Effect of ZrO2 Particles on the Microstructure and Ultrasonic Cavitation Properties of CoCrFeMnNi High-Entropy Alloy Composite Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimen Preparation
2.2. Characterization
2.3. Performance Testing
2.4. Finite Element Model
3. Results
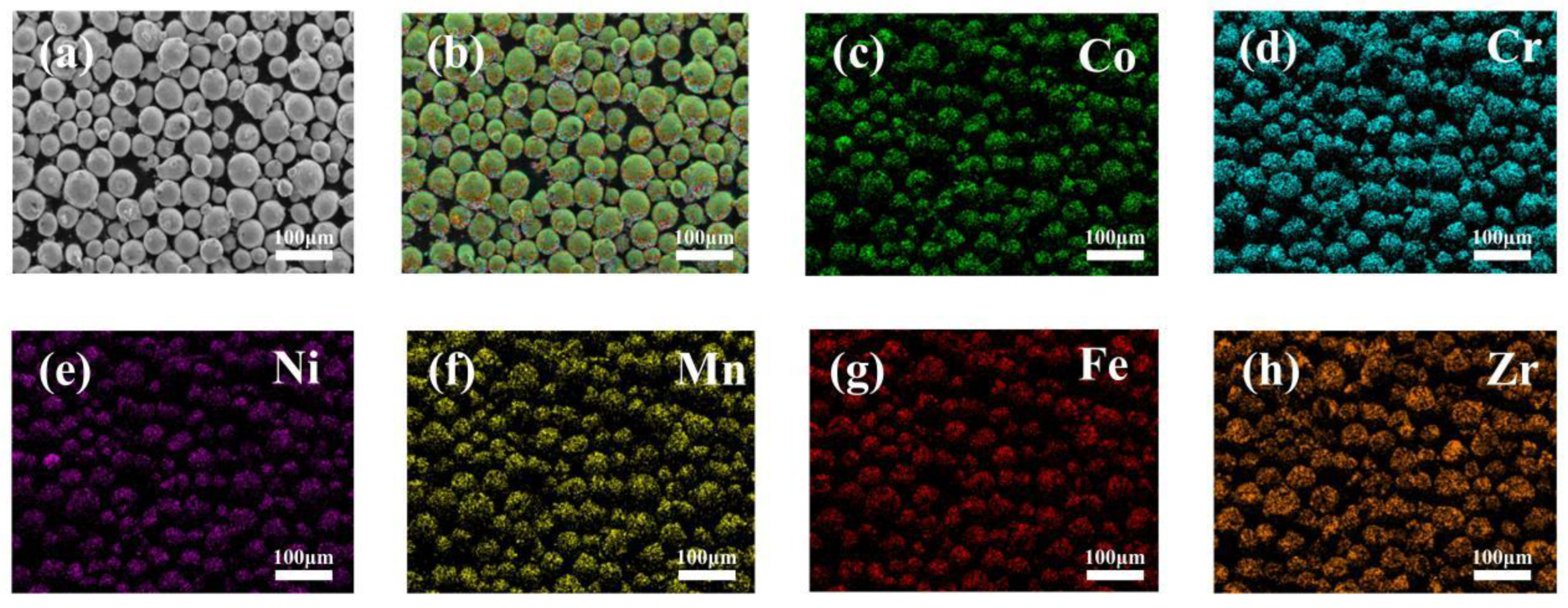
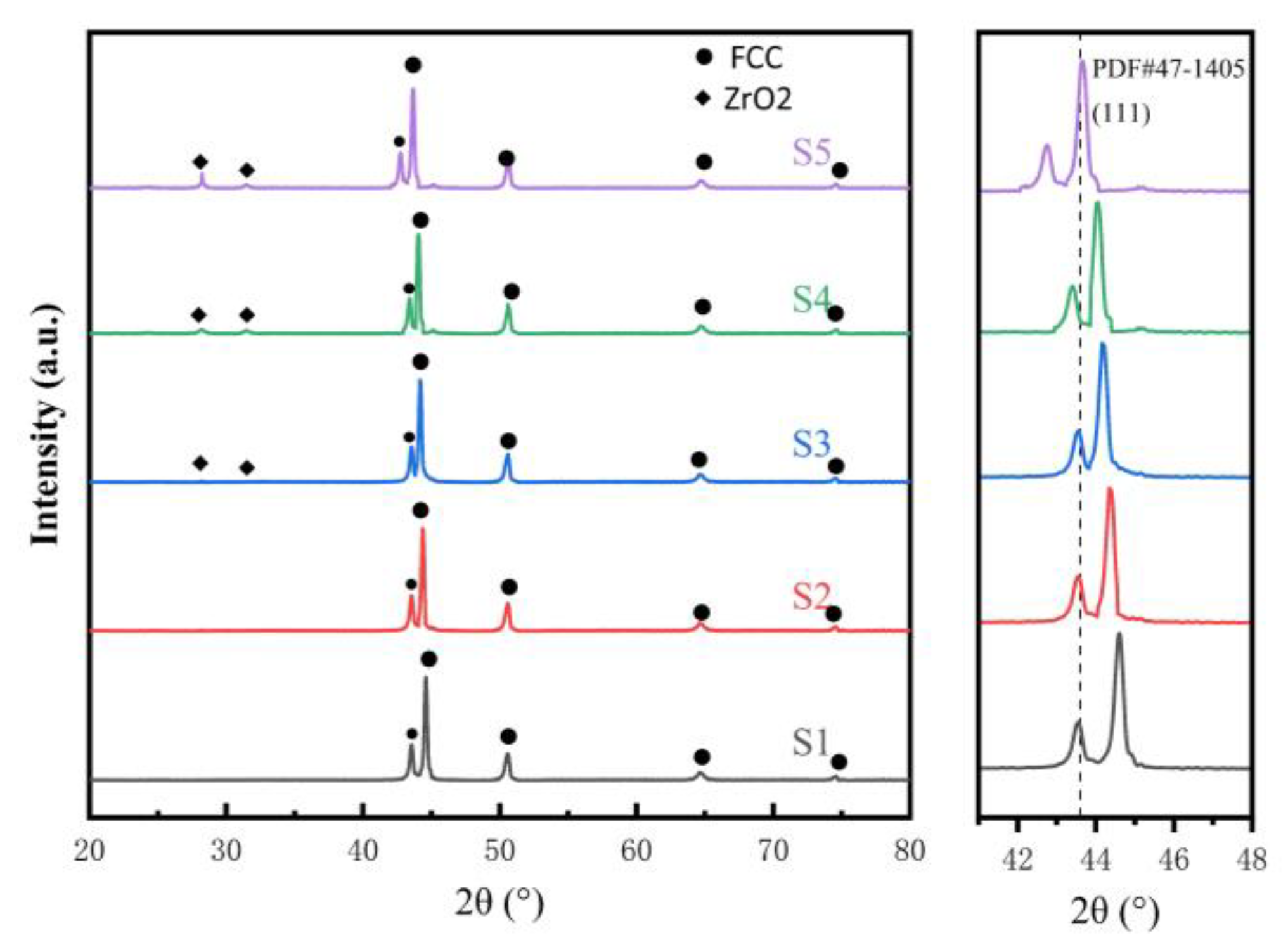
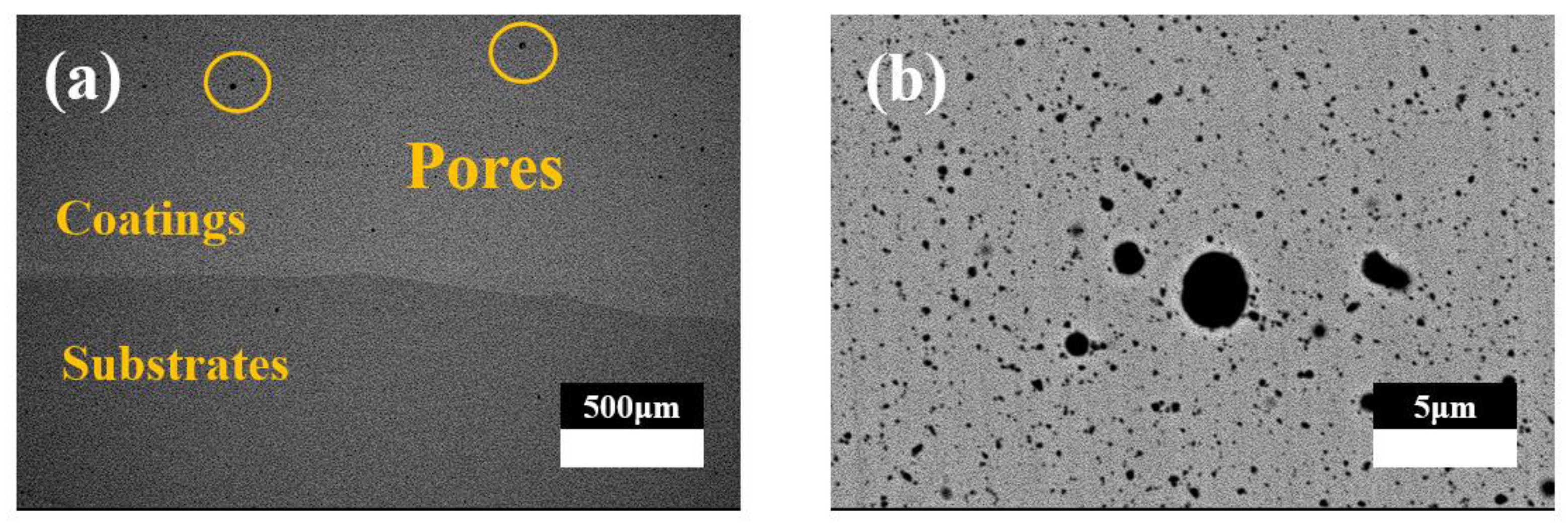
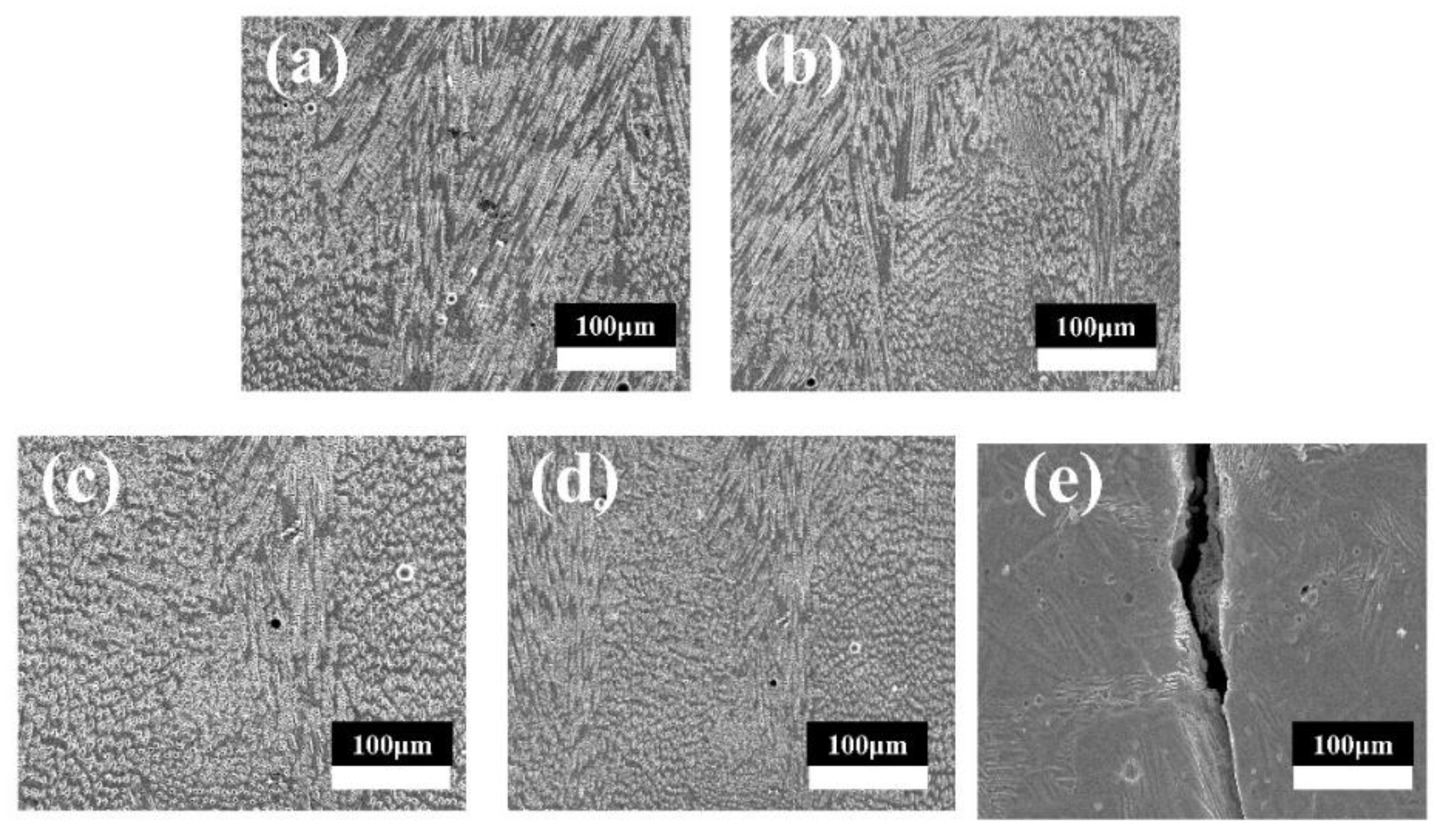
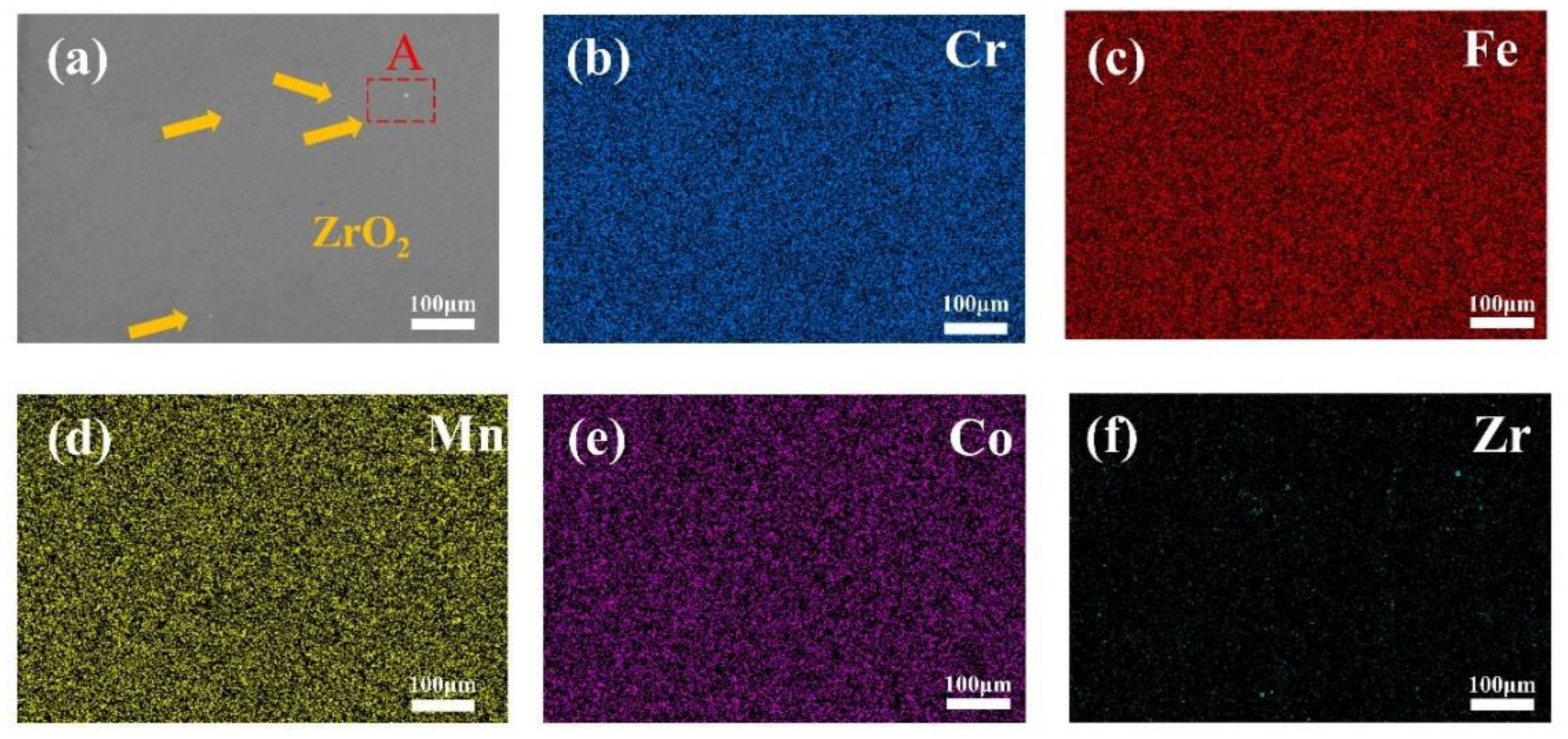
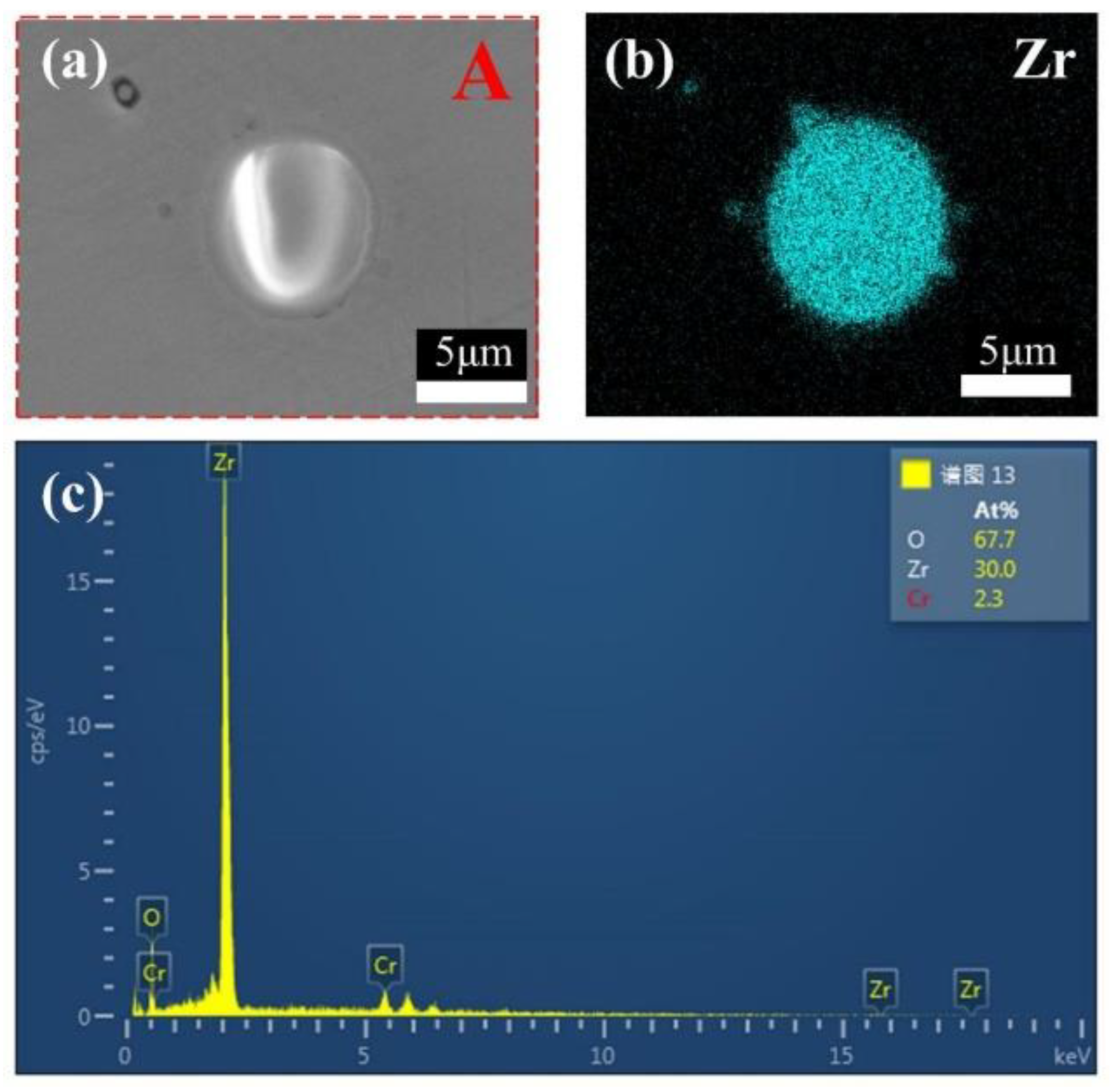
3.1. Phase Composition and Microstructure
3.2. Microhardness
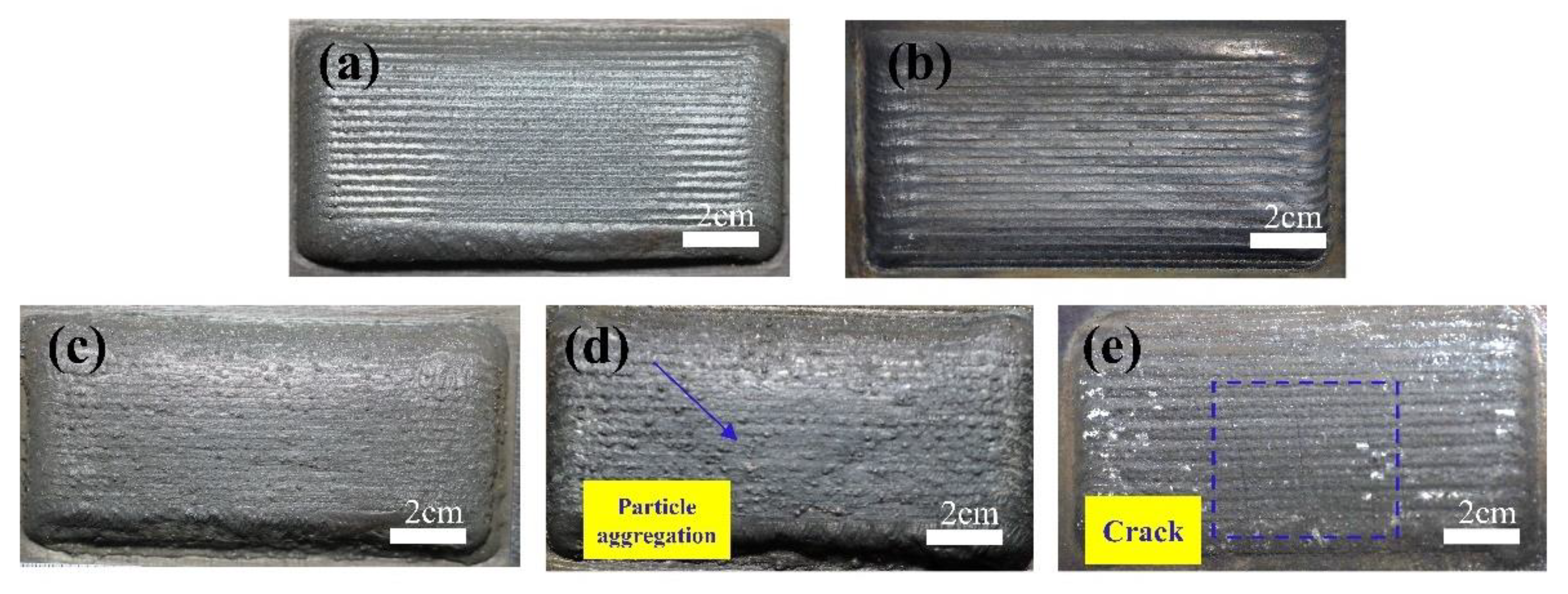
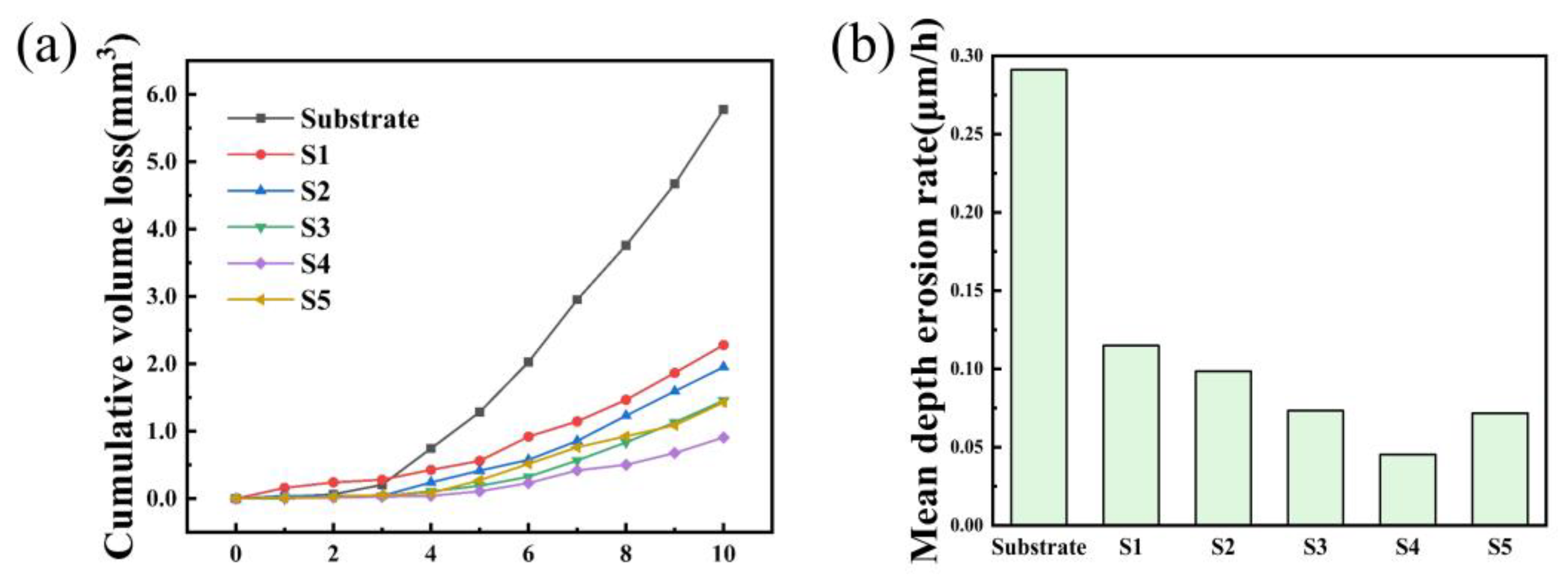
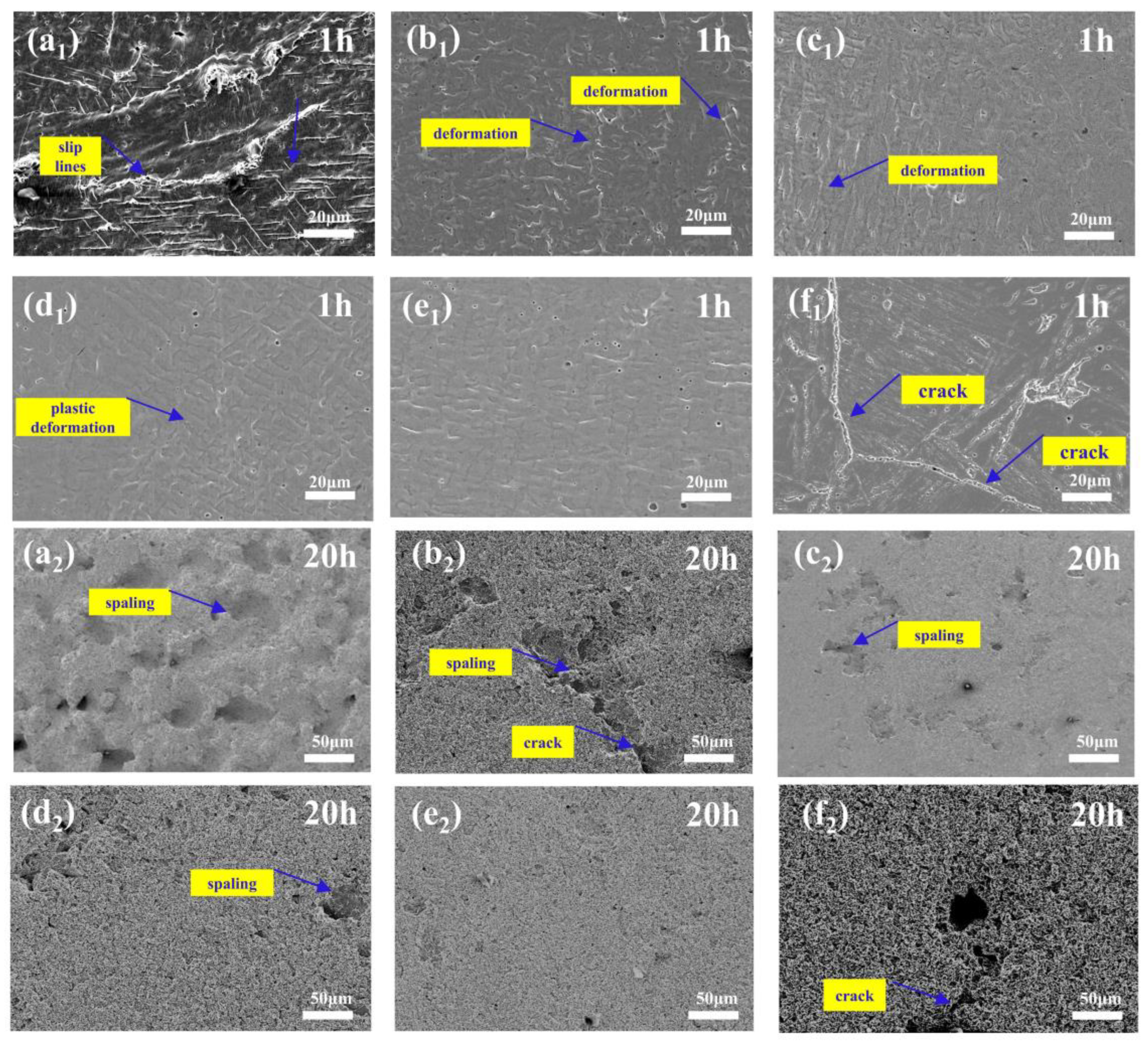
3.3. Cavitation Erosion Behavior
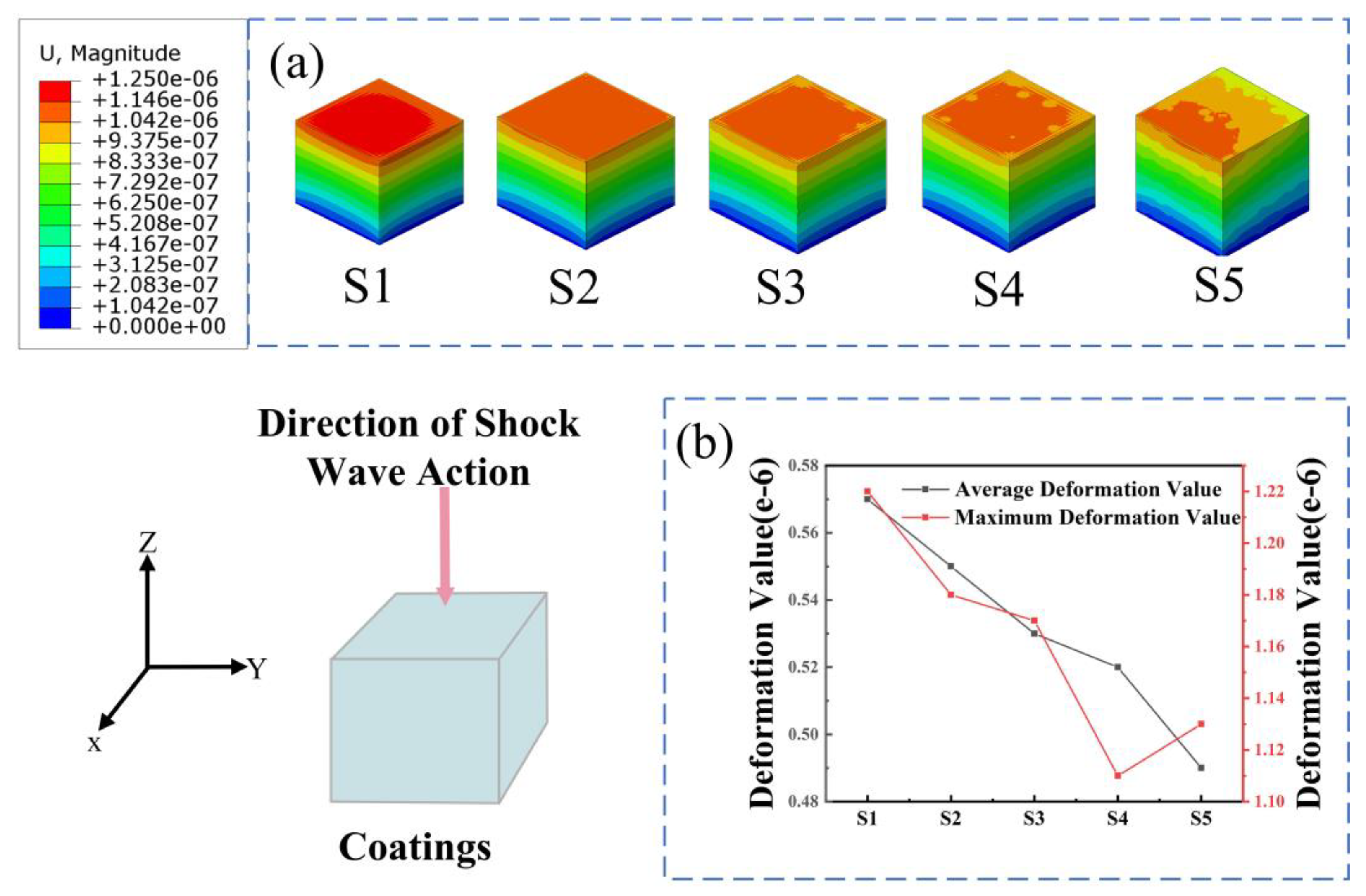
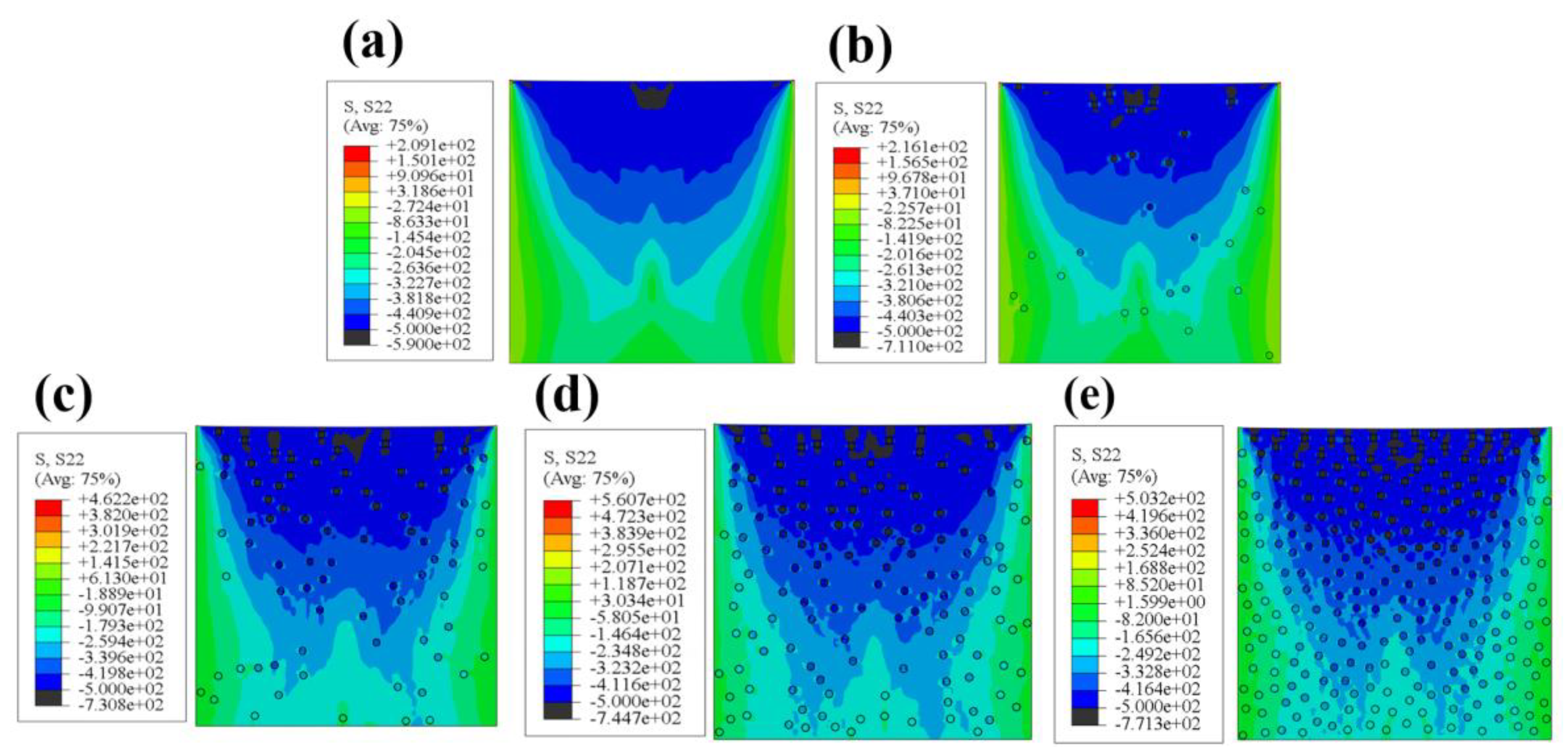
3.4. Finite Element Simulation Results
4. Conclusions
- (1)
- CoCrFeMnNi-xZrO2 high-entropy alloy composite coatings with different ZrO2 contents were prepared on the surface of 0Cr13Ni5Mo martensitic stainless steel by a laser melting method. The phase structure of the composite coating consists of a simple FCC structure and a small amount of ZrO2 phase. The introduction of ZrO2 caused lattice distortion in the coating, and the 2θ of (111)FCC shifted towards lower angles. The five high-entropy alloy composite coatings were metallurgically bonded to the substrate, the elements were uniformly distributed without segregation, and the ZrO2 particles were uniformly distributed in the substrate.
- (2)
- The microhardness of the composite coatings increased significantly with the increase in the content of added ZrO2. When X = 10%, the coating’s microhardness reached 348 HV, which was 95.53% higher than the HEA without ZrO2 added. This increase in microhardness was mainly due to fine grain strengthening and diffusion strengthening.
- (3)
- The composite coatings have better cavitation resistance than the substrate. Large areas of spalling and cracks appeared on the surface of 0Cr13Ni5Mo after 10 h cavitation. HEACs maintained a relatively flat surface. The addition of ZrO2 prolonged the incubation period of the high-entropy alloys. With the addition of 5% wt.% ZrO2, the composite coatings of high-entropy alloys showed the best cavitation resistance, with a cumulative volume loss rate of only 15.74% of that of the substrate at 10 h.
- (4)
- The finite element simulation results show that after the addition of ZrO2, the average and maximum deformations produced by the composite coatings undergoing cavitation erosion were reduced, the stresses were more concentrated on ZrO2 particles, and the stresses on the matrix underwent a decrease. The introduction of the second-phase particles subjected them to higher compressive stresses, which further improved the cavitation erosion resistance of the material. When the proportion of ZrO2 reached 10 percent, the higher concentration of stress on the particles had a deleterious effect.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.G.; Luo, S.Z.; Ke, W. Cavitation erosion–corrosion behaviour of CrMnB stainless overlay and 0Cr13Ni5Mo stainless steel in 0.5M NaCl and 0.5M HCL solutions. Tribol. Int. 2008, 41, 1181–1189. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, Y.; Hong, S.; Cheng, J.; Qiao, L.; Cheng, J.; Zhu, S.J.S.; Technology, C. Ultrasonic cavitation erosion behaviors of high-velocity oxygen-fuel (HVOF) sprayed AlCoCrFeNi high-entropy alloy coating in different solutions. Surf. Coat. Technol. 2021, 409, 126899. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Juan, Y.; Lu, Z.; Jia, W. Evolution in microstructure and corrosion behavior of AlCoCrxFeNi high-entropy alloy coatings fabricated by laser cladding. J. Alloys Compd. 2019, 775, 1–14. [Google Scholar] [CrossRef]
- Haosheng, C.; Shihan, L. Inelastic damages by stress wave on steel surface at the incubation stage of vibration cavitation erosion. Wear 2009, 266, 69–75. [Google Scholar] [CrossRef]
- Sreedhar, B.; Albert, S.; Pandit, A. Cavitation damage: Theory and measurements—A review. Wear 2017, 372, 177–196. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Mukherjee, S.; Singh, S.; Singh, H.; Grewal, H.S. Exceptionally high cavitation erosion and corrosion resistance of a high entropy alloy. Ultrason. Sonochem 2018, 41, 252–260. [Google Scholar] [CrossRef]
- Yin, D.; Liang, G.; Fan, S.; Li, S. Ultrasonic Cavitation Erosion Behavior of AlCoCr(x)CuFe High Entropy Alloy Coatings Synthesized by Laser Cladding. Materials 2020, 13, 4067. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhao, D.; Chen, Y.; Guan, S.; Liu, Y.; Liu, L.; Peng, S.; Kong, F.; Poplawsky, J.D.; et al. Strong yet ductile nanolamellar high-entropy alloys by additive manufacturing. Nature 2022, 608, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, L.; Zhang, S.; Wu, C.L.; Zhang, C.H.; Sun, X.Y. Corrosion and cavitation erosion behaviors of laser clad FeNiCoCr high-entropy alloy coatings with different types of TiC reinforcement. Surf. Coat. Technol. 2023, 471, 129910. [Google Scholar] [CrossRef]
- Ren, X.; Sun, W.; Sheng, Z.; Liu, M.; Hui, H.; Xiao, Y. Effects of Nano-CeO2 on Microstructure and Properties of WC/FeCoNiCrMo0.2 Composite High Entropy Alloy Coatings by Laser Cladding. Nanomaterials 2023, 13, 1104. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Liu, B.; Yi, D.; Yang, X.; Zhang, W.; Chen, G.; Liu, Y.; Bai, P. Influence of NbC particles on microstructure and mechanical properties of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. J. Alloys Compd. 2019, 788, 485–494. [Google Scholar] [CrossRef]
- Zong, L.; Xu, L.; Luo, C.; Li, Z.; Zhao, Y.; Xu, Z.; Zhu, C.; Wei, S. Fabrication of nano-ZrO2 strengthened WMoNbTaV refractory high-entropy alloy by spark plasma sintering. Mater. Sci. Eng. A 2022, 843, 143113. [Google Scholar] [CrossRef]
- Lepule, M.L.; Obadele, B.A.; Andrews, A.; Olubambi, P.A. Corrosion and wear behaviour of ZrO2 modified NiTi coatings on AISI 316 stainless steel. Surf. Coat. Technol. 2015, 261, 21–27. [Google Scholar] [CrossRef]
- Stambolova, I.; Dimitrov, O.; Shipochka, M.; Yordanov, S.; Blaskov, V.; Vassilev, S.; Jivov, B.; Simeonova, S.; Balashev, K.; Grozev, N.; et al. Corrosion resistance of sol-gel ZrO2 coatings deposited on stainless steel. J. Phys. Conf. Ser. 2020, 1492, 012025. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Prasad, P.R.; Aljuwayid, A.M.; Rosaiah, P.; Sudhani, H.P.; Shim, J. Enhanced solar light-induced performance of a step-scheme heterojunction nanostructure (C3N4/ZrO2) for mixed dye degradation and methanol oxidation. Mater. Sci. Semicond. Process. 2024, 177, 108342. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Guo, Z.; Yang, Z.; Qian, W.; Chen, Y.; Li, H.; Zhao, Q.; Xing, Y.; Zhao, Y. Improved corrosion resistance of ZrO2/MgO coating for magnesium alloys by manipulating the pore structure. J. Mater. Res. Technol. 2023, 24, 2403–2415. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, J.; Gong, Y.; Zhao, D.; Zhang, H.; Chen, D.; Wang, M.; Wang, H. Insight into continuous growth mechanisms of ZrO2-Al2O3 composite PEO coatings with superior cavitation erosion resistance. Surf. Coat. Technol. 2024, 477, 130355. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, L.; Zhang, S.; Zhang, C.H.; Sun, X.Y.; Chen, H.T.; Bai, X.L.; Wu, C.L. Effect of synergistic cavitation erosion-corrosion on cavitation damage of CoCrFeNiMn high entropy alloy layer by laser cladding. Surf. Coat. Technol. 2023, 472, 129940. [Google Scholar] [CrossRef]
- Park, J.M.; Moon, J.; Bae, J.W.; Jang, M.J.; Park, J.; Lee, S.; Kim, H.S. Strain rate effects of dynamic compressive deformation on mechanical properties and microstructure of CoCrFeMnNi high-entropy alloy. Mater. Sci. Eng. A 2018, 719, 155–163. [Google Scholar] [CrossRef]
- Luo, C.; Gu, J. Surface Properties and Cavitation Erosion Resistance of Cast Iron Subjected to Laser Cavitation Treatment. Metals 2023, 13, 1793. [Google Scholar] [CrossRef]
- Peshkovsky, S.L.; Peshkovsky, A.S. Shock-wave model of acoustic cavitation. Ultrason. Sonochem 2008, 15, 618–628. [Google Scholar] [CrossRef]
- Garen, W.; Hegedűs, F.; Kai, Y.; Koch, S.; Meyerer, B.; Neu, W.; Teubner, U. Shock wave emission during the collapse of cavitation bubbles. Shock. Waves 2016, 26, 385–394. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Han, B.; Li, M.; Hu, C.; Wang, J. Comparative studies on microstructure and properties of CoCrFeMnNi high entropy alloy coatings fabricated by high-speed laser cladding and normal laser cladding. J. Alloys Compd. 2023, 947, 169517. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Sun, J.; Lu, Y. The relationship between thermo-mechanical history, microstructure and mechanical properties in additively manufactured CoCrFeMnNi high entropy alloy. J. Mater. Sci. Technol. 2021, 77, 187–195. [Google Scholar] [CrossRef]
- Nguyen, T.; Huang, M.; Li, H.; Tran, V.; Yang, S. Microstructure and tensile properties of duplex phase Al0.25FeMnNiCrCu0.5 high entropy alloy fabricated by laser melting deposition. J. Alloys Compd. 2021, 871, 159521. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, W.; Liu, W.; Liu, C.; Geng, J.; Wang, H.; Bian, H. Effect of Sm2O3 particles on microstructure and properties of FeCoNiCrMn composite coating by laser cladding. Mater. Chem. Phys. 2024, 317, 129168. [Google Scholar] [CrossRef]
- Kwok, C.T.; Man, H.C.; Cheng, F.T.; Lo, K.H. Developments in laser-based surface engineering processes: With particular reference to protection against cavitation erosion. Surf. Coat. Technol. 2016, 291, 189–204. [Google Scholar] [CrossRef]
- Yu, D.T.; Zhao, T.; Wu, C.L.; Zhang, S.; Zhang, C.H.; Chen, H.T.; Wang, R. Effects of W element on the microstructure, wear and cavitation erosion behavior of CoCrFeNiMnW high entropy alloy coatings by laser cladding. Mater. Chem. Phys. 2024, 323, 129630. [Google Scholar] [CrossRef]
- Heathcock, C.J.; Ball, A.; Protheroe, B.E. Cavitation erosion of cobalt-based Stellite® alloys, cemented carbides and surface-treated low alloy steels. Wear 1981, 74, 11–26. [Google Scholar] [CrossRef]





| Parameter | Values |
|---|---|
| Sample | CoCrFeMnNi-xZrO2 |
| Laser power (W) | 4200 |
| Scanning velocity (mm/s) | 25 |
| Spot diameter (mm) | 5 |
| Power feed rate (g/min) | 36 |
| Protective gas flow rate (L/min) | 10 |
| Overlap rate (%) | 71.4% |
| Focal length (mm) | 18 |
| Coating Samples | Fe | Cr | Ni | Mn | Co | Zr | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | at.% | wt.% | at.% | wt.% | at.% | wt.% | at.% | wt.% | at.% | wt.% | at.% | |
| S1 | 23.79 | 23.88 | 18.22 | 19.61 | 19.95 | 19.05 | 18.53 | 18.88 | 19.51 | 18.55 | \ | |
| S2 | 22.65 | 22.61 | 18.19 | 19.68 | 20.06 | 19.23 | 18.78 | 19.23 | 19.92 | 19.07 | 0.40 | 0.18 |
| S3 | 22.99 | 22.37 | 17.81 | 19.62 | 18.84 | 18.39 | 18.73 | 19.53 | 19.80 | 19.24 | 1.83 | 0.85 |
| S4 | 23.47 | 22.96 | 17.47 | 19.35 | 18.50 | 18.15 | 18.53 | 19.42 | 19.27 | 18.83 | 2.76 | 1.29 |
| S5 | 24.85 | 24.85 | 17.67 | 19.89 | 17.22 | 17.18 | 16.68 | 17.77 | 17.83 | 17.71 | 5.75 | 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, D.; Chang, J.; Wang, Y.; Ma, N.; Zhao, J.; Zhao, H.; Wang, M. Effect of ZrO2 Particles on the Microstructure and Ultrasonic Cavitation Properties of CoCrFeMnNi High-Entropy Alloy Composite Coatings. Coatings 2024, 14, 1235. https://doi.org/10.3390/coatings14101235
Yin D, Chang J, Wang Y, Ma N, Zhao J, Zhao H, Wang M. Effect of ZrO2 Particles on the Microstructure and Ultrasonic Cavitation Properties of CoCrFeMnNi High-Entropy Alloy Composite Coatings. Coatings. 2024; 14(10):1235. https://doi.org/10.3390/coatings14101235
Chicago/Turabian StyleYin, Danqing, Junming Chang, Yonglei Wang, Ning Ma, Junnan Zhao, Haoqi Zhao, and Meng Wang. 2024. "Effect of ZrO2 Particles on the Microstructure and Ultrasonic Cavitation Properties of CoCrFeMnNi High-Entropy Alloy Composite Coatings" Coatings 14, no. 10: 1235. https://doi.org/10.3390/coatings14101235






