Antimicrobial Stewardship in General Practice: A Scoping Review of the Component Parts
Abstract
:1. Introduction
1.1. Aim of This Scoping Review
1.2. Scoping Review Question
2. Results
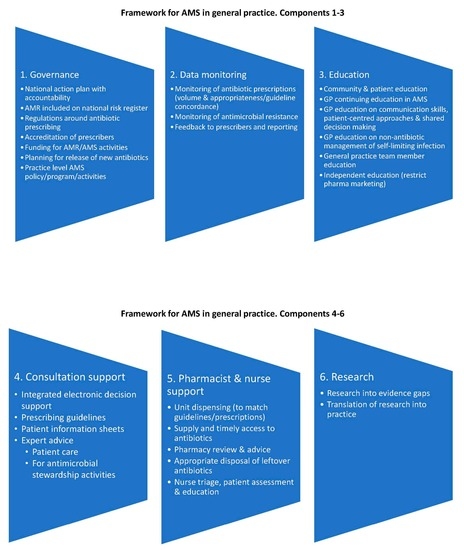
2.1. The Identified Components
2.1.1. Governance
2.1.2. Monitoring and Feedback
2.1.3. Education
2.1.4. Consultation Support
2.1.5. Pharmacy and Nursing Approaches
2.1.6. Research
3. Discussion
4. Materials and Methods
4.1. Selection Criteria
- Hospitals, including their emergency departments and outpatient (specialist) clinics, residential care including nursing or aged care homes; veterinary clinics;
- Other community prescribers (e.g., nurse practitioners, dentists, other medical specialists, veterinarians);
- Patients or community members; animals; the environment;
- Settings where antibiotics were frequently available without a prescription.
4.2. Search Strategy
4.3. Data Collection, Charting and Identification of AMS Components
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C.; ESCMID Study Group for Antimicrobial stewardshiP. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Health and Care Excellence. Antimicrobial stewardship: Systems and processes for effective antimicrobial medicine use. Full guideline: Methods, evidence and recommendations. In NICE Guideline; NICE: London, UK, 2015. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial consumption: 2017. In Annual Epidemiological Report 2017; ECDC: Stockholm, Sweden, 2018. [Google Scholar]
- Australian Commission on Safety and Quality in Health Care. AURA 2019: Third Australian Report on Antimicrobial Use and Resistance in Human Health; ACSQHC: Sydney, Australia, 2019. [Google Scholar]
- Government of Canada. Human Antimicrobial Use Report 2012/2013; Public Health Agency of Canada: Guelph, Ontario, 2014.
- Suda, K.J.; Hicks, L.A.; Roberts, R.M.; Hunkler, R.J.; Matusiak, L.M.; Schumock, G.T. Antibiotic expenditures by medication, class, and healthcare setting in the United States, 2010–2015. Clin. Infect. Dis. 2018, 66, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Dolk, F.C.K.; Pouwels, K.B.; Smith, D.R.M.; Robotham, J.V.; Smieszek, T. Antibiotics in primary care in England: Which antibiotics are prescribed and for which conditions? J. Antimicrob. Chemother. 2018, 73, ii2–ii10. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.R.; Pollack, A.J.; Plejdrup Hansen, M.; Glasziou, P.P.; Looke, D.F.; Britt, H.C.; Del Mar, C.B. Antibiotics for acute respiratory infections in general practice: Comparison of prescribing rates with guideline recommendations. Med. J. Aust. 2017, 207, 65–69. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Dolk, F.C.K.; Smith, D.R.M.; Robotham, J.V.; Smieszek, T. Actual versus ’ideal’ antibiotic prescribing for common conditions in English primary care. J. Antimicrob. Chemother. 2018, 73, 19–26. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M., Jr.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [Green Version]
- O′Neill, J.; The Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; HM Government and Wellcome Trust: London, UK, 2014. [Google Scholar]
- Hansen, M.P.; Hoffmann, T.C.; McCullough, A.R.; van Driel, M.L.; Del Mar, C.B. Antibiotic resistance: What are the opportunities for primary care in alleviating the crisis? Front. Public Health 2015, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Ranji, S.R.; Steinman, M.A.; Shojania, K.G.; Gonzales, R. Interventions to reduce unnecessary antibiotic prescribing: A systematic review and quantitative analysis. Med. Care 2008, 46, 847–862. [Google Scholar] [CrossRef]
- Squires, J.E.; Sullivan, K.; Eccles, M.P.; Worswick, J.; Grimshaw, J.M. Are multifaceted interventions more effective than single-component interventions in changing health-care professionals’ behaviours? An overview of systematic reviews. Implement. Sci. 2014, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Kochling, A.; Loffler, C.; Reinsch, S.; Hornung, A.; Bohmer, F.; Altiner, A.; Chenot, J.F. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: A systematic review. Implement. Sci. 2018, 13, 47. [Google Scholar] [CrossRef]
- Arnold, S.R.; Straus, S.E. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst. Rev. 2005, 4, CD003539. [Google Scholar] [CrossRef] [PubMed]
- Drekonja, D.M.; Filice, G.A.; Greer, N.; Olson, A.; MacDonald, R.; Rutks, I.; Wilt, T.J. Antimicrobial stewardship in outpatient settings: A systematic review. Infect. Control Hosp. Epidemiol. 2015, 36, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Walley, J.; Chou, R.; Tucker, J.D.; Harwell, J.I.; Wu, X.; Yin, J.; Zou, G.; Wei, X. Interventions to reduce childhood antibiotic prescribing for upper respiratory infections: Systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 1162–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coxeter, P.; Del Mar, C.B.; McGregor, L.; Beller, E.M.; Hoffmann, T.C. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst. Rev. 2015, CD010907. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.C.; Tamma, P.D.; Cosgrove, S.E.; Miller, M.A.; Sateia, H.; Szymczak, J.; Gurses, A.P.; Linder, J.A. Ambulatory antibiotic stewardship through a human factors engineering approach: A systematic review. J. Am. Board Fam. Med. 2018, 31, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Ostini, R.; Hegney, D.; Jackson, C.; Williamson, M.; Mackson, J.M.; Gurman, K.; Hall, W.; Tett, S.E. Systematic review of interventions to improve prescribing. Ann. Pharm. 2009, 43, 502–513. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O′Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Arksey, H.; O′Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.; Allen, P.; Peckham, S.; Goodwin, N. Asking the right questions: Scoping studies in the commissioning of research on the organisation and delivery of health services. Health Res. Policy Syst. 2008, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Ashiru-Oredope, D.; Hopkins, S.; English Surveillance Programme for Antimicrobial Utilization Resistance Oversight Group. Antimicrobial stewardship: English Surveillance Programme for Antimicrobial Utilization and Resistance (ESPAUR). J. Antimicrob. Chemother. 2013, 68, 2421–2423. [Google Scholar] [CrossRef] [Green Version]
- Del Mar, C.B.; Scott, A.M.; Glasziou, P.P.; Hoffmann, T.; van Driel, M.L.; Beller, E.; Phillips, S.M.; Dartnell, J. Reducing antibiotic prescribing in Australian general practice: Time for a national strategy. Med. J. Aust. 2017, 207, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Essack, S.; Pignatari, A.C. A framework for the non-antibiotic management of upper respiratory tract infections: Towards a global change in antibiotic resistance. Int. J. Clin. Pract. Suppl. 2013, 67, 4–9. [Google Scholar] [CrossRef] [PubMed]
- McNulty, C.A. Optimising antibiotic prescribing in primary care. Int. J. Antimicrob. Agents 2001, 18, 329–333. [Google Scholar] [CrossRef]
- Wang, S.; Pulcini, C.; Rabaud, C.; Boivin, J.M.; Birge, J. Inventory of antibiotic stewardship programs in general practice in France and abroad. Med. Mal. Infect. 2015, 45, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Ashiru-Oredope, D.; Sharland, M.; Charani, E.; McNulty, C.; Cooke, J.; ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart–Then Focus. J. Antimicrob. Chemother. 2012, 67 (Suppl. 1), i51–i63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molstad, S.; Erntell, M.; Hanberger, H.; Melander, E.; Norman, C.; Skoog, G.; Lundborg, C.S.; Söderström, A.; Torell, E.; Cars, O. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect. Dis. 2008, 8, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Molstad, S.; Lofmark, S.; Carlin, K.; Erntell, M.; Aspevall, O.; Blad, L.; Hanberger, H.; Hedin, K.; Hellman, J.; Norman, C.; et al. Lessons learnt during 20 years of the Swedish strategic programme against antibiotic resistance. Bull. World Health Organ. 2017, 95, 764–773. [Google Scholar] [CrossRef]
- Sanchez, G.V.; Fleming-Dutra, K.E.; Roberts, R.M.; Hicks, L.A. Core elements of outpatient antibiotic stewardship. MMWR Recomm. Rep. 2016, 65, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Australian Commission on Safety and Quality in Health Care. Antimicrobial Stewardship in Australian Health Care; ACSQHC: Sydney, Australia, 2018. [Google Scholar]
- British Society for Antimicrobial Chemotherapy; ESCMID Study Group for Antimicrobial Stewardship; European Society of Clinical Microbiology and Infectious Diseases. Antimicrobial Stewardship: From Principles to Practice; BSAC: Birmingham, UK, 2018. [Google Scholar]
- European Commission. EU Guidelines for the Prudent Use of Antimicrobials in Human Health; ECDC: Solna, Sweden, 2017. [Google Scholar]
- The UK Faculty of Public Health; The Royal College of Physicians; The Royal Pharmaceutical Society; The Royal College of Nursing; The Royal College of General Practitioners. Joint Statement on Antimicrobial Resistance; FPH; RCP; RPS; RCN; RCGP: London, UK, 2014. [Google Scholar]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Gulliford, M.C.; Dregan, A.; Moore, M.V.; Ashworth, M.; Staa, T.V.; McCann, G.; Charlton, J.; Yardley, L.; Little, P.; McDermott, L. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: Survey of 568 UK general practices. BMJ Open 2014, 4, e006245. [Google Scholar] [CrossRef]
- Goossens, H.; Ferech, M.; Vander Stichele, R.V.; Elseviers, M.; ESAC Project Grp. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Zanichelli, V.; Monnier, A.A.; Gyssens, I.C.; Adriaenssens, N.; Versporten, A.; Pulcini, C.; Le Marechal, M.; Tebano, G.; Vlahovic-Palcevski, V.; Stanic Benic, M.; et al. Variation in antibiotic use among and within different settings: A systematic review. J. Antimicrob. Chemother. 2018, 73, vi17–vi29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, H.J.; Walker, A.J.; Mahtani, K.R.; Goldacre, B. Time trends and geographical variation in prescribing of antibiotics in England 1998–2017. J. Antimicrob. Chemother. 2019, 74, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Tonkin-Crine, S.; Pavitt, S.H.; McEachan, R.R.C.; Douglas, G.V.A.; Aggarwal, V.R.; Sandoe, J.A.T. Factors associated with antibiotic prescribing for adults with acute conditions: An umbrella review across primary care and a systematic review focusing on primary dental care. J. Antimicrob. Chemother. 2019, 74, 2139–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanichelli, V.; Tebano, G.; Gyssens, I.C.; Vlahovic-Palcevski, V.; Monnier, A.A.; Stanic Benic, M.; Harbarth, S.; Hulscher, M.; Pulcini, C.; Huttner, B.D. Patient-related determinants of antibiotic use: A systematic review. Clin. Microbiol. Infect. 2019, 25, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aromataris, E.; Munn, Z. (Eds.) Joanna Briggs Institute Reviewer’s Manual, 4th ed.; JBI: Adelaide, Australia, 2017. [Google Scholar]
- Tonkin-Crine, S.K.; Tan, P.S.; van Hecke, O.; Wang, K.; Roberts, N.W.; McCullough, A.; Hansen, M.P.; Butler, C.C.; Del Mar, C.B. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: An overview of systematic reviews. Cochrane Database Syst. Rev. 2017, 9, CD012252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Veritas Health Innovation. Covidence Systematic Review Software, 1059; Veritas Health Innovation: Melbourne, Australia, 2018. [Google Scholar]
- QSR International Pty Ltd. NVivo Qualitative Data Analysis Software, 12 Plus; QSR International Pty Ltd.: Melbourne, Australia, 2018. [Google Scholar]
- Ryan, G.W.; Bernard, H.R. Techniques to identify themes. Field Methods 2003, 15, 85–109. [Google Scholar] [CrossRef] [Green Version]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef] [Green Version]

| Component/Author, Date | Ashiru-Oredope, 2012 [30] | Ashiru-Oredope, 2013 [25] | ACSQHC, 2018 [34] | BSAC, 2018 [35] | Del Mar, 2017 [26] | Essack, 2013 [27] | European Commission, 2017 [36] | Keller, 2018 [20] | McNulty, 2001 [28] | Molstad, 2008 [31] | Molstad, 2017 [32] | NICE, 2015 [2] | Sanchez, 2016 [33] | UK Faculty [37] | Wang, 2015 [29] | WHO, 2015 [38] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.1.1. Governance | ||||||||||||||||
| National action plan, policy or strategy | x | x | x | x | x | x | x | x | x | |||||||
| AMR included on national risk register | x | |||||||||||||||
| Regulations around AMS and antibiotic prescribing | x | x | x | x | x | x | x | x | x | x | ||||||
| Accreditation of prescribers | x | x | ||||||||||||||
| Funding for AMR/AMS | x | x | x | x | x | |||||||||||
| Planning for release of new antibiotics | x | x | x | x | ||||||||||||
| Practice level AMS policy/program/activities | x | x | x | x | x | x | ||||||||||
| 2.1.2. Monitoring and Feedback | ||||||||||||||||
| Monitoring of antibiotic prescriptions | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| Monitoring of antimicrobial resistance | x | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Feedback to prescribers and reporting | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| 2.1.3. Education | ||||||||||||||||
| Community and patient education about AMR and AMS | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
| GP continuing education in AMS and AMR | x | x | x | x | x | x | x | x | x | x | x | x | x | |||
| GP education on communication skills, patient-centred approaches and shared decision making | x | x | x | x | x | x | x | x | x | |||||||
| GP education on non-antibiotic management of self-limiting infection | x | x | x | x | x | x | x | x | x | |||||||
| GP education on delayed prescribing/watchful waiting | x | x | x | x | x | x | x | x | x | x | ||||||
| General practice team member education | x | x | x | x | x | x | ||||||||||
| Independent education (restrict pharma marketing) | x | x | x | x | x | x | ||||||||||
| 2.1.4. Consultation Support | ||||||||||||||||
| Prescribing guidelines | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| Point of care tests | x | x | x | x | x | x | x | x | x | x | x | |||||
| Microbiology testing and reporting | x | x | x | x | x | x | x | x | x | |||||||
| Allergy testing | x | x | ||||||||||||||
| Electronic decision support for prescribers | x | x | x | x | x | x | x | x | ||||||||
| Expert advice | x | x | x | x | x | x | ||||||||||
| Decision support for use with patients | x | x | x | x | x | x | x | x | x | x | ||||||
| 2.1.5. Pharmacy and Nursing Approaches | ||||||||||||||||
| Unit dispensing | x | x | x | x | ||||||||||||
| Supply of and timely access to antibiotics | x | x | x | x | NA | x | ||||||||||
| Pharmacy review and advice | x | x | x | x | x | x | ||||||||||
| Appropriate disposal of left-over antibiotics | x | x | NA | |||||||||||||
| Nurse triage, patient assessment and education | x | x | x | x | x | x | ||||||||||
| 2.1.6. Research | ||||||||||||||||
| Research into AMR/AMS gaps, translation into practice | x | x | x | x | x | x | x | x | x | x | ||||||
| Research into context, culture of general practice and behaviour change strategies | x | x | x | x | x | x | x | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawes, L.; Buising, K.; Mazza, D. Antimicrobial Stewardship in General Practice: A Scoping Review of the Component Parts. Antibiotics 2020, 9, 498. https://doi.org/10.3390/antibiotics9080498
Hawes L, Buising K, Mazza D. Antimicrobial Stewardship in General Practice: A Scoping Review of the Component Parts. Antibiotics. 2020; 9(8):498. https://doi.org/10.3390/antibiotics9080498
Chicago/Turabian StyleHawes, Lesley, Kirsty Buising, and Danielle Mazza. 2020. "Antimicrobial Stewardship in General Practice: A Scoping Review of the Component Parts" Antibiotics 9, no. 8: 498. https://doi.org/10.3390/antibiotics9080498
APA StyleHawes, L., Buising, K., & Mazza, D. (2020). Antimicrobial Stewardship in General Practice: A Scoping Review of the Component Parts. Antibiotics, 9(8), 498. https://doi.org/10.3390/antibiotics9080498