Investigation of the Corrosion–Wear Interaction Behavior of 8Cr4Mo4V Bearing Steel at Various Corrosion Intervals
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Characterization
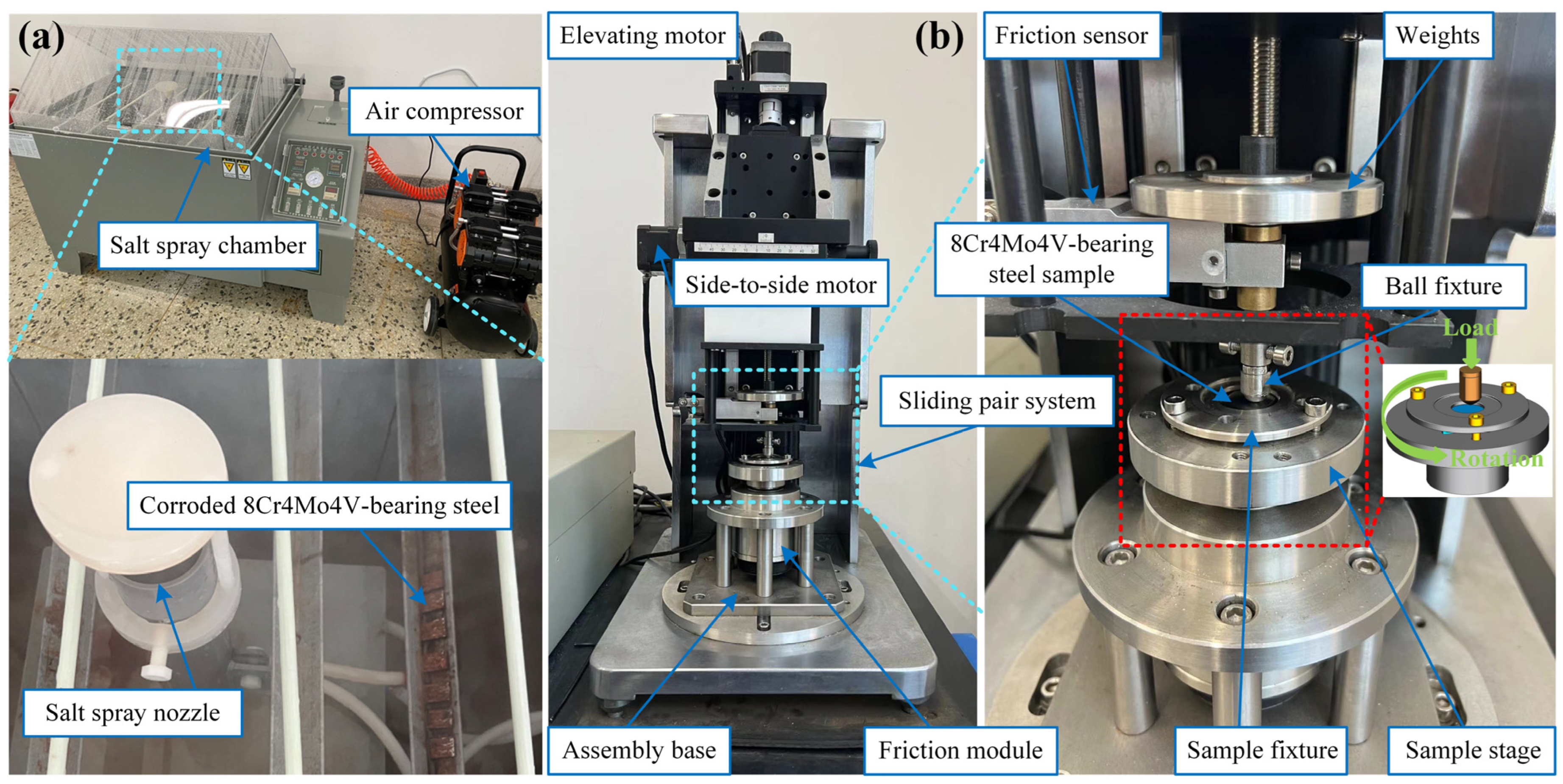
2.2. Neutral Salt Spray Test
2.3. Tribological Experiment
3. Results and Discussion
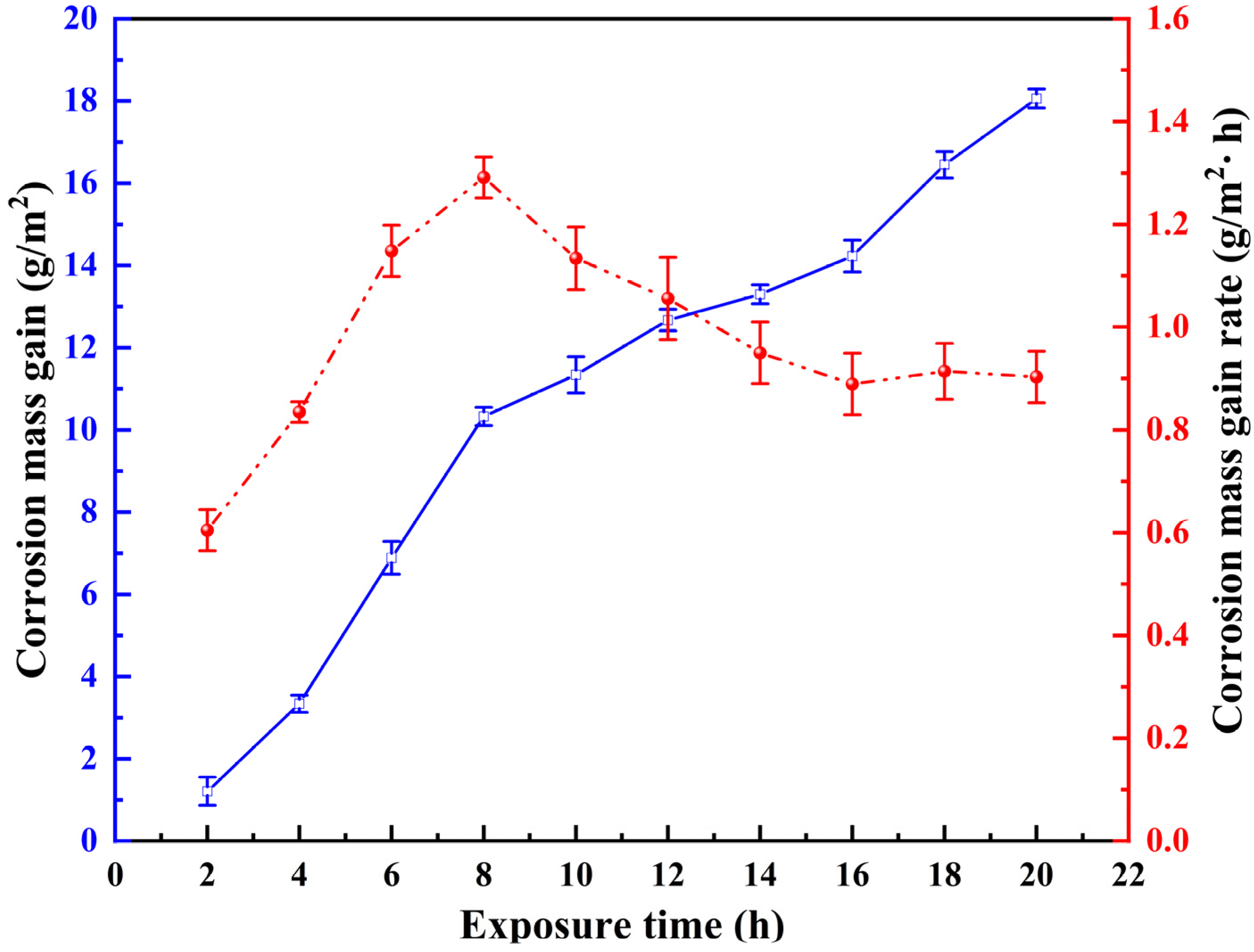
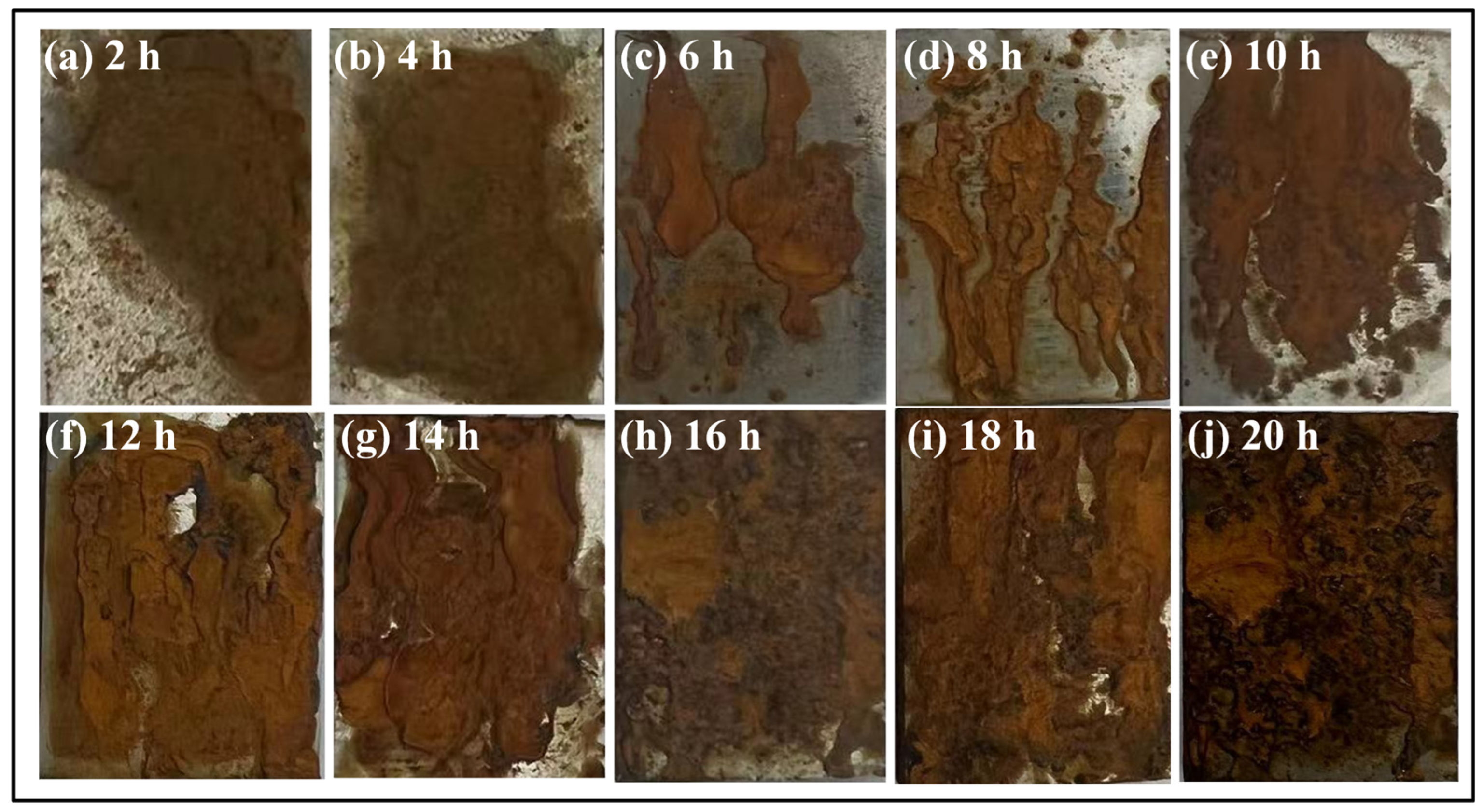
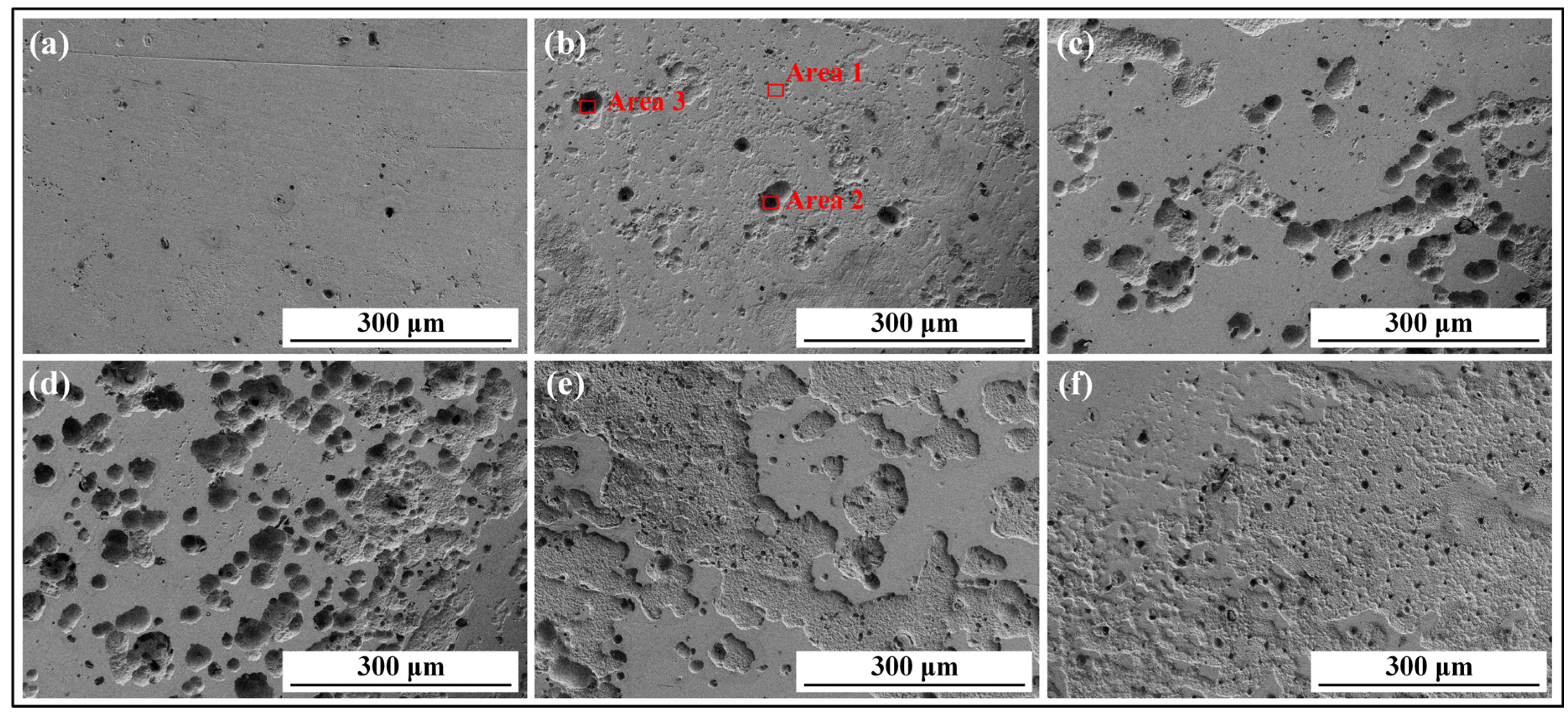
3.1. Influence of Corrosion Interval on 8Cr4Mo4V Bearing Steel Surface Morphologies
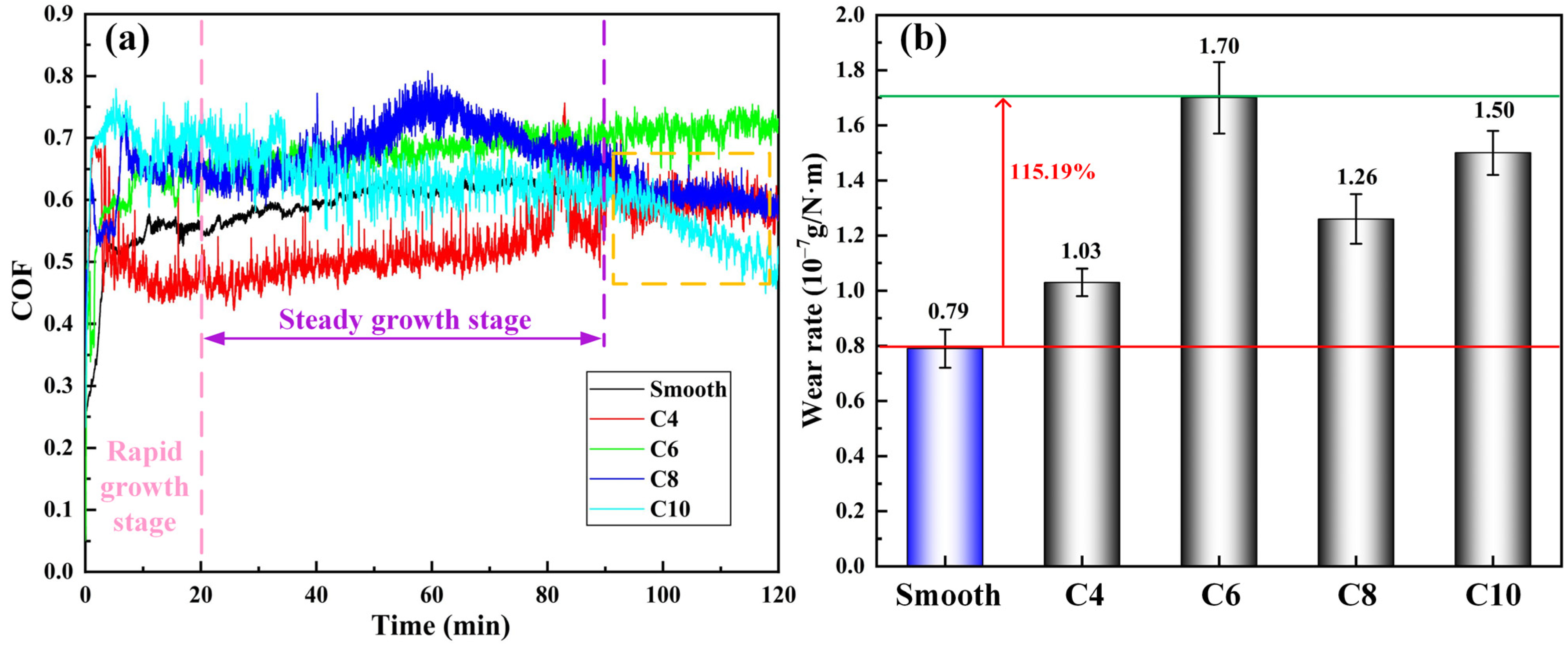
3.2. Influence of Corrosion on 8Cr4Mo4V Bearing Steel Tribological Performance
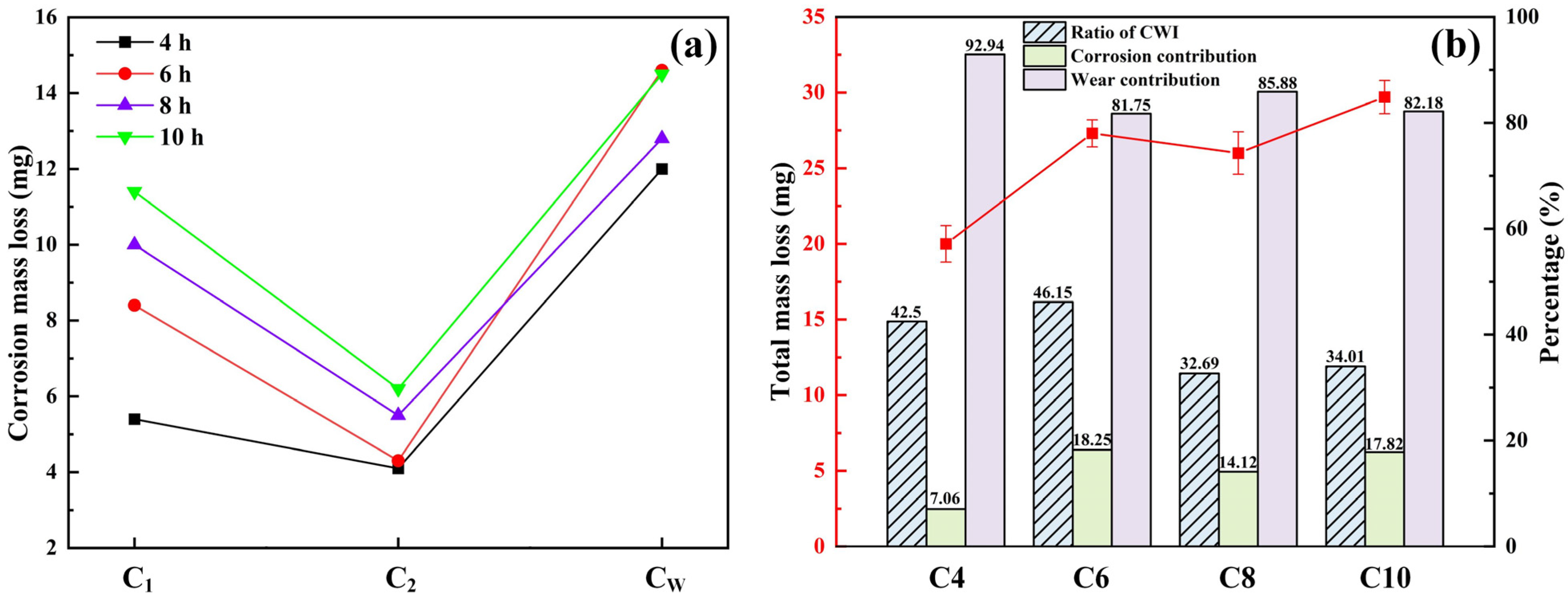
3.3. Quantitative Characterization of 8Cr4Mo4V Bearing Steel Corrosion–Wear Interaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Items |
| CWI | Corrosion–wear interaction |
| RCF | Rolling contact fatigue resistance |
| HCPEB | High energy current pulsed electron beam |
| NAB | Nickel aluminum bronze |
| NSS | Neutral salt spray |
| SEM | Scanning electron microscope |
| EDS | Energy-dispersive spectroscopy |
| rpm | Revolutions per minute |
| COF | Coefficient of friction |
| T2 | Total material losses in corrosion–wear–corrosion process |
| T1 | Material losses in corrosion–wear process |
| W1 | Material losses of pure wear |
| C1 | Material losses of pure corrosion for the first phase |
| C2 | Material losses of pure corrosion for the second phase |
| WC | Wear component |
| ∆WC | Wear increment |
| CW | Corrosion component |
| ∆CW | Corrosion increment |
| Y | Material loss caused by the interaction of corrosion and wear |
| N | Ratio of CWI |
| Q | Corrosion contribution |
| P | Wear contribution |
References
- Jiang, H.W.; Song, Y.R.; Wu, Y.C.; Shan, D.B.; Zong, Y.Y. Macrostructure, microstructure and mechanical properties evolution during 8Cr4Mo4V steel roller bearing inner ring forging process. Mat. Sci. Eng. A 2020, 798, 140196. [Google Scholar] [CrossRef]
- Elsheikh, A.H.; Yu, J.G.; Sathyamurthy, R.; Tawfik, M.M.; Shanmugan, S.; Essa, F.A. Improving the tribological properties of AISI M50 steel using Sns/Zno solid lubricants. J. Alloys Compd. 2020, 821, 153494. [Google Scholar] [CrossRef]
- Zhang, B.H.; Liu, H.T.; Zhang, M.L.; Dai, C.Y.; Xie, Z.W.; Ma, X.X.; Sun, Y.Z. New layering strategy and the gradient microstructure distribution of surface metamorphic layer for 8Cr4Mo4V steel by grinding. Arch. Civ. Mech. Eng. 2024, 24, 42. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Steels for bearings. Prog. Mater. Sci. 2012, 57, 268–435. [Google Scholar] [CrossRef]
- Zhao, C.; Long, R.S.; Zhang, Y.M.; Wang, Y.B.; Wang, Y.Y. Influence of characteristic parameters on the tribological properties of vein-bionic textured cylindrical roller thrust bearings. Tribol. Int. 2022, 175, 107861. [Google Scholar] [CrossRef]
- Litwin, W.; Dymarski, C. Experimental research on water-lubricated marine stern tube bearings in conditions of improper lubrication and cooling causing rapid bush wear. Tribol. Int. 2016, 99, 449–455. [Google Scholar] [CrossRef]
- Liu, Y.W.; Zhao, H.T.; Wang, Z.Y. Initial corrosion behavior of carbon steel and weathering steel in nansha marine atmosphere. Acta Metall. Sin. 2020, 56, 1247–1254. [Google Scholar]
- Dong, B.J.; Liu, W.; Zhang, T.Y.; Chen, L.J.; Fan, Y.M.; Zhao, Y.G.; Yang, W.J.; Banthukul, W. Corrosion failure analysis of low alloy steel and carbon steel rebar in tropical marine atmospheric environment: Outdoor exposure and indoor test. Eng. Fail. Anal. 2021, 129, 105720. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.G.; Gao, M.C.; Zhang, C.; Zhang, T.; Yang, H.J.; Wang, Z.H.; Qiao, J.W. Tribological properties of AlCrCuFeNi2 high-entropy alloy in different conditions. Metall. Mater. Trans A 2016, 47A, 3312–3321. [Google Scholar] [CrossRef]
- Wood, R.J.K. Marine wear and tribocorrosion. Wear 2017, 376, 893–910. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.C.; Wang, X.T.; Jiang, Q.T.; Li, Y.T.; Huang, Y.L.; Yang, L.H. Research on dynamic marine atmospheric corrosion behavior of AZ31 magnesium alloy. Metals 2022, 12, 1886. [Google Scholar] [CrossRef]
- Li, N.; Zhang, W.F.; Xu, H.; Cai, Y.K.; Yan, X.J. Corrosion behavior and mechanical properties of 30CrMnSiA high-strength steel under an indoor accelerated harsh marine atmospheric environment. Materials 2022, 15, 629. [Google Scholar] [CrossRef]
- Lu, Y.F.; Dong, J.H.; Ke, W. Effects of Cl- ions on the corrosion behaviour of low alloy steel in deaerated bicarbonate solutions. J. Mater. Sci. Technol. 2016, 32, 341–348. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Srinivas, M.; Roy, M. Microstructural developments during erosion of tribological steels. Mater. Charact. 2016, 113, 43–51. [Google Scholar] [CrossRef]
- Xu, F.J.; Guo, G.W.; Tang, G.Z.; Ma, X.M.; Wang, L.Q.; Ozur, G.E.; Yukimura, K. Microstructure modifications and corrosion behaviors of Cr4Mo4V steel treated by high current pulsed electron beam. Mater. Chem. Phys. 2011, 126, 904–908. [Google Scholar] [CrossRef]
- Essa, F.A.; Elsheikh, A.H.; Yu, J.G.; Elkady, O.A.; Saleh, B. Studies on the effect of applied load, sliding speed and temperature on the wear behavior of M50 steel reinforced with Al2O3 and/or graphene nanoparticles. J. Mater. Res. Technol. 2021, 12, 283–303. [Google Scholar] [CrossRef]
- Zhang, C.W.; Peng, B.; Wang, L.Q.; Ma, X.X.; Gu, L. Thermal-induced surface damage of M50 steel at rolling-sliding contacts. Wear 2019, 420, 116–122. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Subhash, G.; Arakere, N.; Allison, B.D.; Mccoy, B. Influence of residual stress and temperature on the cyclic hardening response of M50 high-strength bearing steel subjected to rolling contact fatigue. J. Eng. Mater. Technol. 2016, 138, 021003. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, L.; Tang, G.Z.; Mao, Y.Z. Wear transition of CrN coated M50 steel under high temperature and heavy load. Coatings 2017, 7, 202. [Google Scholar] [CrossRef]
- Wang, F.; Qian, D.S.; Hua, L.; Lu, X.H. The effect of prior cold rolling on the carbide dissolution, precipitation and dry wear behaviors of M50 bearing steel. Tribol. Int. 2019, 132, 253–264. [Google Scholar] [CrossRef]
- Huang, Q.P.; Shi, X.L.; Ma, J. Tribological behavior of surface bionic rhombic-textured M50 steel containing SnAgCu and MXene-Nb2C under dry sliding conditions. J. Mater. Eng. Perform. 2021, 30, 9390–9402. [Google Scholar] [CrossRef]
- Essa, F.A.; Zhang, Q.X.; Huang, X.J. Investigation of the effects of mixtures of WS2 and ZnO solid lubricants on the sliding friction and wear of M50 steel against silicon nitride at elevated temperatures. Wear 2017, 374, 128–141. [Google Scholar] [CrossRef]
- Yan, Y.; Neville, A.; Dowson, D.; Williams, S. Tribocorrosion in implants—Assessing high carbon and low carbon Co-Cr-Mo alloys by in situ electrochemical measurements. Tribol. Int. 2006, 39, 1509–1517. [Google Scholar] [CrossRef]
- Rajahram, S.S.; Harvey, T.J.; Wood, R.J.K. Erosion-corrosion resistance of engineering materials in various test conditions. Wear 2009, 267, 244–254. [Google Scholar] [CrossRef]
- Jiang, J.; Stack, M.M.; Neville, A. Modelling the tribo-corrosion interaction in aqueous sliding conditions. Tribol. Int. 2002, 35, 669–679. [Google Scholar] [CrossRef]
- Zhang, B.B.; Wang, J.Z.; Yuan, J.Y.; Yan, F.Y. Tribocorrosion behavior of nickel aluminum bronze in seawater: Identification of corrosion-wear components and effect of pH. Mater. Corros. 2018, 69, 106–114. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, M.; Cai, F.; Liu, M.; Su, X.; Xu, G. Corrosion performance of a corrosion-resistant rail steel in the simulated subsea tunnel environment. Corros. Rev. 2021, 39, 561–571. [Google Scholar] [CrossRef]
- Su, X.; Xu, G.; Zhu, M.; Zhang, Q.; Cai, F.; Liu, M. Investigation on the interaction between corrosion and wear of U68CuCr rail steel with different corrosion periods. Wear 2023, 516, 204598. [Google Scholar] [CrossRef]
- Hou, X.B.; Wang, Y.X.; Dai, L.Y.; Yang, Y.H.; Du, J.H.; Wang, Y.J.; Wan, H. Study on the corrosion and wear behaviors of cylinder liner in marine diesel engine burning low sulfur fuel oil. Eng. Fail. Anal. 2023, 147, 107151. [Google Scholar] [CrossRef]
- Wang, W.L.; Cui, W.F.; Xiao, Z.T.; Qin, G.W. The improved corrosion and wear properties of Ti-Zr based alloys with oxide coating in simulated seawater environment. Surf. Coat. Technol. 2022, 439, 128415. [Google Scholar] [CrossRef]
- Xu, Z.B.; Huang, Z.Y.; Wang, Y.C.; Lin, C.X.; Xu, X. Friction and wear behavior of C17200 copper-beryllium alloy in dry and wet environments. J. Mater. Eng. Perf. 2021, 30, 7545–7551. [Google Scholar] [CrossRef]
- Ding, H.R.; Zhou, G.H.; Dai, Z.D.; Bu, Y.F.; Jiang, T.Y. Corrosion wear behaviors of 2024Al in artificial rainwater and seawater at fretting contact. Wear 2009, 267, 292–298. [Google Scholar] [CrossRef]
- Jun, C. Corrosion wear characteristics of TC4, 316 stainless steel, and Monel K500 in artificial seawater. Rsc Adv. 2019, 7, 23835–23845. [Google Scholar] [CrossRef]
- Simsek, D. An investigate on hot wear and corrosion performance of Al matrix hybrid composites produced by mechanical alloying method. Arab. J. Sci. Eng. 2022, 47, 15477–15487. [Google Scholar] [CrossRef]
- Li, Z.C.; Wang, Y.X.; Cheng, X.Y.; Zeng, Z.X.; Li, J.L.; Lu, X.; Wang, L.P.; Xue, Q.J. Continuously growing ultrathick CrN coating to achieve high load-bearing capacity and good tribological property. ACS Appl. Mater. Interfaces 2018, 10, 2965–2975. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, L.; Feng, Y.P.; Wang, Z.H.; Yang, M.; Cheng, L.; Liu, J.; Wu, K.M. Effect of retained austenite on corrosion behavior of ultrafine bainitic steel in marine environment. Acta Metall. Sin. (Engl. Lett.) 2023, 36, 717–731. [Google Scholar] [CrossRef]
- Sun, M.H.; Yang, X.J.; Du, C.W.; Liu, Z.Y.; Li, Y.; Wu, Y.M.; San, H.Y.; Su, X.D.; Li, X.G. Distinct beneficial effect of Sn on the corrosion resistance of Cr–Mo low alloy steel. J. Mater. Sci. Technol. 2021, 81, 175–189. [Google Scholar] [CrossRef]
- Wu, F.H.; Zhang, N.; Sun, Y.B.; Li, X.; Liu, Z.J.; Yang, Y.L. Effect of applied load on tribological behaviour of Ti-48Al-2Cr-2Nb alloy under oil-lubricated condition. Wear 2024, 538, 205210. [Google Scholar] [CrossRef]
- Komotori, J.; Hisamori, N.; Ohmori, Y. The corrosion/wear mechanisms of Ti-6Al-4V alloy for different scratching rates. Wear 2007, 263, 412–418. [Google Scholar] [CrossRef]




| Steel | C | Cr | Mo | V | Si | Mn | S | Fe |
|---|---|---|---|---|---|---|---|---|
| 8Cr4Mo4V | 0.75~0.85 | 3.75~4.25 | 4.00~4.50 | 0.90~1.10 | ≤0.25 | ≤0.35 | ≤0.20 | Balance |
| Groups | C | O | Fe | Mo | Cl | V | Cr |
|---|---|---|---|---|---|---|---|
| Area 1 | 5.65 | 2.15 | 32.94 | 34.85 | 0.40 | 12.60 | 11.41 |
| Area 2 | 13.79 | 10.92 | 47.86 | 12.71 | 2.28 | 0.10 | 12.31 |
| Area 3 | 19.90 | 5.51 | 39.13 | 4.08 | 5.19 | 11.78 | 14.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Ying, L.; Nie, C.; Zhu, T.; Tang, R.; Liu, R. Investigation of the Corrosion–Wear Interaction Behavior of 8Cr4Mo4V Bearing Steel at Various Corrosion Intervals. Coatings 2024, 14, 1245. https://doi.org/10.3390/coatings14101245
Zhao C, Ying L, Nie C, Zhu T, Tang R, Liu R. Investigation of the Corrosion–Wear Interaction Behavior of 8Cr4Mo4V Bearing Steel at Various Corrosion Intervals. Coatings. 2024; 14(10):1245. https://doi.org/10.3390/coatings14101245
Chicago/Turabian StyleZhao, Chao, Lixia Ying, Chongyang Nie, Tianlin Zhu, Rongxiang Tang, and Ruxin Liu. 2024. "Investigation of the Corrosion–Wear Interaction Behavior of 8Cr4Mo4V Bearing Steel at Various Corrosion Intervals" Coatings 14, no. 10: 1245. https://doi.org/10.3390/coatings14101245





