |
| E. coli | IIE PW 8059 8 TEM | The same |
| E. coli | IIE PW 8123 9 TEM | The same |
| E. coli | IIE PW 8147 10 STX-M | The same |
| E. coli | IIE WLM 11 8194 12 TEM | The same |
| E. coli | IIE NB 13 8215 14 TEM | The same |
| E. coli | IIE NB 8224 15 STX-M | The same |
| E. coli | IIE Co 16 8316 17 STX-M | The same |
| E. coli | IIE Ca 18 8320 19 STX-M | The same |
| E. coli | IIE Co 8356 20 TEM | The same |
| E. coli | IIE Ca 8341 21 TEM | The same |
| E. coli | IIE Pi 22 8420 23 SHV | The same |
| E. coli | IIE WP 24 8436 25 SHV | The same |
| E. coli | IIE LH 26 8508 27 OXA | The same |
| E. coli | IIE Egg 28 8517 29 OXA | The same |
| E. coli | IIE LH 8525 30 STX-M | The same |
| E. coli | IIE Egg 8532 31 STX-M | The same |
| E. coli | IIE BC 32 8648 TEM | The same |
| E. coli | IIE BC 8650 STX-M | The same |
| E. coli | IIE BC 8675 OXA | The same |
| E. coli | IIE BC 8689 SHV | The same |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machulin, A.V.; Abramov, V.M.; Kosarev, I.V.; Deryusheva, E.I.; Priputnevich, T.V.; Panin, A.N.; Manoyan, A.M.; Chikileva, I.O.; Abashina, T.N.; Blumenkrants, D.A.; et al. A Novel Bifidobacterium longum Subsp. longum T1 Strain from Cow’s Milk: Homeostatic and Antibacterial Activity against ESBL-Producing Escherichia coli. Antibiotics 2024, 13, 924. https://doi.org/10.3390/antibiotics13100924
Machulin AV, Abramov VM, Kosarev IV, Deryusheva EI, Priputnevich TV, Panin AN, Manoyan AM, Chikileva IO, Abashina TN, Blumenkrants DA, et al. A Novel Bifidobacterium longum Subsp. longum T1 Strain from Cow’s Milk: Homeostatic and Antibacterial Activity against ESBL-Producing Escherichia coli. Antibiotics. 2024; 13(10):924. https://doi.org/10.3390/antibiotics13100924
Chicago/Turabian StyleMachulin, Andrey V., Vyacheslav M. Abramov, Igor V. Kosarev, Evgenia I. Deryusheva, Tatiana V. Priputnevich, Alexander N. Panin, Ashot M. Manoyan, Irina O. Chikileva, Tatiana N. Abashina, Dmitriy A. Blumenkrants, and et al. 2024. "A Novel Bifidobacterium longum Subsp. longum T1 Strain from Cow’s Milk: Homeostatic and Antibacterial Activity against ESBL-Producing Escherichia coli" Antibiotics 13, no. 10: 924. https://doi.org/10.3390/antibiotics13100924








