Experimental Investigation of Corrosion Behavior of Zinc–Aluminum Alloy-Coated High-Strength Steel Wires under Stress Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Artificial Accelerated Corrosion Test Setup
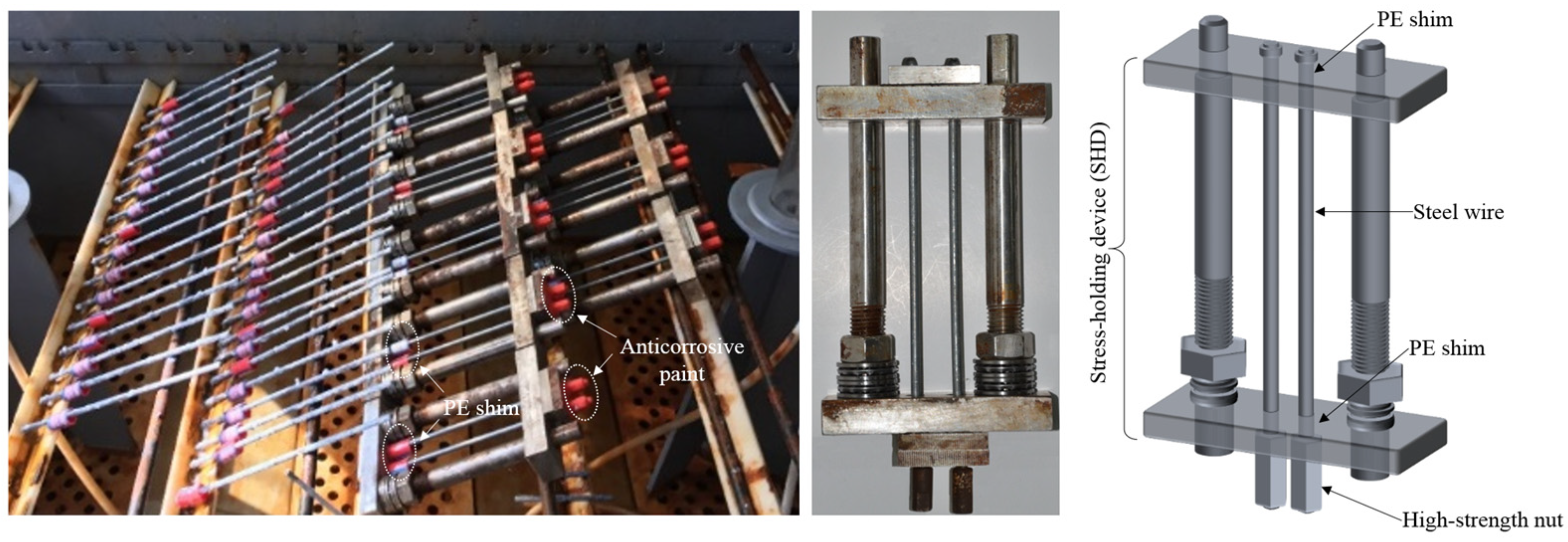
2.3. Preloading Procedure for High-Strength Steel Wire Specimens
2.4. Measurement of Pitting Corrosion
3. Results and Discussion
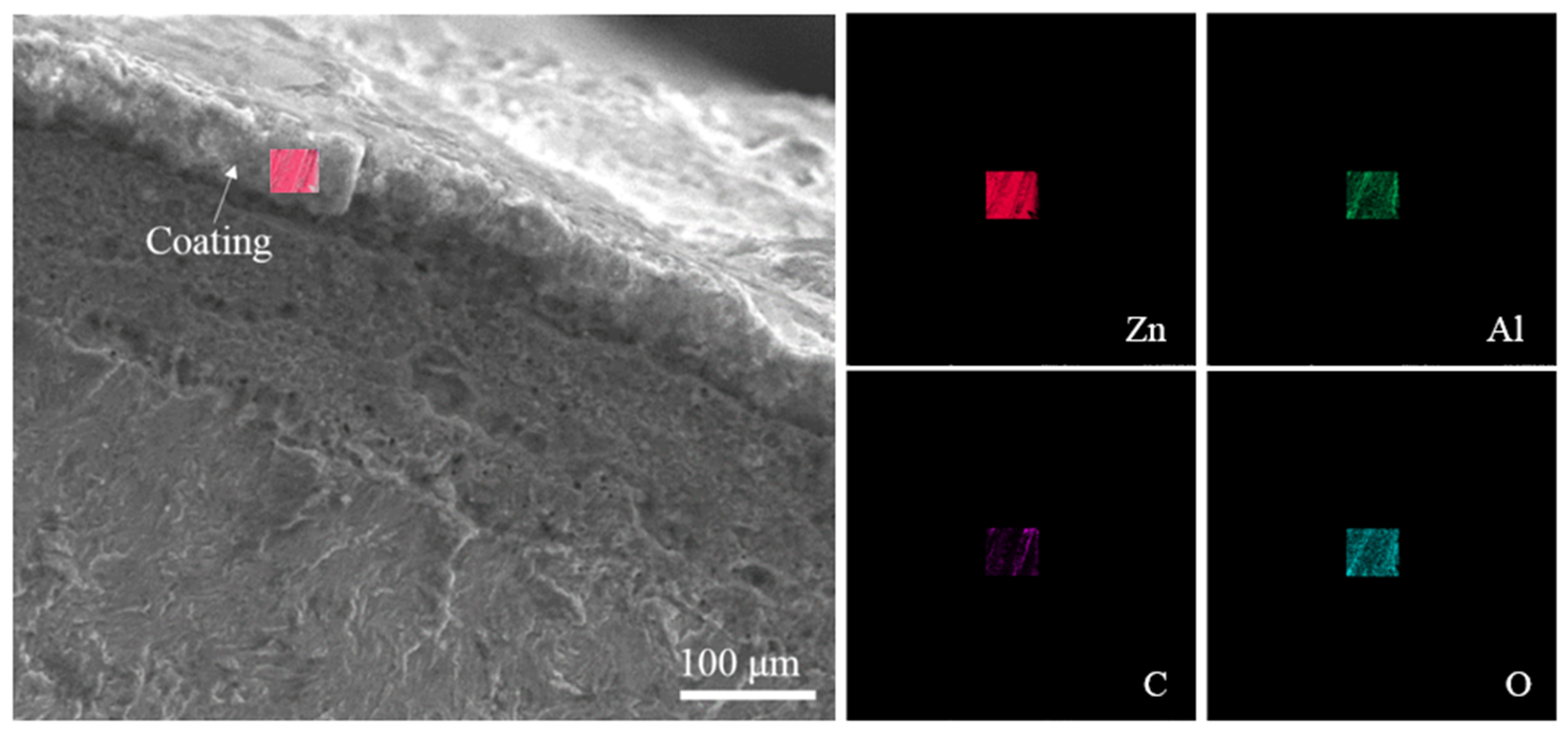
3.1. Microscopic Observation
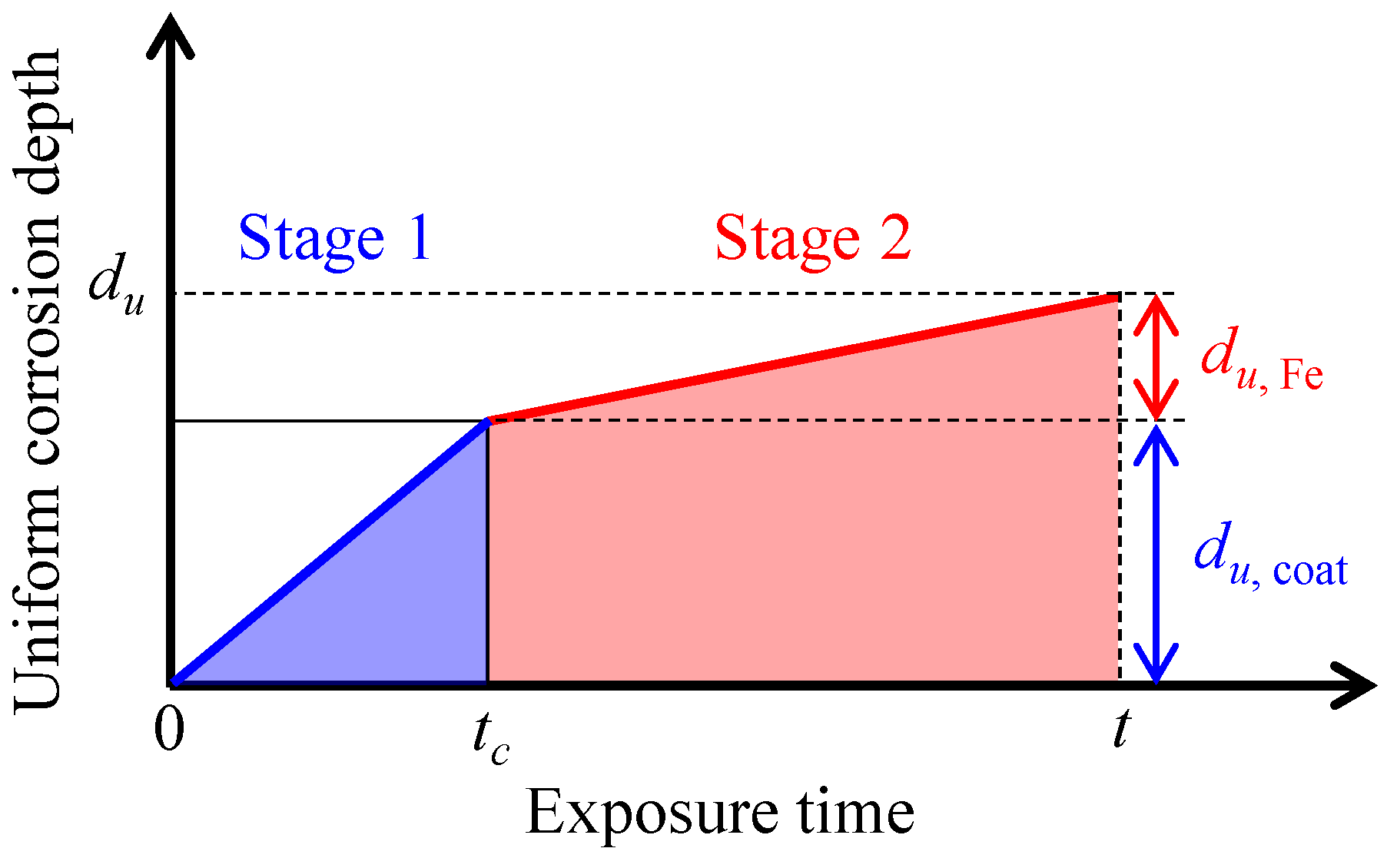
3.2. Corrosion Evolution Kinetics
3.2.1. Non-Stressed Condition
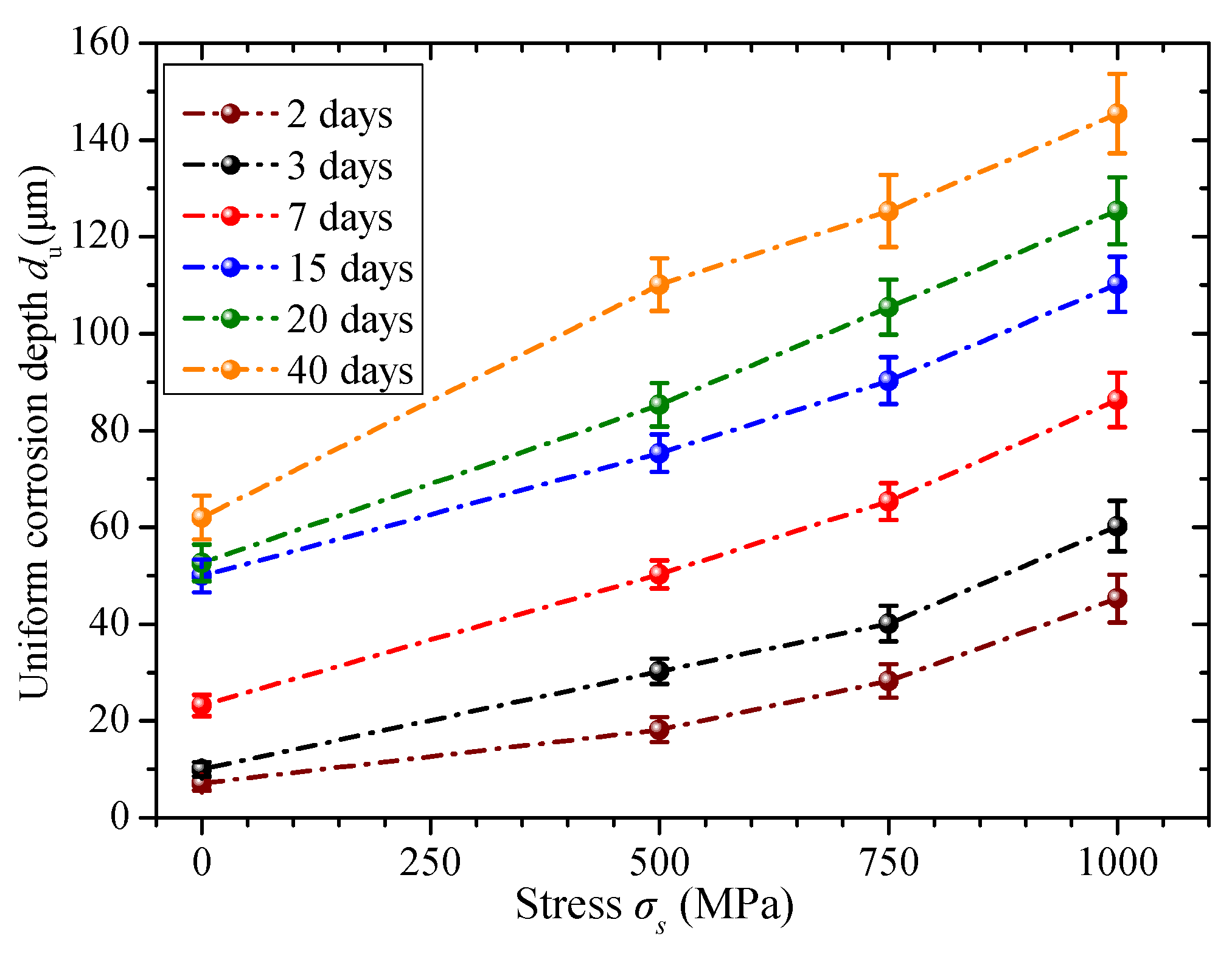
3.2.2. Stress Condition
3.3. Statistical Characteristics of Pitting Corrosion
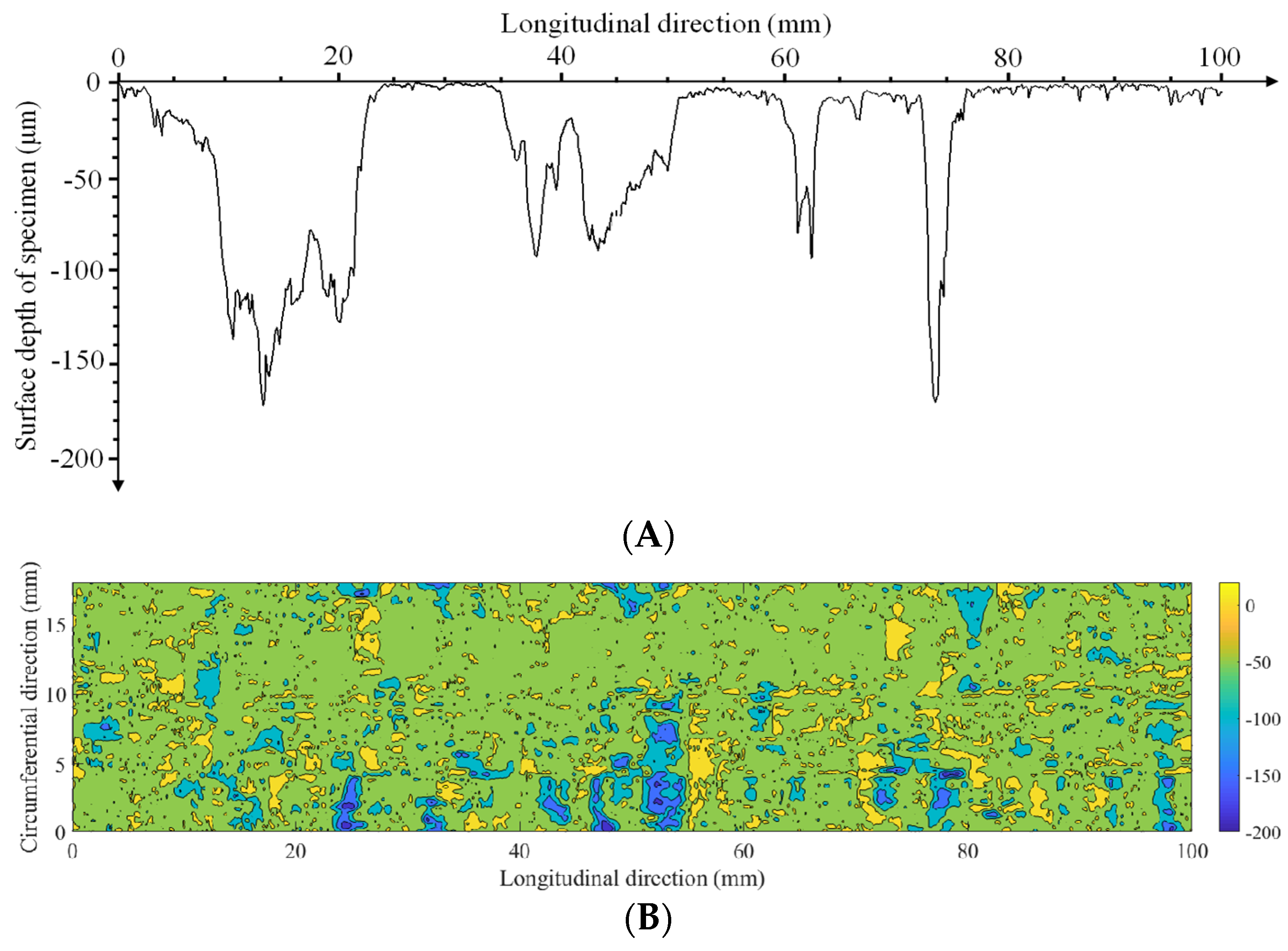
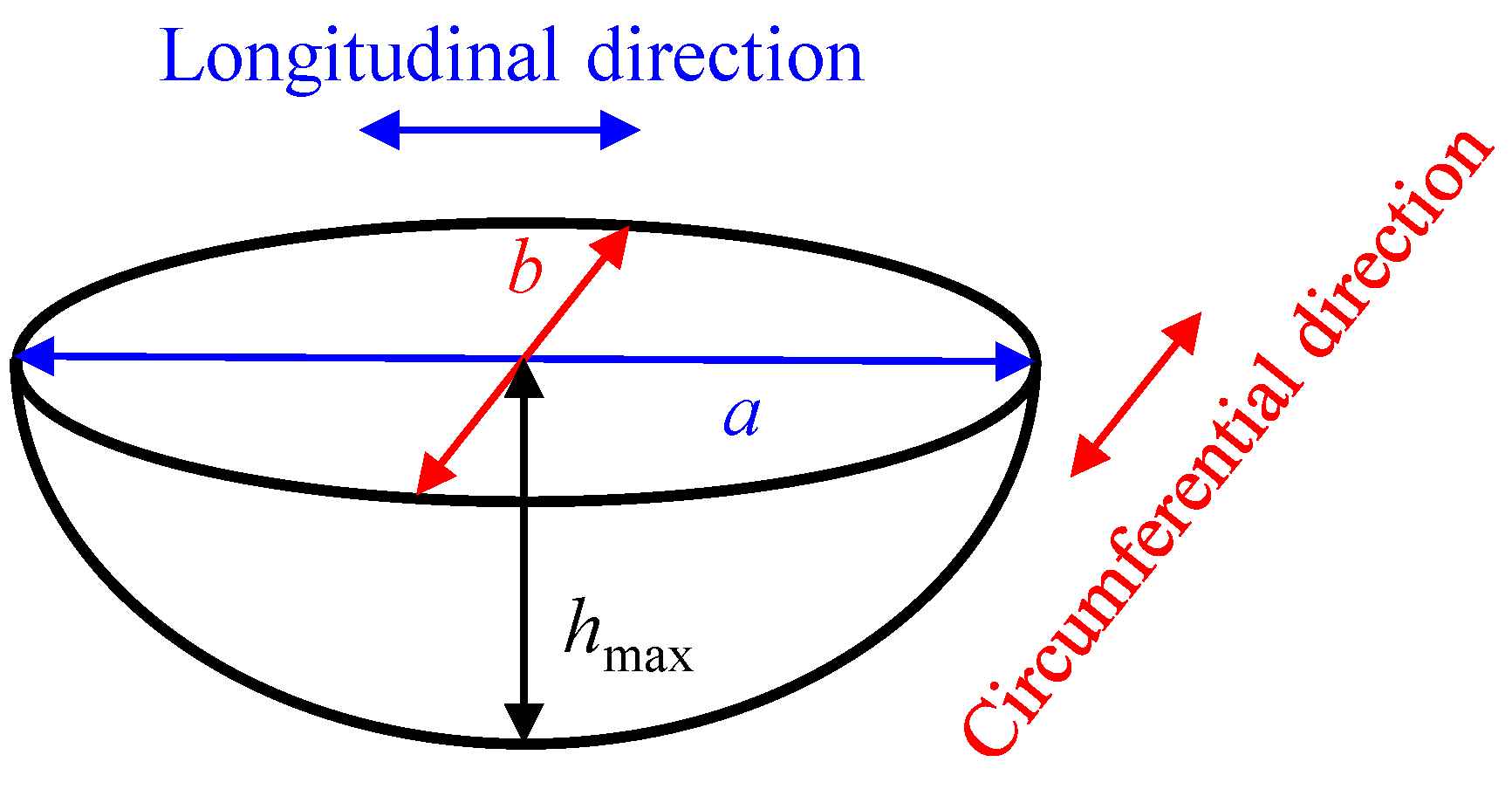
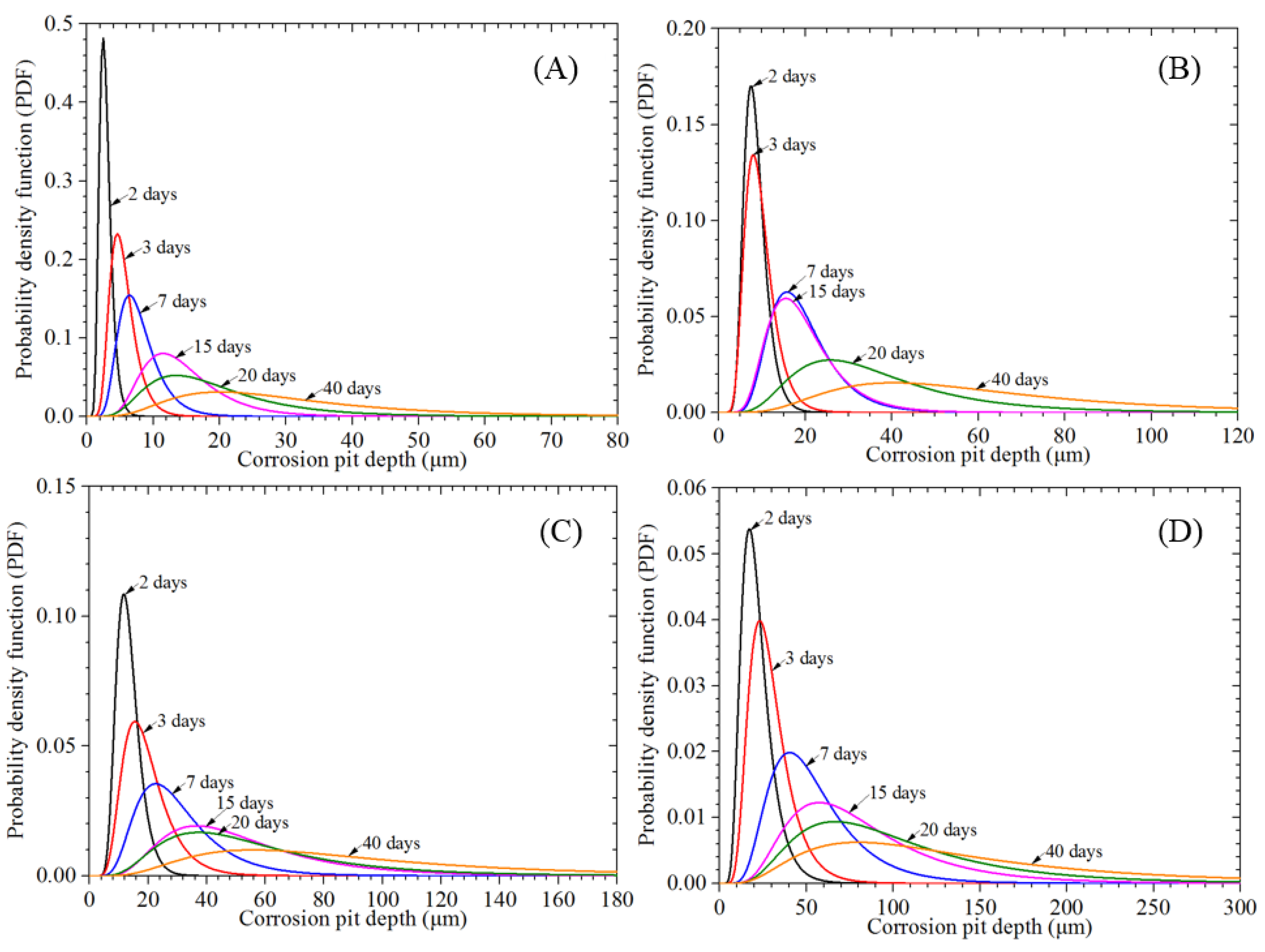
3.3.1. Corrosion Pit Depth
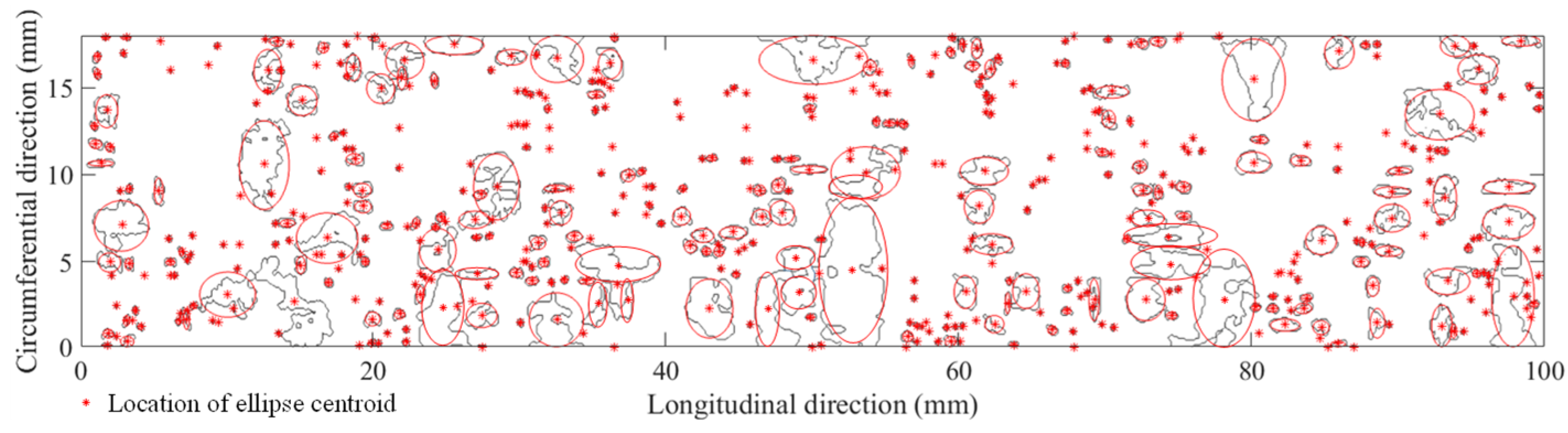
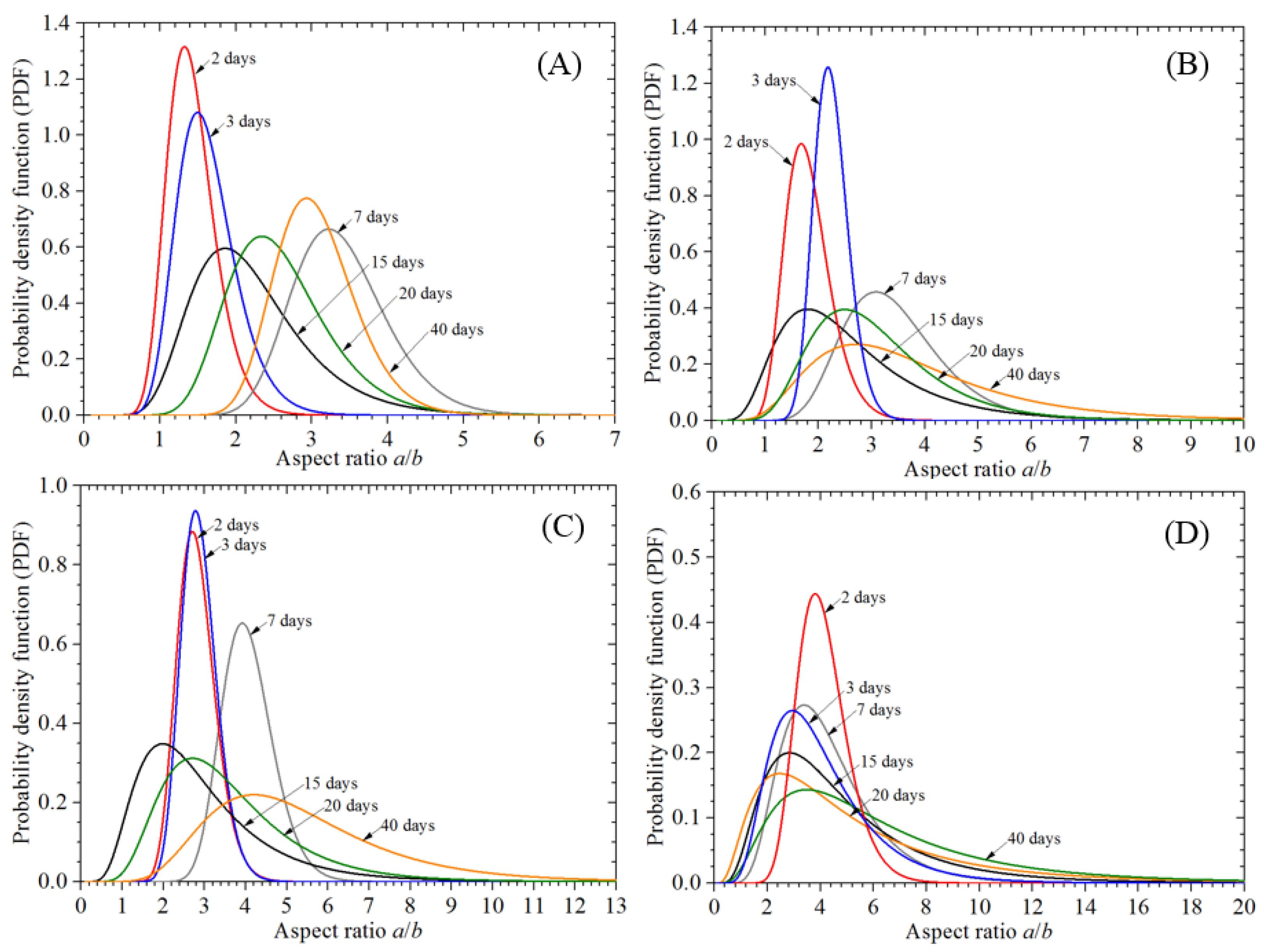
3.3.2. Corrosion Morphology
3.3.3. Block’s Maximum Pitting Factor
4. Conclusions
- 1)
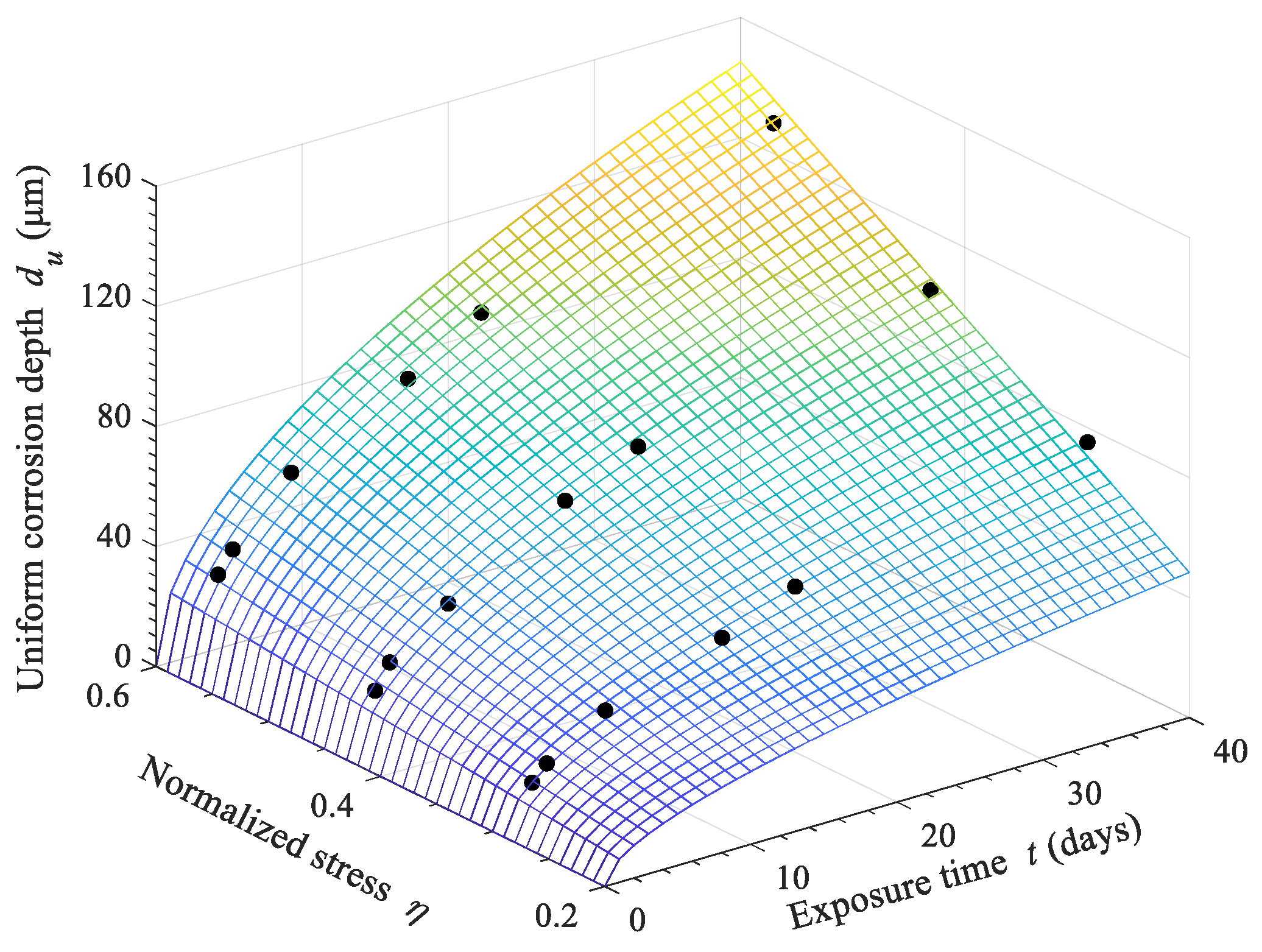
- The corrosion processes of steel wire specimens can be divided into two stages under the non-stressed condition according to the time-dependent uniform corrosion depth. A two-stage model can effectively describe the conversion from coating corrosion to iron matrix corrosion, which is represented by a critical time, tc. The corrosion rate of the coating (3.32 μm/d0.996 for the zinc–aluminum alloy coating) is much higher than that of the iron matrix (~0.5 μm/d). When the exposure time reaches the critical time (tc = 15 d for the zinc–aluminum alloy-coated steel wire), the corrosion rate undergoes a sudden transition to a certain value of ~0.5 μm/d and remains stable.
- 2)
- When considering the stress condition, the critical time is almost indistinguishable. The corrosion rates under stress conditions are always higher than those under non-stressed conditions. It can be concluded that the corrosion behavior changes from uniform corrosion to pitting corrosion under stress conditions. Thus, a continuous power model can be used to describe the corrosion phenomena. In addition, the uniform corrosion depth considering the exposure time and stress condition is formulated using an improved power model.
- 3)
- A semi-ellipsoid model is used to simulate individual corrosion pits, which obeys a lognormal distribution. It is clear that the preloading stress has little effect on corrosion pit growth compared to the exposure time. The variability in the aspect ratio of the corrosion pit depth increases under the stress condition. Statistical results for different block lengths, Lκ, show that the block’s maximum pitting factor under the stress condition still has a spatial scale effect. However, the preloading stress can lead to variation in the location parameter and reduce the variability of the block’s maximum pitting factor, which differs from the behavior of the non-stressed specimens.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miki, C.; Homma, K.; Tominaga, T. High strength and high performance steels and their use in bridge structures. J. Constr. Steel Res. 2002, 58, 3–20. [Google Scholar] [CrossRef]
- He, P.; Jiang, W.; Song, J. Application of Structural Steel and Corrosion Prevention Technology in China. Dev. Appl. Mater. 2022, 37, 96–100. [Google Scholar] [CrossRef]
- Tang, M.C. Super-long span bridges. Struct. Infrastruct. Eng. 2017, 13, 722–730. [Google Scholar] [CrossRef]
- Jarwali, T.; Nakamura, S. Anti-corrosion performance of bridge strands consisting of steel wires galvanised with zinc–aluminum alloy. Struct. Infrastruct. Eng. 2016, 12, 682–694. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhu, P.; Zhang, X.; Wang, H.; He, Y. Experimental and numerical investigations of UHSS wire main cables for suspension bridges. Structures 2022, 38, 1582–1594. [Google Scholar] [CrossRef]
- Sun, G.; Li, X.; Xue, S.; Chen, R. Mechanical properties of Galfan-coated steel cables at elevated temperatures. J. Constr. Steel Res. 2019, 155, 331–341. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Ma, K.; Jiang, L.; Ye, J.; Xu, L. Creep and stress relaxation experiments of 1960-grade high-strength steel wire at elevated temperatures. Structures 2024, 59, 105682. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, X.; Pu, G.; Wang, T.; Guo, Q. Corrosion features and time-dependent corrosion model of Galfan coating of high strength steel wires. Constr. Build. Mater. 2021, 313, 125534. [Google Scholar] [CrossRef]
- Xue, S.; Shen, R.; Chen, W.; Shen, L. The corrosion-fatigue measurement test of the Zn-Al alloy coated steel wire. Structures 2020, 27, 1195–1201. [Google Scholar] [CrossRef]
- Chen, M.; Lu, X.; Zhou, Z.; Du, Y.; Xu, X.; Li, X. Mechanical properties of 2100 MPa parallel wire strands under and after elevated temperature. J. Constr. Steel Res. 2024, 212, 108323. [Google Scholar] [CrossRef]
- Su, M.; Dai, G.; Marx, S.; Liu, W.; Zhang, S. A Brief Review of Developments and Challenges for High-speed Rail Bridges in China and Germany. Struct. Eng. Int. 2019, 29, 160–166. [Google Scholar] [CrossRef]
- He, X.; Wu, T.; Zou, Y.; Chen, Y.F.; Guo, H.; Yu, Z. Recent developments of high-speed railway bridges in China. Struct. Infrastruct. Eng. 2017, 12, 1584–1595. [Google Scholar] [CrossRef]
- Fang, F.; Zhao, Y.; Zhou, L.; Hu, X.; Xie, Z.; Jiang, J. Texture inheritance of cold drawn pearlite steel wires after austenitization. Mat. Sci. Eng. A-Struct. 2014, 618, 505–510. [Google Scholar] [CrossRef]
- Jamoneau, A.; Solas, D.; Bourgon, J.; Morisot, P.; Schmitt, J. Strain localization and delamination mechanism of cold-drawn pearlitic steel wires during torsion. Mat. Sci. Eng. A-Struct. 2021, 814, 141222. [Google Scholar] [CrossRef]
- Bargujer, S.; Suri, N.; Belokar, R. Pearlitic steel wire: High carbon steel based natural nanomaterial by lead patenting process. Mater. Today 2016, 3, 1553–1562. [Google Scholar] [CrossRef]
- Toribio, J.; Ayaso, F.; Fernández-Viña, A. Environmentally-assisted microstructural integrity of commercial cold drawn pearlitic steel wires. Procedia Struct. Integr. 2022, 37, 1001–1006. [Google Scholar] [CrossRef]
- Emamian, Y.; Kolahi, A.; Palizdar, Y. Evaluation of microstructures and mechanical properties of deltha trip steel with different vanadium contents. Results Mater. 2024, 21, 100530. [Google Scholar] [CrossRef]
- Berrocal, C.G.; Lundgren, K.; Löfgren, I. Corrosion of steel bars embedded in fibre reinforced concrete under chloride attack: State of the art. Cem. Concr. Res. 2016, 80, 69–85. [Google Scholar] [CrossRef]
- Fu, C.; Jin, N.; Ye, H.; Jin, X.; Dai, W. Corrosion characteristics of a 4-year naturally corroded reinforced concrete beam with load-induced transverse cracks. Corros. Sci. 2017, 117, 11–23. [Google Scholar] [CrossRef]
- Mohd, H.M.; Kim, D.K.; Kim, D.W.; Paik, J.K. A time-variant corrosion wastage model for subsea gas pipelines. Ships Offshore Struc. 2014, 9, 161–176. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Yu, Y.; Luo, Z.; Liu, W.; Wang, C. Corrosion behavior of X65 pipeline steel: Comparison of wet–Dry cycle and full immersion. Corros. Sci. 2018, 133, 276–287. [Google Scholar] [CrossRef]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structures—A technical review. J. Loss Prevent. Proc. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Pantelakis, S.; Setsika, D.; Chamos, A.; Zervaki, A. Corrosion damage evolution of the aircraft aluminum alloy 2024 T3. Int. J. Struct. Integr. 2016, 7, 25–46. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Karanci, E.; Betti, R. Modeling Corrosion in Suspension Bridge Main Cables. I—Annual Corrosion Rate. J. Bridge Eng. 2018, 23, 04018025. [Google Scholar] [CrossRef]
- Talbot, D.E.J.; Talbot, J.D.R. Corrosion Science and Technology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Suzumura, K.; Nakamura, S.I. Environmental Factors Affecting Corrosion of Galvanized Steel Wires. J. Mater. Civil Eng. 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Wu, S.; Chen, H.; Ramandi, H.L.; Hagan, P.C.; Crosky, A.; Saydam, S. Effects of environmental factors on stress corrosion cracking of cold-drawn high-carbon steel wires. Corros. Sci. 2017, 132, 234–243. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Nakhaie, D.; Zakeri, M.; Moayed, M.H. The effect of dichromate ion on the pitting corrosion of AISI 316 stainless steel Part II: Pit initiation and transition to stability. Corros. Sci. 2015, 94, 420–427. [Google Scholar] [CrossRef]
- Van der Weeën, P.; Zimer, A.M.; Pereira, E.C.; Mascaro, L.H.; Bruno, O.M.; De Baets, B. Modeling pitting corrosion by means of a 3D discrete stochastic model. Corros. Sci. 2014, 82, 133–144. [Google Scholar] [CrossRef]
- Tang, Y.; Zuo, Y.; Wang, J.; Zhao, X.; Niu, B.; Lin, B. The metastable pitting potential and its relation to the pitting potential for four materials in chloride solutions. Corros. Sci. 2014, 80, 111–119. [Google Scholar] [CrossRef]
- Velázquez, J.C.; Hernández-Huerta, J.J.; Diaz-Cruz, M.; Hernández-Sánchez, E.; Cervantes-Tobón, A.; Capula-Colindres, S.I.; Cabrera-Sierra, R. Study on the Influence of Non-Metallic Inclusions on the Pitting Corrosion of API 5L X60 Steel. Coatings 2023, 13, 1040. [Google Scholar] [CrossRef]
- Stewart, M.G.; Al-Harthy, A. Pitting corrosion and structural reliability of corroding RC structures: Experimental data and probabilistic analysis. Reliab. Eng. Syst. Saf. 2008, 93, 373–382. [Google Scholar] [CrossRef]
- Li, F.; Luo, X.; Wang, K.; Ji, Y. Pitting Damage Characteristics on Prestressing Steel Strands by Combined Action of Fatigue Load and Chloride Corrosion. J. Bridge Eng. 2017, 22, 04017023. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzumura, K. Experimental Study on Fatigue Strength of Corroded Bridge Wires. J. Bridge Eng. 2013, 18, 200–209. [Google Scholar] [CrossRef]
- Sun, H.; Xu, J.; Chen, W.; Yang, J. Time-Dependent Effect of Corrosion on the Mechanical Characteristics of Stay Cable. J. Bridge Eng. 2018, 23, 04018019. [Google Scholar] [CrossRef]
- Karuppanasamy, J.; Pillai, R. Statistical distributions for the corrosion rates of conventional and prestressing steel reinforcement embedded in chloride contaminated mortar. Corrosion 2017, 73, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xiao, Y.; Wang, S.; Li, W.; Liu, W.; Du, D. Numerical model for corrosion rate of steel reinforcement in cracked reinforced concrete structure. Constr. Build. Mater. 2018, 180, 55–67. [Google Scholar] [CrossRef]
- Biondini, F.; Camnasio, E.; Palermo, A. Lifetime seismic performance of concrete bridges exposed to corrosion. Struct. Infrastruct. Eng. 2014, 10, 880–900. [Google Scholar] [CrossRef]
- Betti, R.; West, A.C.; Vermaas, G.; Cao, Y. Corrosion and Embrittlement in High-Strength Wires of Suspension Bridge Cables. J. Bridge Eng. 2005, 10, 151–162. [Google Scholar] [CrossRef]
- Yang, W.J.; Yang, P.; Li, X.M.; Feng, W.L. Influence of tensile stress on corrosion behaviour of high-strength galvanized steel bridge wires in simulated acid rain. Mater. Corros. 2015, 63, 401–407. [Google Scholar] [CrossRef]
- Chen, C.; Ma, H.; Wang, F.; Yang, Z.; Zhang, F.; Yan, Z. Influence of Carbon Content on Tensile Properties of Pure High Manganese Austenitic Steel. Coatings 2022, 12, 1622. [Google Scholar] [CrossRef]
- ISO 9227: 2022(E); Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. International Organization for Standardization: Geneva, Switzerland, 2022.
- ASTM G1-03 (2011); Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM: West Conshohocken, PA, USA, 2011.
- Tahara, A.; Shinohara, T. Influence of the alloy element on corrosion morphology of the low alloy steels exposed to the atmospheric environments. Corros. Sci. 2005, 47, 2589–2598. [Google Scholar] [CrossRef]
- Cole, I.S.; Marney, D. The science of pipe corrosion: A review of the literature on the corrosion of ferrous metals in soils. Corros. Sci. 2012, 56, 5–16. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Y. Temporal and Spatial Variation Study on Corrosion of High-Strength Steel Wires in the Suspender of CFST Arch Bridge. Coatings 2024, 14, 628. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Li, H.; Guan, X. Uniform and pitting corrosion modeling for high-strength bridge wires. J. Bridge Eng. 2014, 19, 04014025. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Mahmoodian, M. Effect of Applied Stress on Corrosion and Mechanical Properties of Mild Steel. J. Mater. Civ. Eng. 2019, 31, 04018375. [Google Scholar] [CrossRef]
- Hong, H.; Cao, S.; Fu, J.; Tian, H. Experimental Study on Apparent Corrosion Rule of Main Cable Steel Wires of Suspension Bridge in Holding Force State. J. Highw. Transport. Res. Dev. 2022, 39, 97–102. [Google Scholar] [CrossRef]
- Stewart, M.G. Mechanical behaviour of pitting corrosion of flexural and shear reinforcement and its effect on structural reliability of corroding RC beams. Struct. Saf. 2009, 31, 19–30. [Google Scholar] [CrossRef]
- Valor, A.; Caleyo, F.; Rivas, D.; Hallen, J.M. Stochastic approach to pitting-corrosion-extreme modelling in low-carbon steel. Corros. Sci. 2010, 52, 910–915. [Google Scholar] [CrossRef]





| Yield Strength (MPa) | Ultimate Strength (MPa) | Elastic Modulus (MPa) | Elongation |
|---|---|---|---|
| ≥1750 | ≥1960 | (2.0 ± 0.1) × 105 | ≥4.0% |
| Element | C | Mn | Cr | Si | Al | V | Ni | Cu | P | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.95~ 1.20 | 0.6~ 1.0 | 0.1~ 0.5 | 0.10~ 0.48 | 0.05~ 0.15 | 0.01~ 0.05 | 0.01~ 0.03 | ≤0.05 | ≤0.025 | ≤0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, K.; Liu, X.; Chen, P.; Luo, X.; Liu, Z.; Zha, X.; Zhang, X.; Zhai, J. Experimental Investigation of Corrosion Behavior of Zinc–Aluminum Alloy-Coated High-Strength Steel Wires under Stress Condition. Coatings 2024, 14, 890. https://doi.org/10.3390/coatings14070890
Fang K, Liu X, Chen P, Luo X, Liu Z, Zha X, Zhang X, Zhai J. Experimental Investigation of Corrosion Behavior of Zinc–Aluminum Alloy-Coated High-Strength Steel Wires under Stress Condition. Coatings. 2024; 14(7):890. https://doi.org/10.3390/coatings14070890
Chicago/Turabian StyleFang, Kun, Xiaoyong Liu, Pei Chen, Xianfu Luo, Zhenliang Liu, Xiaoqin Zha, Xinyao Zhang, and Jianfei Zhai. 2024. "Experimental Investigation of Corrosion Behavior of Zinc–Aluminum Alloy-Coated High-Strength Steel Wires under Stress Condition" Coatings 14, no. 7: 890. https://doi.org/10.3390/coatings14070890





