Chitosan and Its Derivatives as a Barrier Anti-Corrosive Coating of 304 Stainless Steel against Corrosion in 3.5% Sodium Chloride Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pre-Treatment of Stainless Steel Plates
2.3. Barrier Inhibitor Coating Synthesis
2.3.1. Morphological Characterization
2.3.2. FTIR Characterization
2.4. Electrochemical Analysis
2.4.1. Electrochemical Impedance Spectroscopy (EIS)
2.4.2. Potentiodynamic Polarization (PDP)
3. Results and Discussion
3.1. Chitosan Coating Characterization
3.1.1. Physicochemical Characterization
3.1.2. Morphological Characterization (SEM-EDS)
3.1.3. Metallographic Analysis of Coating Thickness
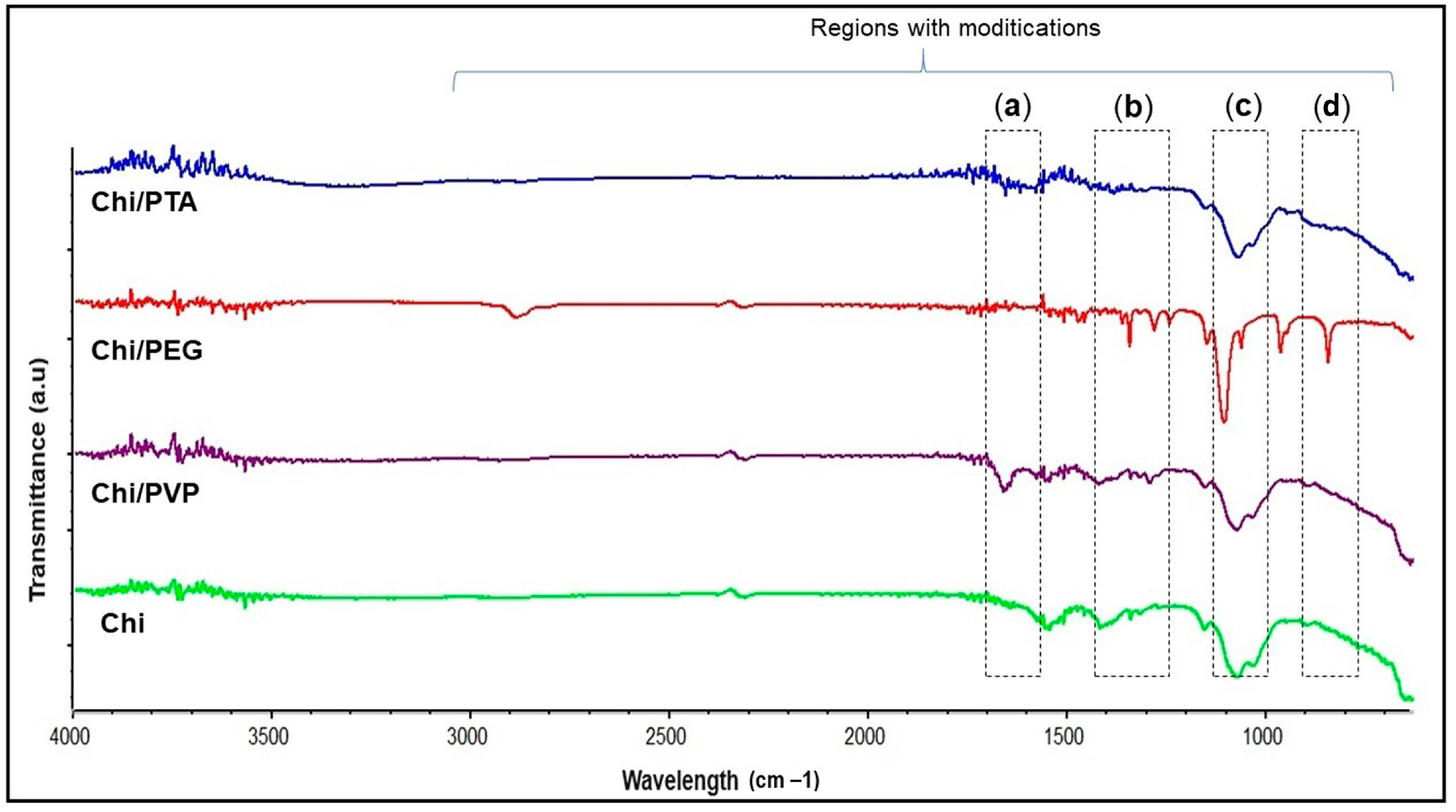
3.1.4. FTIR Structural Analysis
3.2. Coatings Electrochemical Impedance Spectroscopy (EIS)
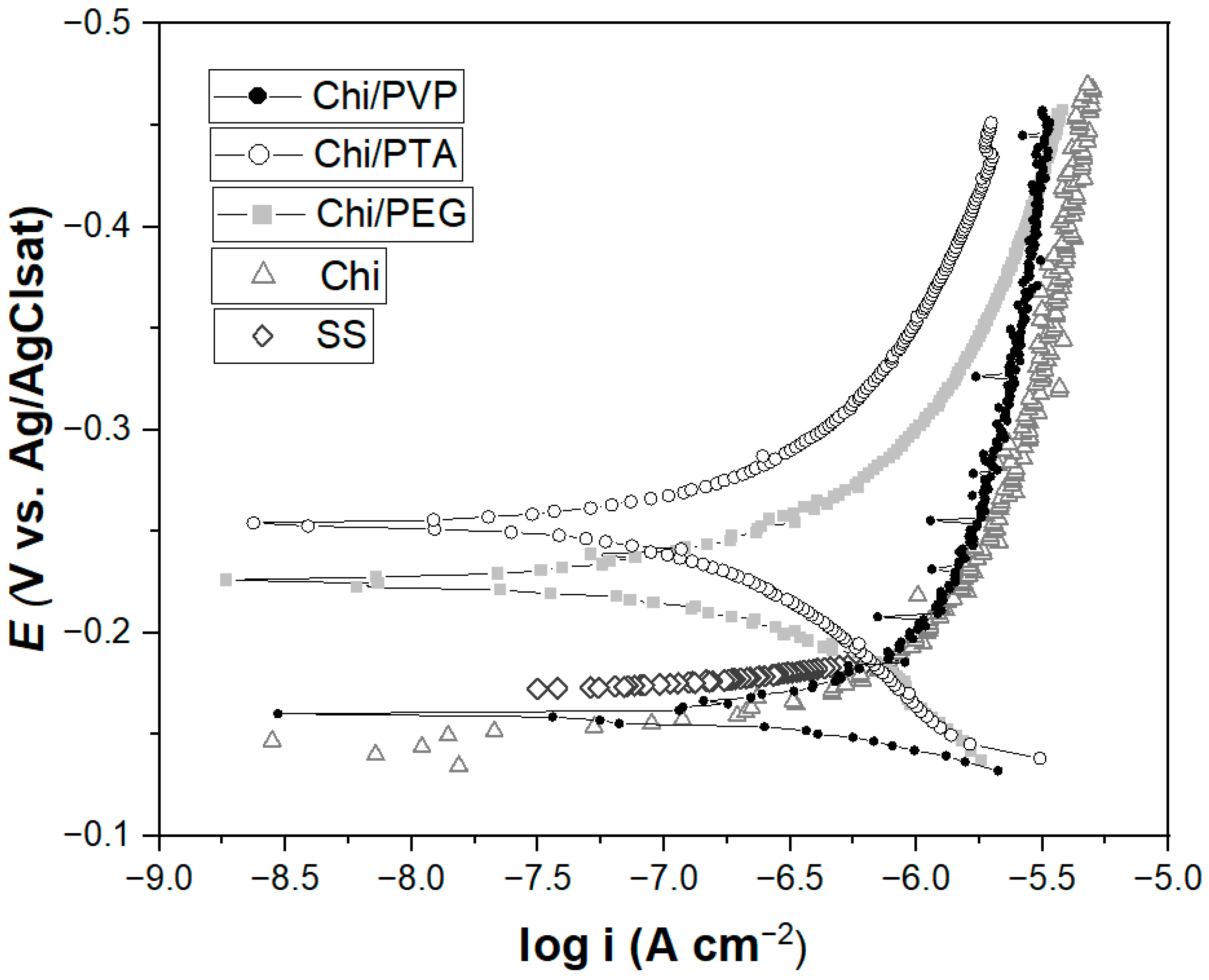
3.3. Coatings Potentiodynamic Polarization Tests (PDP)
3.4. Resistance Corrosion Efficiencies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Agouz, S.A.; Abd El-Aziz, G.B.; Awad, A.M. Solar desalination system using spray evaporation. Energy 2014, 76, 276–283. [Google Scholar] [CrossRef]
- Bogdanov, D.; Gulagi, A.; Fasihi, M.; Breyer, C. Full energy sector transition towards 100% renewable energy supply: Integrating power, heat, transport and industry sectors including desalination. Appl. Energy 2021, 283, 116273. [Google Scholar] [CrossRef]
- Moreno, S.; Hinojosa, J.F.; Dévora-Isiordia, G.E. Exploring water desalination in an arid climate: An experimental and numerical analysis of a compact solar chimney. Desalination 2024, 583, 117671. [Google Scholar] [CrossRef]
- de Castro-Pardo, M.; Martínez, P.F.; Zabaleta, A.P.; Azevedo, J.C. Dealing with water conflicts: A comprehensive review of mcdm approaches to manage freshwater ecosystem services. Land 2021, 10, 469. [Google Scholar] [CrossRef]
- Ghazouani, N.; El-Bary, A.A.; Hassan, G.E.; Becheikh, N.; Bawadekji, A.; Elewa, M.M. Solar Desalination by Humidification–Dehumidification: A Review. Water 2022, 14, 3424. [Google Scholar] [CrossRef]
- Gartner, N.; Kosec, T.; Legat, A. Monitoring the corrosion of steel in concrete exposed to a marine environment. Materials 2020, 13, 407. [Google Scholar] [CrossRef]
- Esmaeilion, F.; Ahmadi, A.; Hoseinzadeh, S.; Aliehyaei, M.; Makkeh, S.A.; Astiaso Garcia, D. Renewable energy desalination; a sustainable approach for water scarcity in arid lands. Int. J. Sustain. Eng. 2021, 14, 1916–1942. [Google Scholar] [CrossRef]
- Kummu, M.; Guillaume, J.H.A.; Moel HDe Eisner, S.; Flörke, M.; Porkka, M. The world ’ s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef]
- Dhaiveegan, P.; Elangovan, N.; Nishimura, T.; Rajendran, N. Corrosion behavior of 316L and 304 stainless steels exposed to industrial-marine-urban environment: Field study. RSC Adv. 2016, 6, 47314–47324. [Google Scholar] [CrossRef]
- Lin, Y.; Cai, S.; Jiang, S.; Xie, D.; Ling, R.; Sun, J.; Wei, J.; Shen, K.; Xu, G. Enhanced corrosion resistance and bonding strength of Mg substituted β-tricalcium phosphate/Mg(OH)2 composite coating on magnesium alloys via one-step hydrothermal method. J. Mech. Behav. Biomed. Mater. 2019, 90, 547–555. [Google Scholar] [CrossRef]
- Hernández, H.H.; Reynoso, A.R.; González, J.T.; Morán, C.G.; Hernández, J.M.; Ruiz, A.M.; Hernández, J.M.; Cruz, R.O. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels. In Electrochemical Impedance Spectroscopy; IntechOpen: London, UK, 2020. [Google Scholar]
- Aljeaban, N.A.; Goni, L.K.M.O.; Alharbi, B.G.; Mazumder, M.A.J.; Ali, S.A.; Chen, T.; Quraishi, M.A.; Al-Muallem, H.A. Polymers Decorated with Functional Motifs for Mitigation of Steel Corrosion: An Overview. Int. J. Polym. Sci. 2020, 2020, 9512680. [Google Scholar] [CrossRef]
- Kadhim, A.; Betti, N.; Al-Bahrani, H.A.; Al-Ghezi, M.K.S.; Gaaz, T.; Kadhum, A.H.; Alamiery, A. A mini review on corrosion, inhibitors and mechanism types of mild steel inhibition in an acidic environment. Int. J. Corros. Scale Inhib. 2021, 10, 861–884. [Google Scholar]
- Aaziz, J.; Abdallah, E.A. Corrosion processes and strategies for protection. In Anti-Corrosive Nanomaterials: Design, Characterization, Mechanisms and Applications; CRC Press: Boca Raton, FL, USA, 2023; pp. 25–56. [Google Scholar]
- Sanni, O.; Sunday Isaac Fayomi, O.; Patricia Idowu Popoola, A. Eco-friendly Inhibitors for Corrosion Protection of Stainless steel: An Overview. In Journal of Physics: Conference Series; Institute of Physics Publishing: Bristol, UK, 2019. [Google Scholar]
- Verma, C.; Quraishi, M.A. Chelation capability of chitosan and chitosan derivatives: Recent developments in sustainable corrosion inhibition and metal decontamination applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100184. [Google Scholar] [CrossRef]
- Singh, P.; Rana, A.; Karak, N.; Kumar, I.; Rana, S.; Kumar, P. Sustainable smart anti-corrosion coating materials derived from vegetable oil derivatives: A review. RSC Adv. 2023, 13, 3910–3941. [Google Scholar] [CrossRef]
- Yang, X.L.; Gao, R.; Dai, W.L.; Fan, K. Influence of tungsten precursors on the structure and catalytic properties of WO3/SBA-I5 in the selective oxidation of cyclopentene to glutaraldehyde. J. Phys. Chem. C 2008, 112, 3819–3826. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, J.; Yu, K.; Chen, C.; Dai, Y.; Qiao, X.; Yan, Y. Improving of in vitro biodegradation resistance in a chitosan coated magnesium bio-composite. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2015, 44, 1862–1865. [Google Scholar]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Versteg, A.; Beraldo, C.H.M.; Spinelli, A.; da Conceição, T.F. Improving the barrier properties of chitosan coatings through Schiff base formation and halloysite incorporation for corrosion protection of commercially pure aluminum (cp-Al). Mater. Today Commun. 2024, 38, 108046. [Google Scholar] [CrossRef]
- Loperena, A.P.; Saidman, S.B.; Forero López, A.D.; Brugnoni, L.I.; Lehr, I.L. Corrosion protection and antibacterial performance of a chitosan/salicylate coating electrogenerated on a magnesium alloy. Results Surf. Interfaces 2024, 16, 100244. [Google Scholar] [CrossRef]
- Ding, F.; Li, H.; Du, Y.; Shi, X. Recent advances in chitosan-based self-healing materials. Res. Chem. Intermed. 2018, 44, 4827–4840. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, M.; Ren, Y.; Lou, R.; Xie, H.; Yu, W.; Liu, X.; Ma, X. Controlling Gel Structure to Modulate Cell Adhesion and Spreading on the Surface of Microcapsules. ACS Appl. Mater. Interfaces 2016, 8, 19333–19342. [Google Scholar] [CrossRef]
- Ramos Avilez, H.V.; Castilla Casadiego, D.A.; Vega Avila, A.L.; Perales Perez, O.J.; Almodovar, J. Production of chitosan coatings on metal and ceramic biomaterials. In Chitosan Based Biomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 255–293. [Google Scholar]
- Galvão, T.L.P.; CBouali, A.; Serdechnova, M.; Yasakau, K.A.; Zheludkevich, M.L.; Tedim, J. Anticorrosion thin film smart coatings for aluminum alloys. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–454. [Google Scholar]
- Cao, Y.; Wu, H.; Wang, X.; Wang, G.; Yang, H. Novel long-acting smart anticorrosion coating based on pH-controlled release polyaniline hollow microspheres encapsulating inhibitor. J. Mol. Liq. 2022, 359, 119341. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Yu, J.; Zhang, Y.; Zhao, Y.; Yuan, Q. Microstructure evolution and corrosion behavior of dissimilar 304/430 stainless steel welded joints. J. Manuf. Process. 2020, 50, 183–191. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Mittal, V. Anti-corrosion behavior of layer by layer coatings of cross-linked chitosan and poly(vinyl butyral) on carbon steel. Cellulose 2015, 22, 3275–3290. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, M.; Li, A.; Zhu, G.; Zhang, Y. Anticorrosion behavior of polyvinyl butyral (PVB)/polymethylhydrosiloxane (PMHS)/chitosan (Ch) environment-friendly assembled coatings. Prog. Org. Coat. 2020, 144, 105662. [Google Scholar] [CrossRef]
- Mina, A.; Caicedo, H.H.; Uquillas, J.A.; Aperador, W.; Gutiérrez, O.; Caicedo, J.C. Biocompatibility behavior of β-tricalcium phosphate-chitosan coatings obtained on 316L stainless steel. Mater. Chem. Phys. 2016, 175, 68–80. [Google Scholar] [CrossRef]
- Umoren, S.A.; Banera, M.J.; Alonso-Garcia, T.; Gervasi, C.A.; Mirífico, M.V. Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 2013, 20, 2529–2545. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Sánchez-Duarte, R.G.; Sánchez-Machado, D.I.; López-Cervantes, J.; Correa-Murrieta, M.A. Adsorption of allura red dye by cross-linked chitosan from shrimp waste. Water Sci. Technol. 2012, 65, 618–623. [Google Scholar] [CrossRef]
- Bahari, H.S.; Ye, F.; Carrillo, E.A.T.; Leliopoulos, C.; Savaloni, H.; Dutta, J. Chitosan nanocomposite coatings with enhanced corrosion inhibition effects for copper. Int. J. Biol. Macromol. 2020, 162, 1566–1577. [Google Scholar] [CrossRef]
- Aguilar-Ruiz, A.A.; Dévora-Isiordia, G.E.; Sánchez-Duarte, R.G.; Villegas-Peralta, Y.; Orozco-Carmona, V.M.; Álvarez-Sánchez, J. Chitosan-Based Sustainable Coatings for Corrosion Inhibition of Aluminum in Seawater. Coatings 2023, 13, 1615. [Google Scholar] [CrossRef]
- ASTM Norma G 106; Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. ASTM: West Conshohocken, PA, USA, 1999.
- ASTM G61-86; Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM: West Conshohocken, PA, USA, 2018.
- Chávez Huerta, A.; Rincón, M.C.; Valbuena, A.C.; López, A. Obtenciòn y caracterización de papel de quitosano. Rev. Iberoam. Polim. 2012, 13, 41–51. Available online: https://www.researchgate.net/publication/318351737_OBTENCION_Y_CARACTERIZACION_DE_PAPEL_DE_QUITOSANO (accessed on 4 October 2023).
- Solano Romero, J.F. Obtención de Quitosano a Partir del Exoesqueleto del Camarón (Infraorden Caridea). Bachelor’s Thesis, Universidad Técnica de Machala, Machala, Ecuador, 2017; pp. 1–54. Available online: https://repositorio.utmachala.edu.ec/handle/48000/11382 (accessed on 3 November 2023).
- Mouaden, K.E.L.; Chauhan, D.S.; Quraishi, M.A.; Bazzi, L. Thiocarbohydrazide-crosslinked chitosan as a bioinspired corrosion inhibitor for protection of stainless steel in 3.5% NaCl. Sustain. Chem. Pharm. 2020, 15, 100213. [Google Scholar] [CrossRef]
- Xhanari, K.; Finšgar, M. Organic corrosion inhibitors for aluminium and its alloys in acid solutions: A review. RSC Adv. 2016, 6, 62833–62857. [Google Scholar] [CrossRef]
- Fekry, A.M.; Ghoneim, A.A.; Ameer, M.A. Electrochemical impedance spectroscopy of chitosan coated magnesium alloys in a synthetic sweat medium. Surf. Coat. Technol. 2014, 238, 126–132. [Google Scholar] [CrossRef]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid sol-gel coatings: Smart and green materials for corrosion mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef]
- Hua, Q.; Zeng, Y.; He, Z.; Xu, Q.; Min, Y. Microstructure, synergistic mechanism and corrosion behavior of tin oxide conversion film modified by chitosan on aluminum alloy surface. Colloids Interface Sci. Commun. 2020, 36, 100262. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally friendly anticorrosive polymeric coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Wade, L.G., Jr. Organic Chemistry. 656 p. Pearson. 2011. Available online: https://www.academia.edu/35083932/Qu%C3%ADmica_organica_Vol_1_Wade_7ma (accessed on 19 March 2023).
- Silva, S.S.; Goodfellow, B.J.; Benesch, J.; Rocha, J.; Mano, J.F.; Reis, R.L. Morphology and miscibility of chitosan/soy protein blended membranes. Carbohydr. Polym. 2007, 70, 25–31. [Google Scholar] [CrossRef]
- Giteru, S.G.; Azam Ali, M.; Oey, I. Elucidating the pH influence on pulsed electric fields-induced self-assembly of chitosan-zein-poly(vinyl alcohol)-polyethylene glycol nanostructured composites. J. Colloid Interface Sci. 2021, 588, 531–546. [Google Scholar] [CrossRef]
- Szőke, F.; Szabó, G.; Simó, Z.; Hórvölgyi, Z.; Albert, E.; Végh, A.G.; Zimányi, L.; Muresan, L.M. Chitosan coatings ionically cross-linked with ammonium paratungstate as anticorrosive coatings for zinc. Eur. Polym. J. 2019, 118, 205–212. [Google Scholar] [CrossRef]
- Baker, A.P.; Hodgson, S.N.B.; Edirisinghe, M.J. Production of tungsten oxide coatings, via sol-gel processing of tungsten anion solutions. Surf. Coat. Technol. 2002, 153, 184–193. [Google Scholar] [CrossRef]
- Pecoraro, C.M.; Santamaria, M.; Bocchetta, P.; Di Quarto, F. Influence of synthesis conditions on the performance of chitosaneHeteropolyacid complexes as membranes for low temperature H2eO2fuel cell. Int. J. Hydrogen Energy 2015, 40, 14616–14626. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.; Freire, C.; Gandini, A.; Ferreira, M.; Zheludkevich, M. Functionalized chitosan-based coatings for active corrosion protection. Surf. Coat. Technol. 2013, 226, 51–59. [Google Scholar] [CrossRef]
- Arwati, I.G.A.; Majlan, E.H.; Alva, S.; Muhammad, W. Effect of Chitosan on the Corrosion Inhibition for Aluminium Alloy in H2SO4 Medium. Energies 2022, 15, 8511. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Li, Y.Y.; Zhu, G.Y.; Liu, H.F.; Zhang, G.A. Two novel chitosan derivatives as high efficient eco-friendly inhibitors for the corrosion of mild steel in acidic solution. Corros. Sci. 2020, 164, 108346. [Google Scholar] [CrossRef]
- Esmailzadeh, M.; Tammari, E.; Safarpour, T.; Razavian, S.M.; Pezzato, L. Anti-corrosion effect of chitin and chitosan nanoparticles in epoxy coatings. Mater. Chem. Phys. 2024, 317, 129097. [Google Scholar] [CrossRef]
- Kirk-Othmer (Ed.) Kirk-Othmer Concise Encyclopedia of Chemical Technology, 2 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2007; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/0471238961 (accessed on 5 November 2022)ISBN 9780471238966. [CrossRef]
- Chandra, S.; Tolpadi, S.K.; Hashmi, S.A. Experimental studies on the ionic (protonic) transport in ammonium para-tungstate pentahydrate. J. Phys. Condens. Matter 1989, 1, 9101–9109. [Google Scholar] [CrossRef]
- Umoren, S.A.; AlAhmary, A.A.; Gasem, Z.M.; Solomon, M.M. Evaluation of chitosan and carboxymethyl cellulose as ecofriendly corrosion inhibitors for steel. Int. J. Biol. Macromol. 2018, 117, 1017–1028. [Google Scholar] [CrossRef]
- Shamsheera, K.O.; Prasad, A.R.; Garvasis, J.; Basheer, S.M.; Joseph, A. Stearic acid grafted chitosan/epoxy blend surface coating for prolonged protection of mild steel in saline environment. J. Adhes. Sci. Technol. 2019, 33, 2250–2264. [Google Scholar]
- Yee, Y.P.; Saud, S.N.; Hamzah, E. Pomelo Peel Extract as Corrosion Inhibitor for Steel in Simulated Seawater and Acidic Mediums. J. Mater. Eng. Perform. 2020, 29, 2202–2215. [Google Scholar] [CrossRef]
- Jena, G.; Anandkumar, B.; Vanithakumari, S.C.; George, R.P.; Philip, J.; Amarendra, G. Graphene oxide-chitosan-silver composite coating on Cu-Ni alloy with enhanced anticorrosive and antibacterial properties suitable for marine applications. Prog. Org. Coat. 2020, 139, 105444. [Google Scholar] [CrossRef]
- Oukhrib, R. Quantum chemical calculations and corrosion inhibition efficiency of biopolymer “chitosan” on copper surface in 3% NaCl. J. Mater. Environ. Sci. 2017, 8, 195–208. Available online: http://www.jmaterenvironsci.com/ (accessed on 3 November 2023).

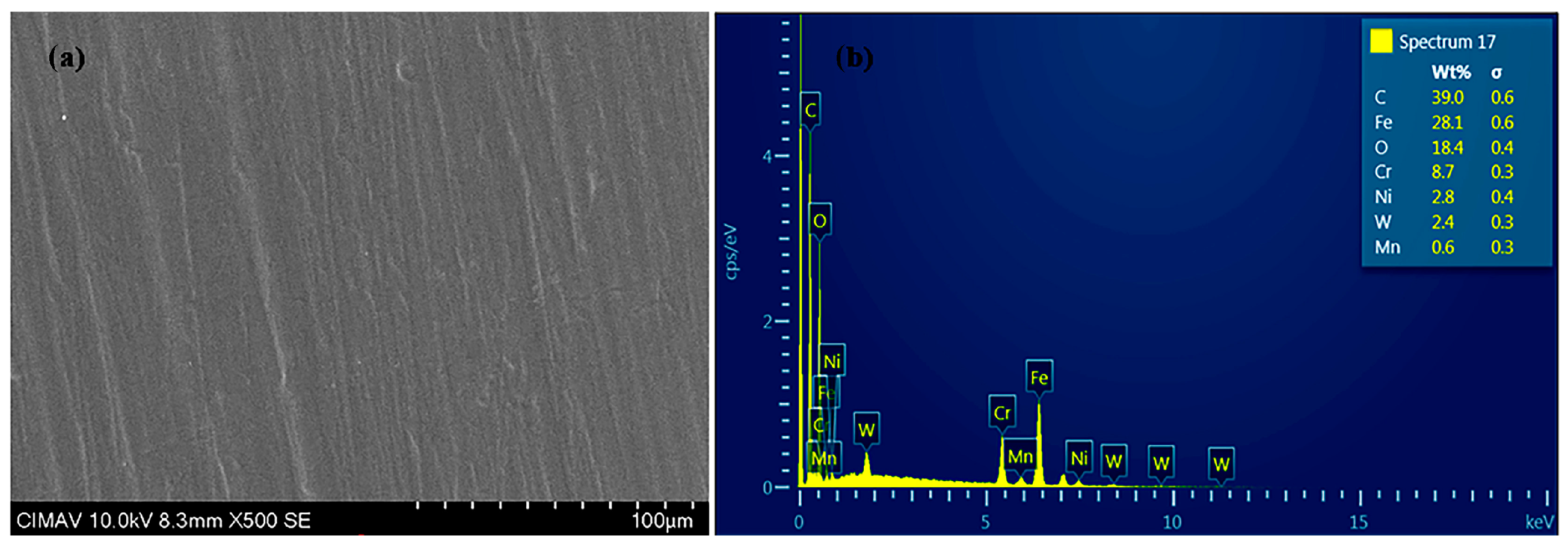

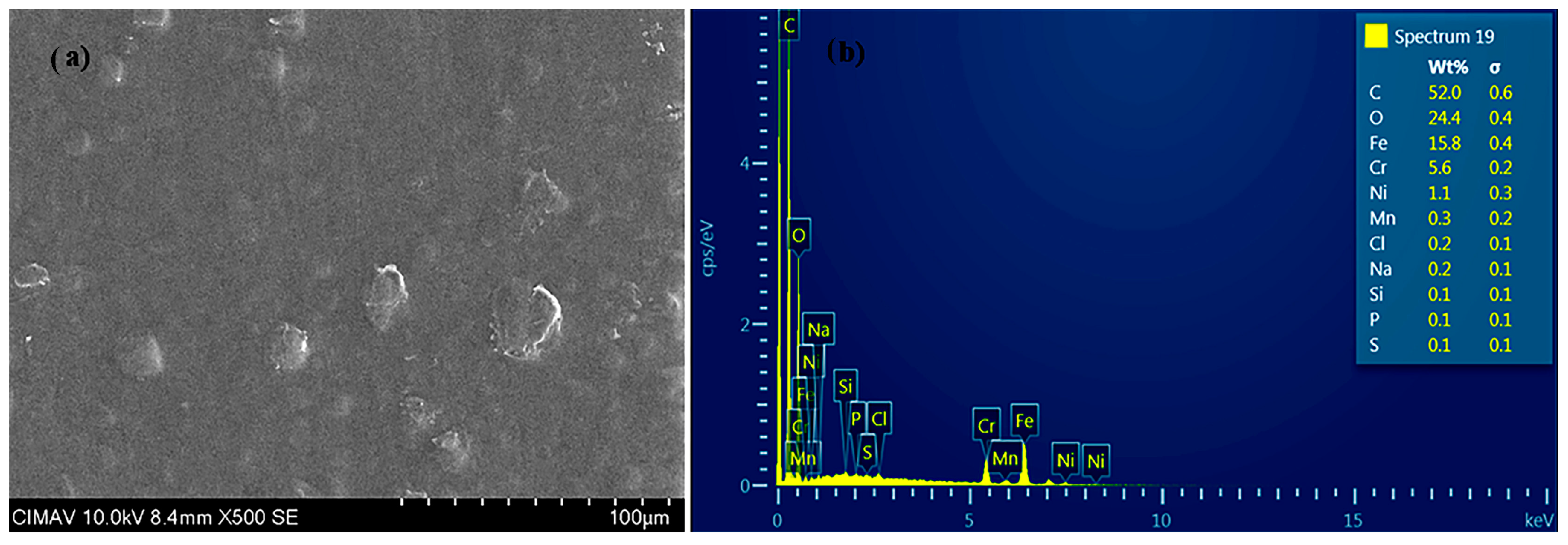
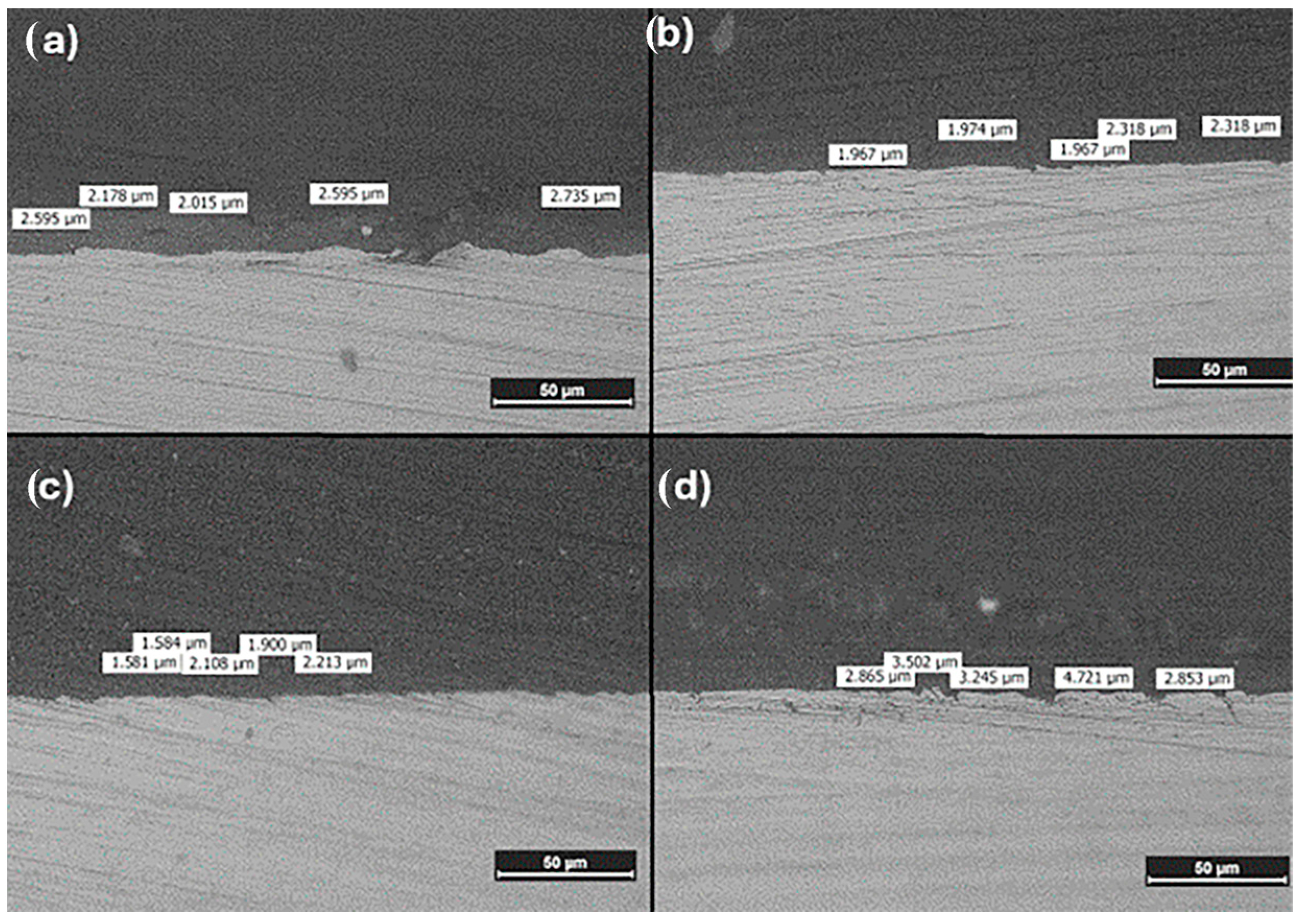


| Coating | Thickness (µm) |
|---|---|
| Chi/PEG | 2.424 ± 0.309 |
| Chi/PTA | 2.109 ± 0.191 |
| Chi/PVP | 1.877 ± 0.292 |
| Chi | 3.437 ± 0.768 |
| Sample | Rs (Ω cm2) | CPE-T (µF/cm2) | CPE-P (µF/cm2) | Rp (Ω cm2) |
|---|---|---|---|---|
| Naked SS | 165.76 | 4.2422 × 10−5 | 0.8948 | 15,960 |
| Chi | 222.1 | 6.15 × 10−5 | 0.8083 | 219,560 |
| Chi/PTA | 225.6 | 7.21 × 10−5 | 0.8465 | 247,840 |
| Chi/PEG | 213.8 | 0.00010655 | 0.8061 | 35,560 |
| Chi/PVP | 227.6 | 9.07 × 10−5 | 0.8264 | 13,520 |
| Samples | Icorr (A/cm2) | −Ecorr (V) | βa (mV/D) | −βc (mV/D) | Corrosion Rate (mmPY) |
|---|---|---|---|---|---|
| SS | 4.14 × 10−6 | −0.323 | 1.33 × 107 | 308.52 | 0.042 |
| Chi | 2.16 × 10−6 | −0.237 | 536.14 | 342.75 | 0.022 |
| Chi/PTA | 1.22 × 10−6 | −0.265 | 1.77 × 107 | 210.34 | 0.014 |
| Chi/PEG | 2.52 × 10−6 | −0.430 | 3.26 × 107 | 118.72 | 0.025 |
| Chi/PVP | 2.90 × 10−6 | −0.205 | 146.1 | 159.75 | 0.023 |
| Coating Type | Efficiency (%) | Reference |
|---|---|---|
| Chitosan/PTA on Zinc | 95% | [50] |
| Chitosan (CS), Carboxymethylcellulose (CMC) on Steel | 45% | [59] |
| Graphene Oxide–Chitosan–Silver on copper nickel alloy | 98% | [62] |
| Chitosan–TiO2 on cupper | 92% | [63] |
| Chitosan–ZnO Steel | 75% | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Ruiz, A.A.; Sánchez-Duarte, R.G.; Orozco-Carmona, V.M.; Devora-Isiordia, G.E.; Villegas-Peralta, Y.; Álvarez-Sánchez, J. Chitosan and Its Derivatives as a Barrier Anti-Corrosive Coating of 304 Stainless Steel against Corrosion in 3.5% Sodium Chloride Solution. Coatings 2024, 14, 1244. https://doi.org/10.3390/coatings14101244
Aguilar-Ruiz AA, Sánchez-Duarte RG, Orozco-Carmona VM, Devora-Isiordia GE, Villegas-Peralta Y, Álvarez-Sánchez J. Chitosan and Its Derivatives as a Barrier Anti-Corrosive Coating of 304 Stainless Steel against Corrosion in 3.5% Sodium Chloride Solution. Coatings. 2024; 14(10):1244. https://doi.org/10.3390/coatings14101244
Chicago/Turabian StyleAguilar-Ruiz, Ana Alejandra, Reyna Guadalupe Sánchez-Duarte, Víctor Manuel Orozco-Carmona, Germán Eduardo Devora-Isiordia, Yedidia Villegas-Peralta, and Jesús Álvarez-Sánchez. 2024. "Chitosan and Its Derivatives as a Barrier Anti-Corrosive Coating of 304 Stainless Steel against Corrosion in 3.5% Sodium Chloride Solution" Coatings 14, no. 10: 1244. https://doi.org/10.3390/coatings14101244










