Determination of the Size of Complex Iron Oxide Nanoparticles Using Various Physical Experimental Methods
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Sezer, N.; Arı, İ.; Bicer, Y.; Koc, M. Superparamagnetic nanoarchitectures: Multimodal functionalities and applications. J. Magn. Magn. Mater. 2021, 538, 168300. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef]
- Nandhini, G.; Shobana, M. Role of ferrite nanoparticles in hyperthermia applications. J. Magn. Magn. Mater. 2022, 552, 169236. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Jiang, L.; Tang, D.; Xu, H.; Zhao, P.; Fu, J.; Zhou, Q.; Chen, Y. 3D printing of functional magnetic materials: From design to applications. Adv. Funct. Mater. 2021, 31, 2102777. [Google Scholar] [CrossRef]
- Weller, D.; Moser, A. Thermal effect limits in ultrahigh-density magnetic recording. IEEE Trans. Magn. 1999, 35, 4423–4439. [Google Scholar] [CrossRef]
- Weiss, W.; Ranke, W. Surface chemistry and catalysis on well-defined epitaxial iron-oxide layers. Prog. Surf. Sci. 2002, 70, 1–151. [Google Scholar] [CrossRef]
- Rytov, R.; Bautin, V.; Usov, N. Towards optimal thermal distribution in magnetic hyperthermia. Sci. Rep. 2022, 12, 3023. [Google Scholar] [CrossRef]
- Usov, N.A.; Gubanova, E.M. Application of magnetosomes in magnetic hyperthermia. Nanomaterials 2020, 10, 1320. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef] [PubMed]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Khizar, S.; Ahmad, N.M.; Zine, N.; Jaffrezic-Renault, N.; Errachid-el-salhi, A.; Elaissari, A. Magnetic nanoparticles: From synthesis to theranostic applications. ACS Appl. Nano Mater. 2021, 4, 4284–4306. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Q. Collective behaviors of magnetic microparticle swarms: From dexterous tentacles to reconfigurable carpets. ACS Nano 2022, 16, 13728–13739. [Google Scholar] [CrossRef]
- Khamidullin, T.; Lounev, I.; Solodov, A.; Vakhitov, I.; Hashemi, S.A.; Dimiev, A.M. Graphene Oxide–Epoxy Composites with Induced Anisotropy of Electrical Properties. J. Phys. Chem. C 2021, 125, 26823–26831. [Google Scholar] [CrossRef]
- Solodov, A.N.; Balkaev, D.; Amirov, R.; Gataullina, R.; Nurtdinova, L.; Yusupov, R.; Kharintsev, S.S.; Dimiev, A.M. Polymer Composites with Magnetically Tunable Optical Anisotropy. ACS Appl. Polym. Mater. 2023, 5, 6338–6345. [Google Scholar] [CrossRef]
- Solodov, A.; Neklyudov, V.V.; Shayimova, J.; Amirov, R.R.; Dimiev, A.M. Magneto-optical properties of the magnetite-graphene oxide composites in organic solvents. ACS Appl. Mater. Interfaces 2018, 10, 40024–40031. [Google Scholar] [CrossRef]
- Solodov, A.N.; Balkaev, D.A.; Shayimova, J.R.; Vakhitov, I.R.; Gataullina, R.M.; Sukhov, A.V.; Burilova, E.A.; Amirova, L.M.; Zhuravleva, Y.I.; Amirov, R.R. Tribological properties of an epoxy polymer containing a magnetically oriented graphene oxide/iron oxide nanoparticle composite. Diam. Relat. Mater. 2023, 138, 110211. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–relaxivity relationships of magnetic nanoparticles for magnetic resonance imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef]
- Tang, Y.; Flesch, R.C.; Jin, T.; Gao, Y.; He, M. Effect of nanoparticle shape on therapeutic temperature distribution during magnetic hyperthermia. J. Phys. D Appl. Phys. 2021, 54, 165401. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.Á.; Orue, I.; Phan, M.-H.; Srikanth, H. Improving the heating efficiency of iron oxide nanoparticles by tuning their shape and size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal decomposition synthesis of iron oxide nanoparticles with diminished magnetic dead layer by controlled addition of oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Luigjes, B.; Woudenberg, S.M.; de Groot, R.; Meeldijk, J.D.; Torres Galvis, H.M.; de Jong, K.P.; Philipse, A.P.; Erné, B.H. Diverging geometric and magnetic size distributions of iron oxide nanocrystals. J. Phys. Chem. C 2011, 115, 14598–14605. [Google Scholar] [CrossRef]
- Kodama, R. Magnetic nanoparticles. J. Magn. Magn. Mater. 1999, 200, 359–372. [Google Scholar] [CrossRef]
- Roca, A.; Morales, M.; O’Grady, K.; Serna, C. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology 2006, 17, 2783. [Google Scholar] [CrossRef]
- Hassellöv, M.; Readman, J.W.; Ranville, J.F.; Tiede, K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 2008, 17, 344–361. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of particles in solution–a perspective on light scattering and comparative technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, E.; Hwang, N.M.; Kang, M.; Kim, S.C.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H. One-nanometer-scale size-controlled synthesis of monodisperse magnetic Iron oxide nanoparticles. Angew. Chem. 2005, 117, 2932–2937. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse mfe2o4 (m = fe, co, mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Chalasani, R.; Vasudevan, S. Form, content, and magnetism in iron oxide nanocrystals. J. Phys. Chem. C 2011, 115, 18088–18093. [Google Scholar] [CrossRef]
- Casula, M.F.; Jun, Y.-W.; Zaziski, D.J.; Chan, E.M.; Corrias, A.; Alivisatos, A.P. The concept of delayed nucleation in nanocrystal growth demonstrated for the case of iron oxide nanodisks. J. Am. Chem. Soc. 2006, 128, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Solodov, A.N.; Shayimova, J.R.; Burilova, E.A.; Shurtakova, D.V.; Zhuravleva, Y.I.; Cherosov, M.A.; Tian, Y.; Kiiamov, A.G.; Amirov, R.R. Understanding the Nucleation and Growth of Iron Oxide Nanoparticle Formation by a “Heating-Up” Process: An NMR Relaxation Study. J. Phys. Chem. C 2021, 125, 20980–20992. [Google Scholar] [CrossRef]
- Chen, D.-X.; Sanchez, A.; Taboada, E.; Roig, A.; Sun, N.; Gu, H.-C. Size determination of superparamagnetic nanoparticles from magnetization curve. J. Appl. Phys. 2009, 105, 083924. [Google Scholar] [CrossRef]
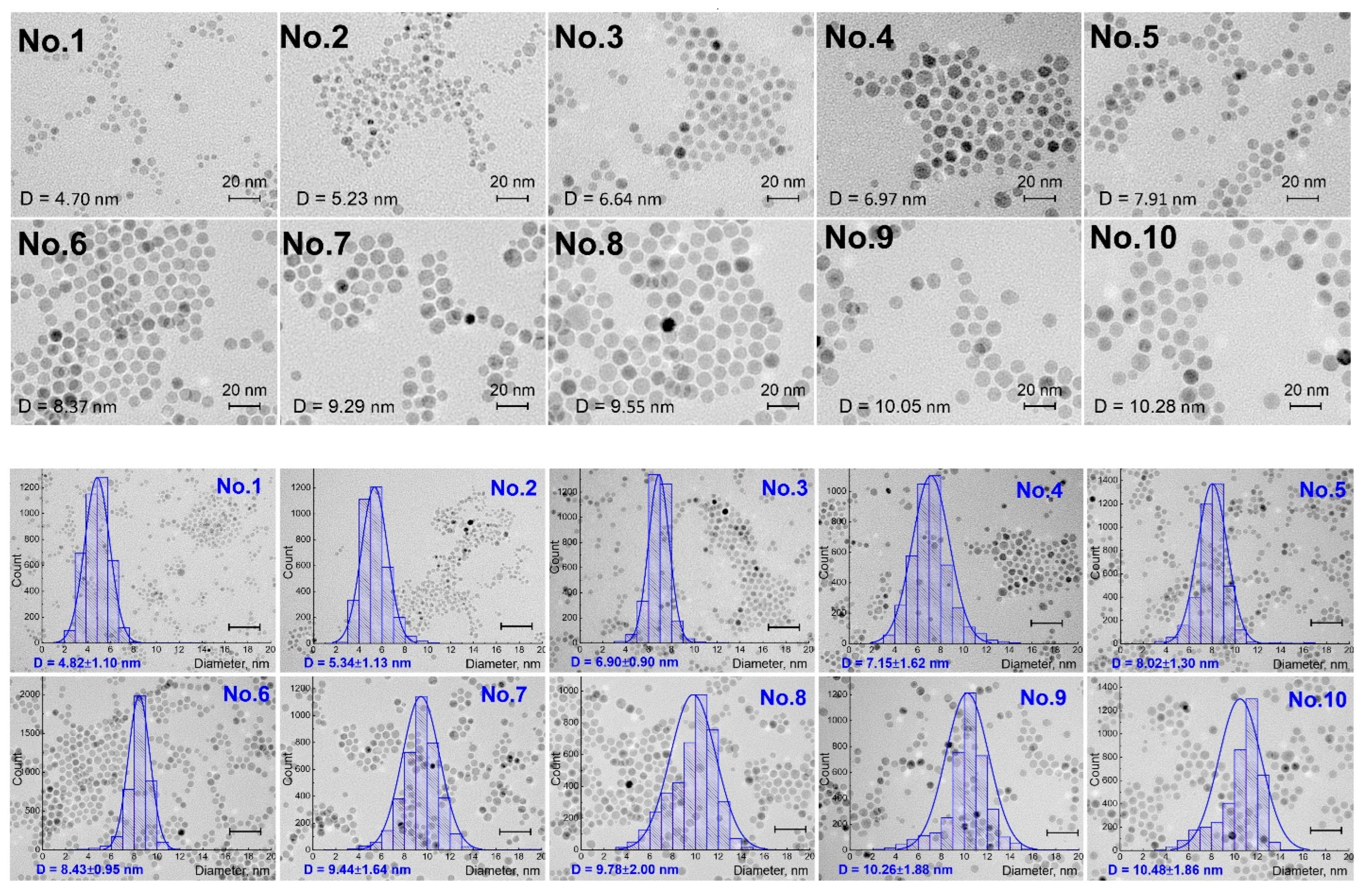
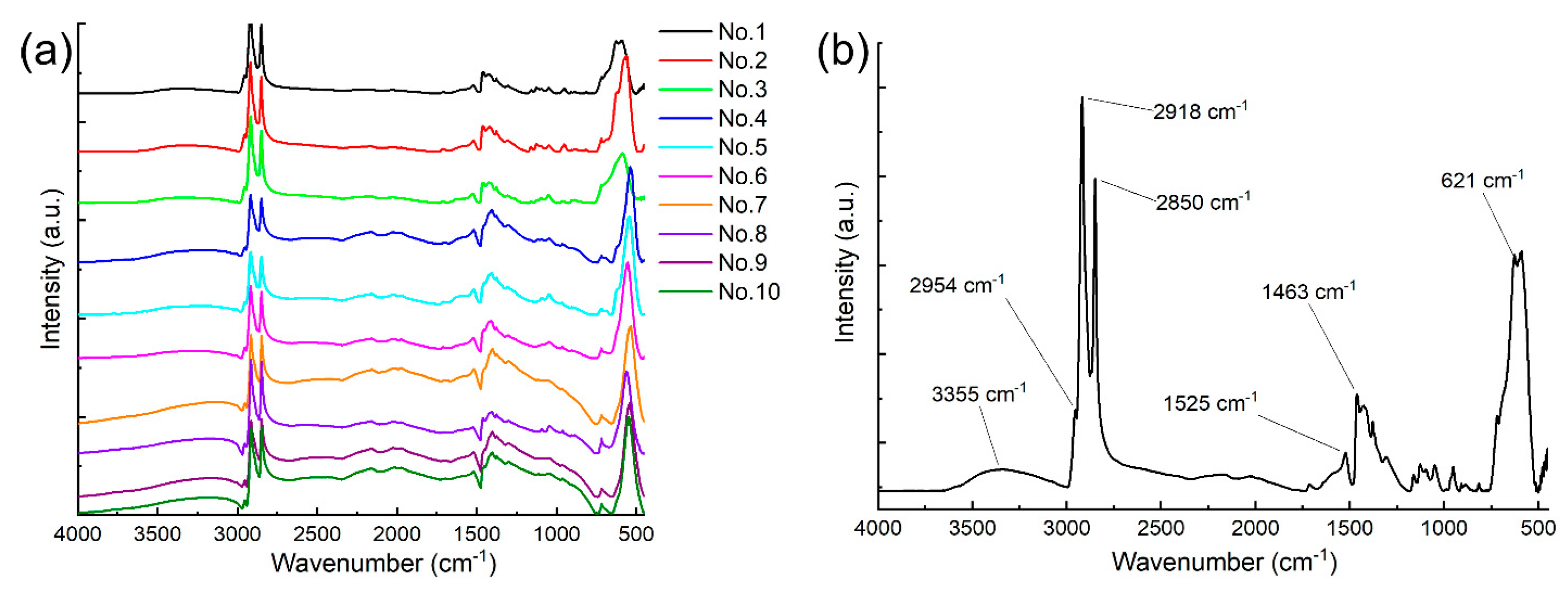

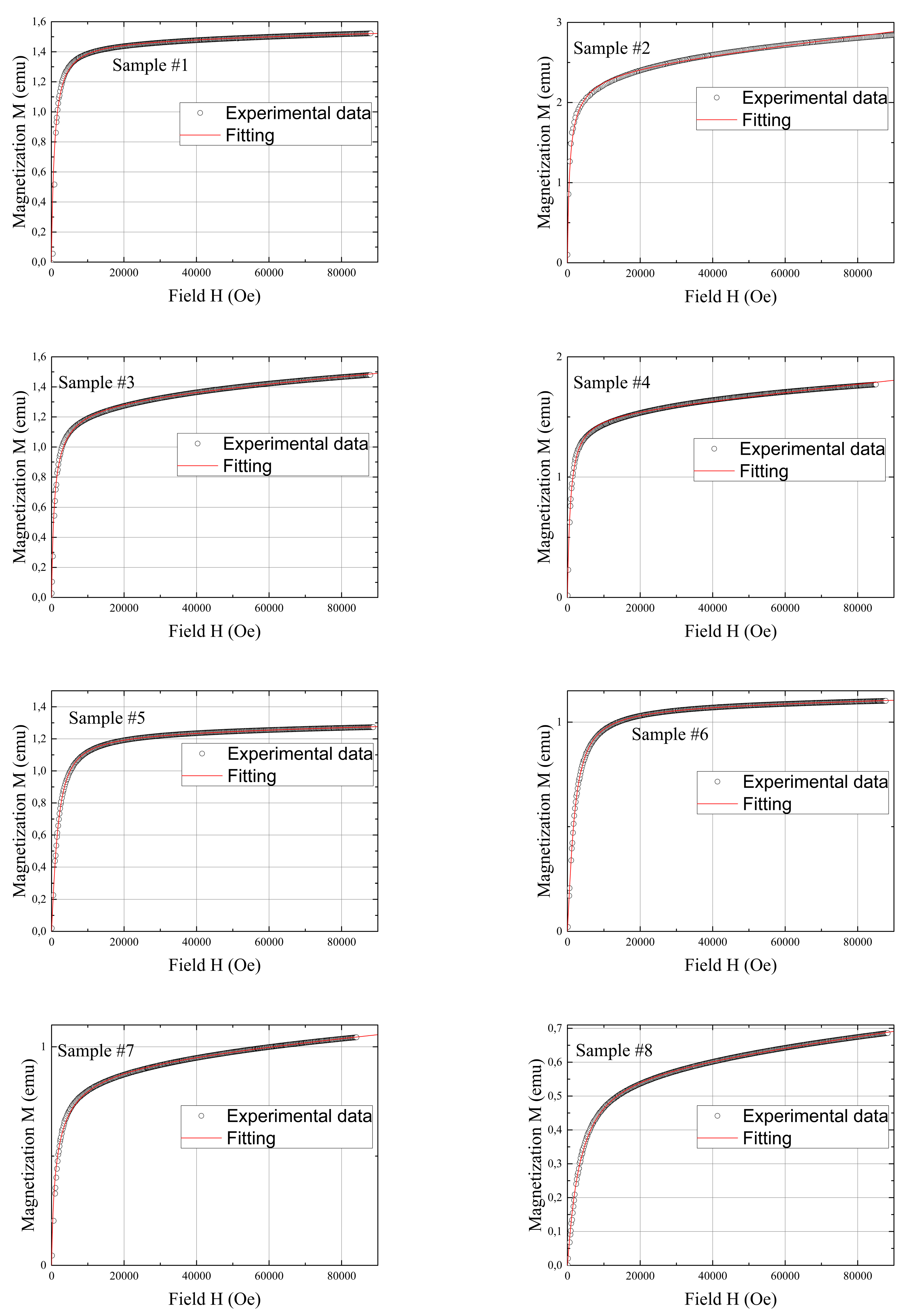

| Sample ID Number | Obtained via TEM, nm | Obtained via XRD, nm | Obtained from the Field Dependencies of Magnetization, nm |
|---|---|---|---|
| 1 | 4.82 ± 1.10 | 4.7 | 3.50 |
| 4.3 | |||
| 4.5 | |||
| 4.7 | |||
| 2 | 5.34 ± 1.13 | 5.6 | 3.33 |
| 4.0 | |||
| 5.3 | |||
| 3 | 6.90 ± 0.90 | 6.6 | 1.80 |
| 7.4 | |||
| 6.5 | |||
| 4 | 7.15 ± 1.62 | 6.8 | 4.50 |
| 6.6 | |||
| 5 | 8.02 ± 1.30 | 4.6 | 4.33 |
| 7.1 | |||
| 4.9 | |||
| 6 | 8.43 ± 0.95 | 4.9 | 4.25 |
| 8.0 | |||
| 7.6 | |||
| 4.8 | |||
| 7 | 9.44 ± 1.64 | 5.4 | 4.00 |
| 7.0 | |||
| 5.3 | |||
| 8 | 9.78 ± 2.00 | 6.3 | 3.30 |
| 8.2 | |||
| 6.5 | |||
| 9 | 10.26 ± 1.88 | 6.3 | 3.33 |
| 10.1 | |||
| 7.4 | |||
| 8.0 | |||
| 10 | 10.48 ± 1.86 | 7.9 | 3.33 |
| 10.3 | |||
| 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiiamov, A.G.; Ivanova, A.G.; Solodov, A.N.; Cherosov, M.A.; Tayurskii, D.A.; Khannanov, A. Determination of the Size of Complex Iron Oxide Nanoparticles Using Various Physical Experimental Methods. Coatings 2023, 13, 1589. https://doi.org/10.3390/coatings13091589
Kiiamov AG, Ivanova AG, Solodov AN, Cherosov MA, Tayurskii DA, Khannanov A. Determination of the Size of Complex Iron Oxide Nanoparticles Using Various Physical Experimental Methods. Coatings. 2023; 13(9):1589. https://doi.org/10.3390/coatings13091589
Chicago/Turabian StyleKiiamov, Airat G., Anna G. Ivanova, Alexander N. Solodov, Mikhail A. Cherosov, Dmitrii A. Tayurskii, and Artur Khannanov. 2023. "Determination of the Size of Complex Iron Oxide Nanoparticles Using Various Physical Experimental Methods" Coatings 13, no. 9: 1589. https://doi.org/10.3390/coatings13091589