Contact Effect of a Methylobacterium sp. Extract on Biofilm of a Mycobacterium chimaera Strain Isolated from a 3T Heater-Cooler System
Abstract
:1. Introduction
2. Results
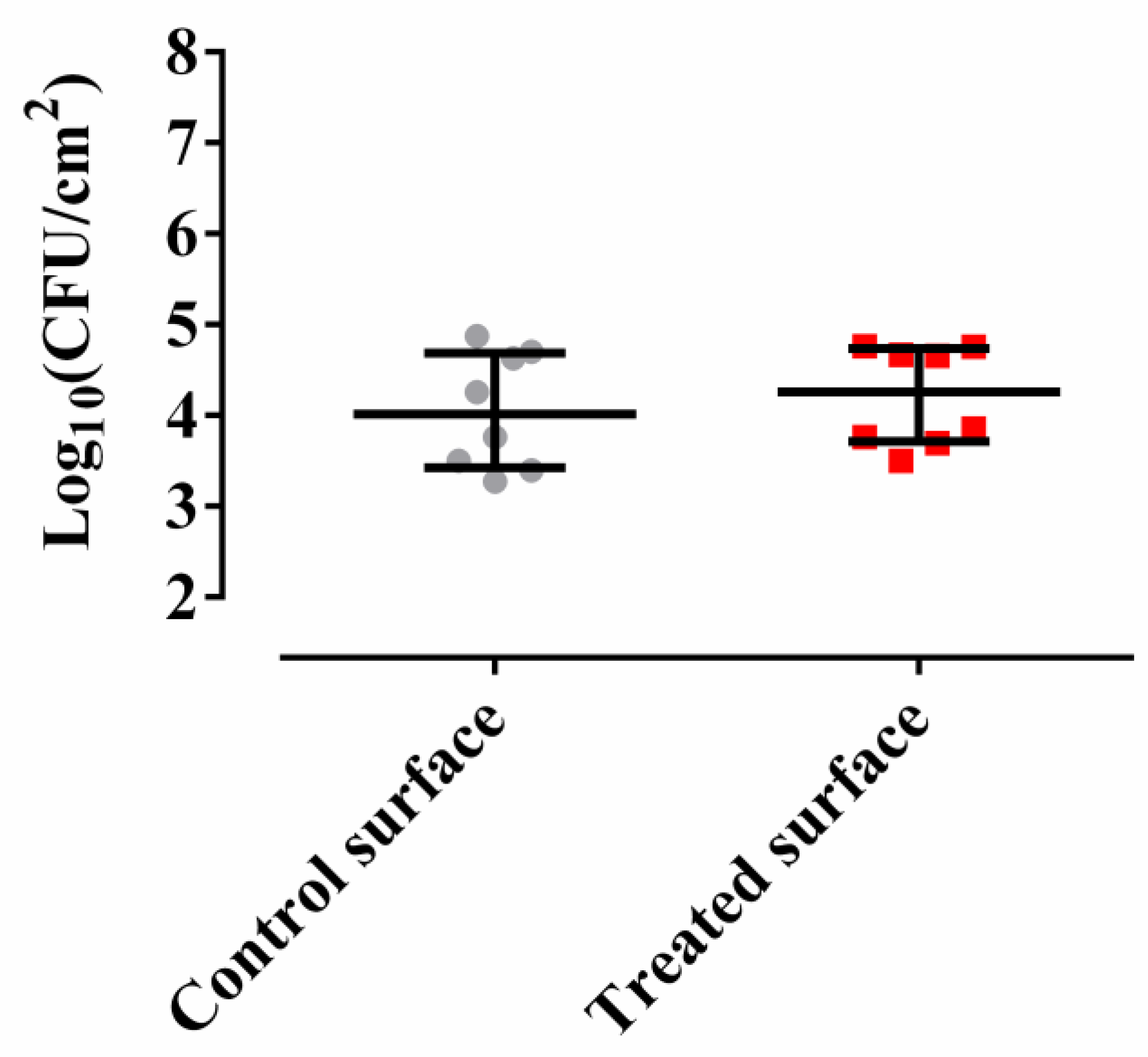
2.1. Methylobacterium sp. CECT 7180 Does Not Inhibit M. chimaera ECMO Adherence
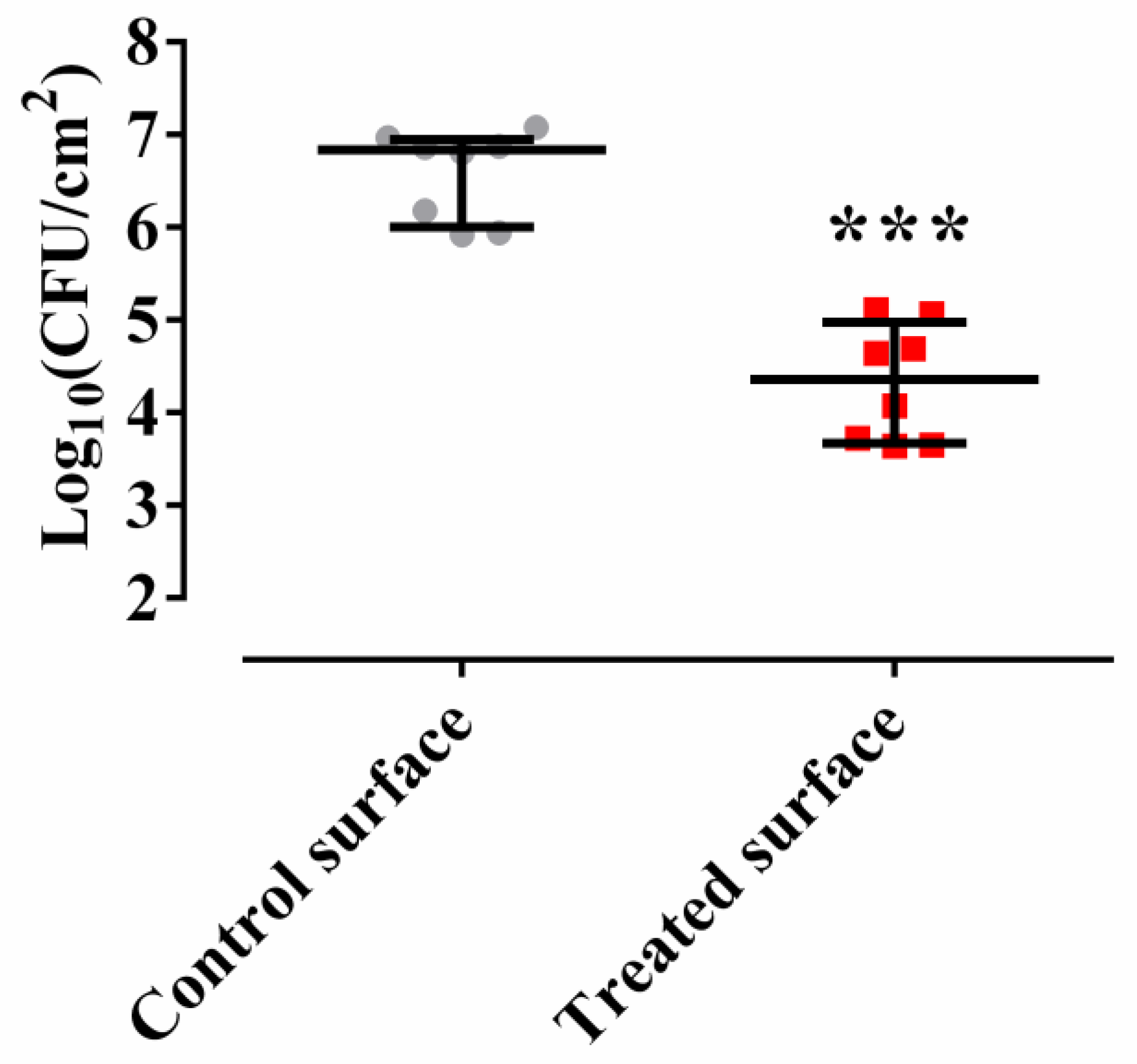
2.2. Methylobacterium sp. CECT 7180 Inhibits the Formation of M. chimaera ECMO Biofilm
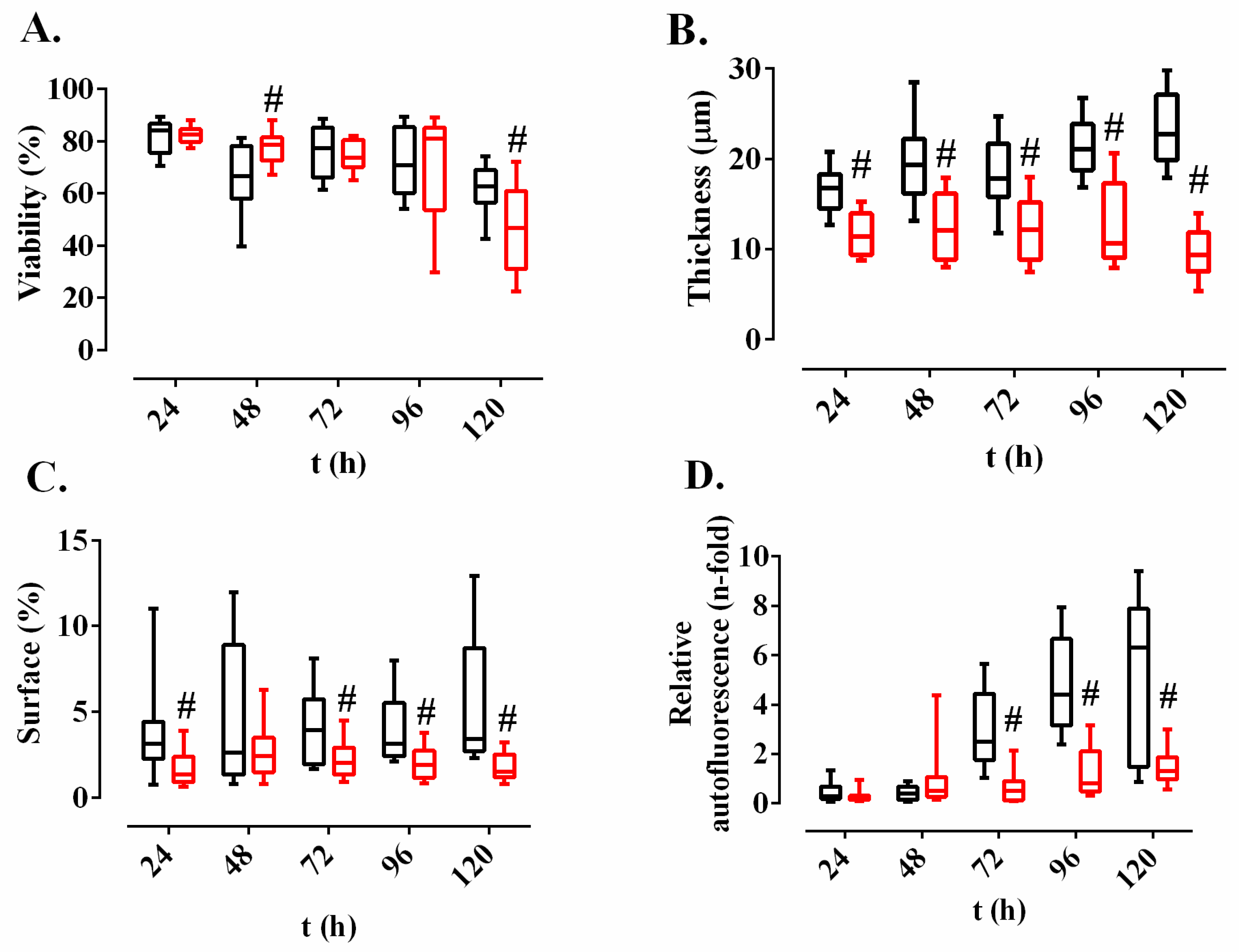
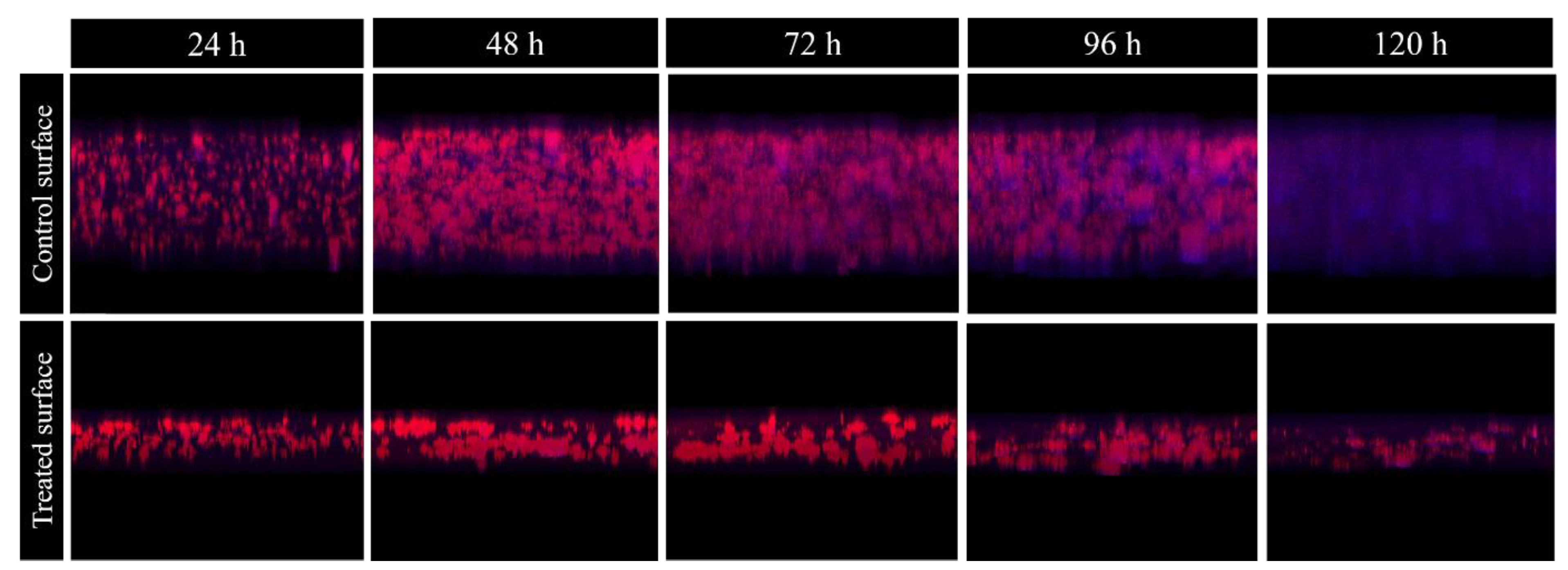
2.3. Effect of Methylobacterium sp. CECT 7180 Extract on M. chimaera ECMO Biofilms Dehydrated for Different Time Periods
2.4. Study of Adhered Proteins of Methylobacterium sp. CECT 7180 Extract
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Methylobacterium sp. CECT 7180 Extract Elaboration
4.3. M. chimaera ECMO Adherence Study
4.4. Inhibition of M. chimaera ECMO Biofilm Formation
4.5. Formation of M. chimaera ECMO Biofilm
4.6. Desiccation Resistance of M. chimaera ECMO Biofilms
4.7. Statistical Analysis
4.8. Study of Adhered Proteins of Methylobacterium sp. CECT 7180 Extract
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diggle, S.P. Microbial communication and virulence: Lessons from evolutionary theory. Micobriology 2010, 156, 3503–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaide Fernández de Vega, F.; Moreno, J.E.; Martín, J.G.; Gutiérrez, J.J.P. Procedimientos en Microbiología Clínica. 2005. ISBN 8460970329. Available online: https://www.seimc.org/contenidos/documentoscientificos/procedimientosmicrobiologia/seimc-procedimientomicrobiologia9a.pdf (accessed on 31 December 2005).
- Mason, R.J.; Slutsky, A.S.; Murray, E.J.; Nadel, J.A.; Gotway, M.B. Nontubercolous mycobacterial infections. In Murray and Nadel’s Textbook of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Faria, S.; Joao, I.; Jordao, L. General overview on nontuberculous mycobacteria, biofilms, and human infection. J. Pathog. 2015, 2015, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.M.; Odell, J.A. Nontuberculous mycobacterial pulmonary infections. J. Thorac. Dis. 2014, 6, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Jarashow, M.C.; Terashita, D.; Balter, S.; Schwartz, B. Mycobacteria Chimaera Infections Associated with Heater-Cooler Unit Use during Cardiopulmonary Bypass Surgery—Los Angeles County, 2012–2016. Am. J. Transplant. 2019, 19, 601–602. [Google Scholar] [CrossRef] [Green Version]
- Acosta, F.; Pérez-lago, L.; Jesús, M.; Serrano, R.; Marín, M.; Kohl, T.A.; Lozano, N.; Niemann, S.; Valerio, M.; Olmedo, M.; et al. Fast update of undetected Mycobacterium chimaera infections to reveal unsuspected cases. J. Hosp. Infect. 2018, 152. [Google Scholar] [CrossRef]
- Casini, B.; Tuvo, B.; Totaro, M.; Baggiani, A.; Privitera, G. Detection and decontamination of Mycobacterium chimaera and other non-tuberculosis mycobacteria in heater—cooler devices used in cardiopulmonary bypass: A Manufacturer and National guidelines summary, and a potential resolution to the problem requirin. Perfusion 2020, 35, 190–196. [Google Scholar] [CrossRef]
- Stefan, P.; Erik, C.; Sax, H.; Bloemberg, G.; Hasse, B.; Sommerstein, R.; Kohler, P.; Achermann, Y. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin. Infect. Dis. 2015, 61, 67–75. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Invasive Cardiovascular Infection by Mycobacterium Chimaera Associated with the 3T Heater-Cooler System Used during Open-Heart Surgery; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2016. [Google Scholar]
- Schreiber, P.W.; Sax, H. Mycobacterium chimaera infections associated with heater–cooler units in cardiac surgery. Curr. Opin. 2017, 30. [Google Scholar] [CrossRef] [Green Version]
- Haller, S.; Höller, C.; Jacobshagen, A.; Hamouda, O.; Abu Sin, M.; Monnet, D.L.; Plachouras, D.; Eckmanns, T. Contamination during production of heater-cooler units by Mycobacterium chimaera potential cause for invasive cardiovascular infections: Results of an outbreak investigation in Germany, April 2015 to February 2016. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.; Moore, G.; Collins, S.; Parks, S.; Garvey, M.I.; Lamagni, T.; Smith, G.; Dawkin, L.; Goldenberg, S.; Chand, M. Microbiological problems and biofilms associated with Mycobacterium chimaera in heater e cooler units used for cardiopulmonary bypass. J. Hosp. Infect. 2017, 96, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Chand, M.; Lamagni, T.; Kranzer, K.; Hedge, J.; Moore, G.; Parks, S.; Collins, S.; Elias, O.; Ahmed, N.; Brown, T.; et al. Insidious risk of severe Mycobacterium chimaera infection in cardiac surgery patients. Clin. Infect. Dis. 2017, 64, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Landolfo, K.; Renew, J.R. Mycobacterium infection from a cardiopulmonary bypass heater- cooler unit in a patient with steroid-induced immunosuppression. Can. J. Anesth. 2017, 64, 513–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, F.; Forteza, A.; Garcia-pavia, P.; Ramos-martinez, A. Successful treatment of healthcare-associated Mycobacterium chimaera prosthetic infective endocarditis: The first Spanish case report. Eur. Heart J. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Tan, N.; Sampath, R.; Saleh, O.M.A.; Tweet, M.S. Disseminated Mycobacterium chimaera infection after cardiothoracic surgery. Open Forum Infect. Dis. 2016, 3, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lin, J.; Feng, Y.; Wang, X.; Mcnally, A. Identification of Mycobacterium chimaera in heater-cooler units in China. Sci. Rep. 2018, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Scriven, J.E.; Scobie, A.; Verlander, N.Q.; Houston, A.; Collyns, T.; Cajic, V.; Kon, O.M.; Mitchell, T.; Rahama, O.; Robinson, A.; et al. Mycobacterium chimaera infection following cardiac surgery in the United Kingdom: Clinical features and outcome of the first 30 cases. Clin. Microbiol. Infect. 2018, 24, 1164–1170. [Google Scholar] [CrossRef] [Green Version]
- Sommerstein, R.; Hasse, B.; Marschall, J.; Sax, H.; Genoni, M.; Schlegel, M.; Widmer, A.F. Global health estimate of invasive mycobacterium chimaera infections associated with heater–cooler devices in cardiac surgery. Emerg. Infect. Dis. 2018, 24, 1–3. [Google Scholar] [CrossRef]
- Sommerstein, R.; Rüegg, C.; Kohler, P.; Bloemberg, G.; Kuster, S.P.; Sax, H. Transmission of Mycobacterium chimaera from heater–cooler units during crdiac surgery despite an ultraclean air ventilation system. Emerg. Infect. Dis. 2016, 22, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Nihn, A.; Weiner, M.; Goldberg, A. Healthcare-associated Mycobacterium chimaera infection subsequent to heater-cooler device exposure during cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1831–1835. [Google Scholar] [CrossRef]
- Kohler, P.; Kuster, S.P.; Bloemberg, G.; Schulthess, B.; Bo, C.; Falk, V.; Frank, M.; Tanner, F.C.; Ro, M.; Wilhelm, M.J.; et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur. Heart J. 2015, 36, 2745–2753. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.J.; Koh, W.; Daley, C.L. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians’ perspectives. Tuberc. Respir. Dis. (Seoul) 2016, 79, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, P.W.; Kuster, S.P.; Hasse, B.; Bayard, C.; Rüegg, C.; Kohler, P.; Keller, P.M.; Bloemberg, G.V.; Maisano, F.; Bettex, D.; et al. Reemergence of Mycobacterium chimaera in heater-cooler units despite intesified clining and disinfection protocol. Emerg. Infect. Dis. 2016, 22, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewardson, A.J.; Stuart, R.L.; Cheng, A.C. Mycobacterium chimaera and cardiac surgery. Med. J. Aust. 2016, 206, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.I.; Phillips, N.; Bradley, C.W.; Holden, E. Decontamination of an extracorporeal membrane oxygenator contaminated with mycobacterium chimaera. Infect. Control. Hosp. Epidemiol. 2017, 38, 1244–1246. [Google Scholar] [CrossRef]
- Martí, M.C.; Bermejo, B.B.; Gil, L.A. Infections with Mycobacterium chimaera and open chest surgery. An unresolved problem. Med. Clin. (Barc). 2019, 152, 317–323. [Google Scholar] [CrossRef]
- Sommerstein, R.; Schreiber, P.W.; Diekema, D.J.; Edmond, M.B.; Hasse, B.; Marschall, J.; Sax, H. Mycobacterium chimaera outbreak associated with Heater-Cooler Devices: Piecing the puzzle together. Infect. Control. Hosp. Epidemiol. 2017, 38. [Google Scholar] [CrossRef] [Green Version]
- Kaelin, M.B.; Kuster, S.P.; Hasse, B.; Schulthess, B.; Imkamp, F.; Halbe, M.; Sander, P.; Sax, H.; Schreiber, P.W. Diversity of nontuberculous mycobacteria in Heater-Cooler Devices–results from prospective surveillance. J. Hosp. Infect. 2020. [Google Scholar] [CrossRef]
- Perkins, K.M.; Lawsin, A.; Hasan, N.A.; Strong, M.; Halpin, A.L.; Rodger, R.R.; Moulton-Meissner, H.; Crist, M.B.; Schwartz, S.; Marders, J.; et al. Mycobacterium Chimaera Contamination of Heater-Cooler Devices Used in Cardiac Surgery—United States. In MMWR. Morbidity and Mortality Weekly Report 2016; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016; Volume 65. [Google Scholar]
- Esteban, J.; García-coca, M. Mycobacterium biofilms. Front. Microbiol. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- García-Coca, M.; Rodríguez-Sevilla, G.; Aguilera-Correa, J.-J.; Esteban-Moreno, J.; Muño-Egea, M.-C. Inhibition of Mycobacterium chelonae and Mycobacterium fortuitum biofilms by Methylobacterium sp. In Proceedings of the European Congress of Clinical Micobiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018. [Google Scholar]
- Patt, T.E.; Cole, G.C.; Hanson, R.S. Methylobacterium, a new genus of facultatively methylotrophic bacteria. Int. J. Syst. Bacteriol. 1976, 26, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Egea, M.C.M.; Ji, P.; Pruden, A.; Falkinham, J.O. Inhibition of adherence of mycobacterium avium to plumbing surface biofilms of Methylobacterium spp. Pathogens 2017, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Balachandran, C.; Duraipandiyan, V.; Ignacimuthu, S. Cytotoxic (A549) and antimicrobial effects of Methylobacterium sp. isolate (ERI-135) from Nilgiris forest soil, India. Asian Pac. J. Trop. Biomed. 2012, 2, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Tejesvi, M.V.; Andersen, B. MB1533 is a defensin-like antimicrobial peptide from the intracellular meristem endophyte of Scots Pine Methylobacterium extorquens DSM13060. J. Microb. Biochem. Technol. 2015, 08, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Photolo, M.M.; Mavumengwana, V.; Sitole, L.; Tlou, M.G. Antimicrobial and Antioxidant Properties of a Bacterial Endophyte, Methylobacterium radiotolerans MAMP 4754, Isolated from Combretum erythrophyllum seeds. Int. J. Microbiol. 2020, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Egea, M.-C.; García-Pedrazuela, M.; Mahillo, I.; García, M.J.; Esteban, J. Autofluorescence as a tool for structural analysis of biofilms formed by nonpigmented rapidly growing Mycobacteria. Appl. Environ. Microbiol. 2013, 79, 1065–1067. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, Q.; Tang, X.; An, Y.; Li, S.; Xu, H.; Li, Y.; Wang, X.; Luan, W.; Wang, Y.; et al. Effects of CwlM on autolysis and biofilm formation in Mycobacterium tuberculosis and Mycobacterium smegmatis. Int. J. Med. Microbiol. 2019, 309, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.M.; Petchiappan, A.; Chatterji, D. Quorum sensing and biofilm formation in Mycobacteria: Role of c-di-GMP and methods to study this second messenger. Int. Union Biochem. Mol. Biol. 2014, 66, 823–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.K.; Mishra, A.; Jha, B. Anti-quorum sensing and anti-biofilm activity of Delftia tsuruhatensis extract by attenuating the quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.Y.; Wu, L.; Yew, W.S. Directed evolution of a quorum-quenching lactonase from Mycobacterium avium subsp. paratuberculosis K-10 in the amidohydrolase superfamily. Biochemistry 2009, 48, 4344–4353. [Google Scholar] [CrossRef]
- Milon, P.; Carotti, M.; Konevega, A.L.; Wintermeyer, W.; Rodnina, M.V.; Gualerzi, C.O. The ribosome-bound initiation factor 2 recruits initiator tRNA to the 30S initiation complex. EMBO Rep. 2010, 11, 312–316. [Google Scholar] [CrossRef]
- Delvillani, F.; Papiani, G.; Dehó, G.; Briani, F. S1 ribosomal protein and the interplay between translation and mRNA decay. Nucleic Acids Res. 2011, 39, 7702–7715. [Google Scholar] [CrossRef] [Green Version]
- Savelsbergh, A.; Rodnina, M.V.; Wintermeyer, W. Distinct functions of elongation factor G in ribosome recycling and translocation. RNA 2009, 15, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Rodríguez, J.; Sabater-Muñoz, B.; Montagud-Martínez, R.; Berlanga, V.; Alvarez-Ponce, D.; Wagner, A.; Fares, M.A. The molecular chaperone dnak is a source of mutational robustness. Genome Biol. Evol. 2016, 8, 2979–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkinham, J.O. Challenges of NTM drug development. Front. Microbiol. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chistoserdova, L.; Crowther, G.J.; Vorholt, J.A.; Skovran, E.; Portais, J.C.; Lidstrom, M.E. Identification of a fourth formate dehydrogenase in Methylobacterium extorquens AM1 and confirmation of the essential role of formate oxidation in methylotrophy. J. Bacteriol. 2007, 189, 9076–9081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujii, A.; Nishino, T. Mechanism of transition from xanthine dehydrogenase to xanthine oxidase: Effect of guanidine-HCL or urea on the activity. Nucleosides Nucleotides Nucleic Acids 2008, 27, 881–887. [Google Scholar] [CrossRef]
- Bonini, M.G.; Miyamoto, S.; Di Mascio, P.; Augusto, O. Production of the carbonate radical anion during xanthine oxidase turnover in the presence of bicarbonate. J. Biol. Chem. 2004, 279, 51836–51843. [Google Scholar] [CrossRef] [Green Version]
- Blombach, B.; Takors, R. CO2—Intrinsic product, essential substrate, and regulatory trigger of microbial and mammalian production processes. Front. Bioeng. Biotechnol. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Tomioka, H.; Sato, K.; Sano, C.; Akaki, T.; Shimizu, T.; Kajitani, H.; Saito, H. Effector molecules of the host defence mechanism against Mycobacterium avium complex: The evidence showing that reactive oxygen intermediates, reactive nitrogen intermediates, and free fatty acids each alone are not decisive in expression of macrophage an. Clin. Exp. Immunol. 1997, 109, 248–254. [Google Scholar] [CrossRef]
- Danielyan, K.E.; Simonyan, A.A. Protective abilities of pyridoxine in experimental oxidative stress settings in vivo and in vitro. Biomed. Pharmacother. 2017, 86, 537–540. [Google Scholar] [CrossRef]
- Dick, T.; Manjunatha, U.; Kappes, B.; Gengenbacher, M. Vitamin B6 biosynthesis is essential for survival and virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2010, 78, 980–988. [Google Scholar] [CrossRef]
- Falkinham, J.O. Disinfection and cleaning of heater–cooler units: Suspension- and biofilm-killing. J. Hosp. Infect. 2020. [Google Scholar] [CrossRef]
- Witko, A. Effect of natural and physical agents. Effect of desiccation. In Inhibition and Destruction of the Microbial Cell; Academic Press: Cambridge, MA, USA, 2003; p. 425. ISBN 3904144987. [Google Scholar]
- Pitcher, R.S.; Green, A.J.; Brzostek, A.; Korycka-Machala, M.; Dziadek, J.; Doherty, A.J. NHEJ protects mycobacteria in stationary phase against the harmful effects of desiccation. DNA Repair (Amst). 2007, 6, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, P.; Kumar, A. The extracellular matrix of mycobacterial biofilms: Could we shorten the treatment of mycobacterial infections? Microb. Cell 2019, 6, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Mai-Prochnow, A.; Webb, J.S.; Ferrari, B.C.; Kjelleberg, S. Ecological advantages of autolysis during the development and dispersal of Pseudoalteromonas tunicata biofilms. Appl. Environ. Microbiol. 2006, 72, 5414–5420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Coca, M.; Perez-Domingo, A.; Esteban-Moreno, J.; Muñoz-Egea, M.-C. Effect of Methylobacterium sp. combined with clarithromycin on Mycobacterium abscessus biofilms. In Proceedings of the European Congress of Clinical Micobiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018. [Google Scholar]
- García-Coca, M.; Rodríguez-Sevilla, G.; Pérez-Domingo, A.; Aguilera-Correa, J.-J.; Esteban, J.; Muñoz-Egea, M.-C. Inhibition of Mycobacterium abscessus, M. chelonae, and M. fortuitum biofilms by Methylobacterium sp. J. Antibiot. (Tokyo) 2019. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Conesa-Buendía, F.M.; Llamas-Granda, P.; Larrañaga-Vera, A.; Wilder, T.; Largo, R.; Herrero-Beaumont, G.; Cronstein, B.; Mediero, A. Tenofovir causes bone loss via decreased bone formation and increased bone resorption, which can be counteracted by dipyridamole in mice. J. Bone Miner. Res. 2019, 34, 923–938. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]

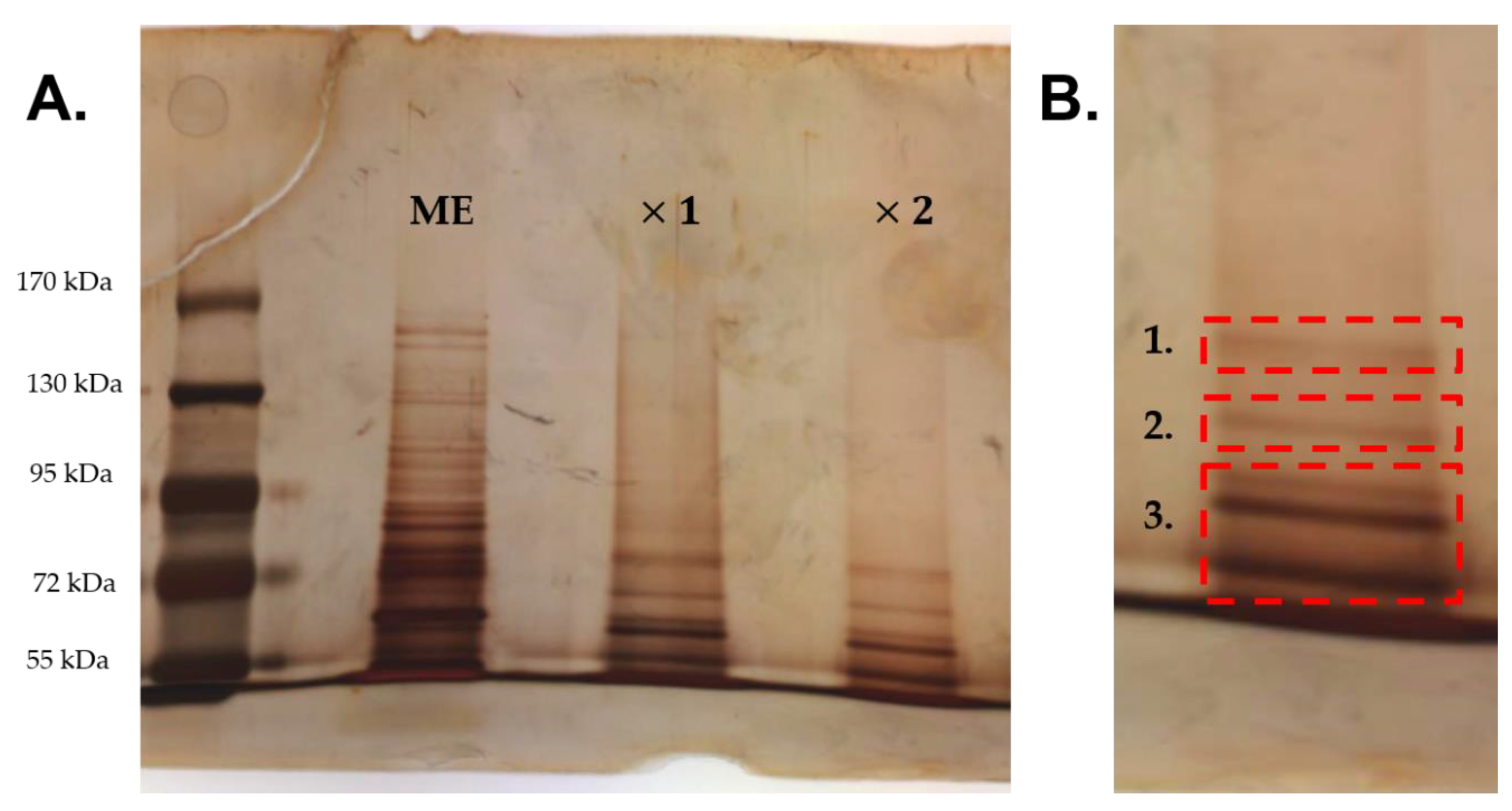
| Band | UniProt ID | Peptide Sequences | Protein Name | Species | Score a (p < 0.05) |
|---|---|---|---|---|---|
| 1 | A0A512JN39 | K.LAAEDPSFR.V K.LAAEDPSFR.V K.LAAEDPSFR.V | Elongation factor G | Methylobacterium gnaphalii | 82 |
| A0A2R4WM33 | R.GSRATVSLPR.A | Glutathione-dependent formaldehyde dehydrogenase | Methylobacterium currus | 50 | |
| A0A5A8ABH1 | R.AEFAESAR.A | Uncharacterized protein | Methylobacterium sp. P1-11 | 37 | |
| A0A0X1SN19 | K.TLEDLR.D | Glycerol kinase | Methylobacterium sp. DM1 | 37 | |
| A0A2R4WQJ0 | R.VALANQR.Q | SLBB domain-containing protein | Methylobacterium currus | 31 | |
| 2 | A0A389MW96 | R.LDSLDQR.V K.AIGAPAEAGR.- R.SGFAGSTPAGSAR.G R.AGLPYADSLTALR.G R.AAAASAAAGLSADAFK.A R.QAADAGKAEAQEAAR.A | Uncharacterized protein (translation initiation factor 2) b | Methylobacterium sp. | 198 |
| A0A2U8WMK9 | K.VIENAEGAR.T R.TTDLMQASMK.L R.TTPSIVAFTDDGER.L | Chaperone protein DnaK | Methylobacterium terrae | 98 | |
| A0A0X1SM13 | R.GSRATVSLPR.A | Glutamine amidotransferase | Methylobacteriumsp. AMS5 | 50 | |
| A0A0J6SME0 | R.SQVDIRPVR.D R.AQVLDVDVEKER.I | 30S ribosomal protein S1 | Methylobacterium tarhaniae | 47 | |
| A0A1E4DI60 | M.IDSELR.R | 4-hydroxy-tetrahydrodipicolinate synthase | Methylobacterium sp. SCN 67-24 | 43 | |
| A0A2R4WQJ0 | R.VALANQR.Q | SLBB domain-containing protein | Methylobacterium currus | 43 | |
| A0A0X1SN19 | K.TLEDLR.D | Glycerol kinase | Methylobacterium sp. AMS5 | 41 | |
| A0A0C6EZC1 | R.EAGEVLR.G | Glycosyl transferase | Methylobacterium aquaticum | 39 | |
| A0A389MNS2 | R.TEFAPADAK.L R.AVPGVVDVVR.I | Oxidoreductase (xanthine oxidase family protein molybdopterin-binding subunit) b | Methylobacterium sp. | 38 | |
| A0A0C6F942 | R.FSVLSR.L | Serine/threonine protein kinase | Methylobacterium aquaticum | 38 | |
| B0ULW9 | R.LLIDVK.E | Glycosyl transferase group 1 | Methylobacterium sp. (strain 4-46) | 37 | |
| B0UGC4 | R.LERELSEARR.K | Alanine--tRNA ligase | Methylobacterium sp. (strain 4-46) | 37 | |
| 3 | A0A389MRA9 | K.IVADNNLK.L K.AMGGDLEAQSR.R R.SVMADVLQNAVNEANQK.I | Trigger factor | Methylobacterium sp. | 135 |
| A0A0X1SM13 | R.GSRATVSLPR.A | Glutamine amidotransferase | Methylobacterium sp. AMS5 | 54 | |
| A0A0Q4WX57 | R.VLSELGTR.A | Chaperone protein HtpG | Methylobacterium sp. Leaf91 | 41 | |
| A0A2V3TYL6 | R.RDISVTNPSR.R | Small-conductance mechanosensitive channel | Methylobacterium sp. B4 | 32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradal, I.; Esteban, J.; Mediero, A.; García-Coca, M.; Aguilera-Correa, J.J. Contact Effect of a Methylobacterium sp. Extract on Biofilm of a Mycobacterium chimaera Strain Isolated from a 3T Heater-Cooler System. Antibiotics 2020, 9, 474. https://doi.org/10.3390/antibiotics9080474
Pradal I, Esteban J, Mediero A, García-Coca M, Aguilera-Correa JJ. Contact Effect of a Methylobacterium sp. Extract on Biofilm of a Mycobacterium chimaera Strain Isolated from a 3T Heater-Cooler System. Antibiotics. 2020; 9(8):474. https://doi.org/10.3390/antibiotics9080474
Chicago/Turabian StylePradal, Inés, Jaime Esteban, Arancha Mediero, Marta García-Coca, and John Jairo Aguilera-Correa. 2020. "Contact Effect of a Methylobacterium sp. Extract on Biofilm of a Mycobacterium chimaera Strain Isolated from a 3T Heater-Cooler System" Antibiotics 9, no. 8: 474. https://doi.org/10.3390/antibiotics9080474
APA StylePradal, I., Esteban, J., Mediero, A., García-Coca, M., & Aguilera-Correa, J. J. (2020). Contact Effect of a Methylobacterium sp. Extract on Biofilm of a Mycobacterium chimaera Strain Isolated from a 3T Heater-Cooler System. Antibiotics, 9(8), 474. https://doi.org/10.3390/antibiotics9080474






