Eco-Friendly Disperse Dyeing and Functional Finishing of Nylon 6 Using Supercritical Carbon Dioxide
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabric and Dyes

2.2. Dyeing Apparatus

2.3. Procedures
2.3.1. ScCO2 Dyeing
2.3.2. Aqueous Dyeing

2.4. Measurements
2.5. Statistical Analysis
3. Results and Discussion
3.1. Dyeing Properties of Hydazonopropanenitrile Dyes
3.1.1. Effect of Dye Concentration
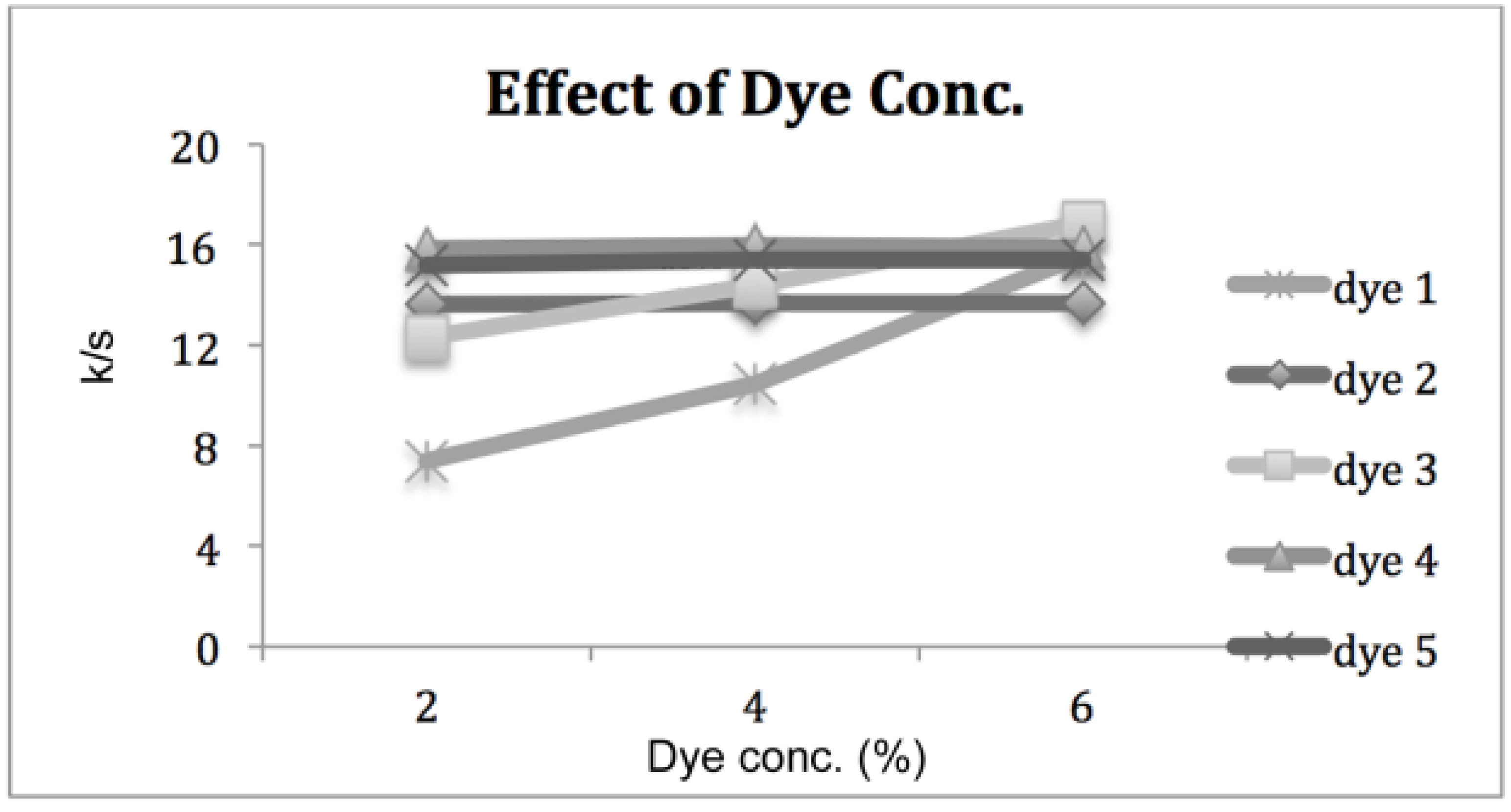

3.1.2. Effect of Dyeing Temperature

3.1.3. Effect of Dyeing Pressure
3.1.4. Effect of Dyeing Time

3.2. Color Fastness
| Dye | ScCO2 Dyeing | Aqueous Dyeing | ||||||
|---|---|---|---|---|---|---|---|---|
| Rubbing Fastness | Washing Fastness | Light Fastness | Rubbing Fastness | Washing Fastness | Light Fastness | |||
| Color Change | Staining | Color Change | Staining | |||||
| 1 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 1–2 |
| 2 | 4–5 | 5 | 5 | 4 | 5 | 5 | 5 | 1 |
| 3 | 4–5 | 5 | 5 | 4–5 | 2–3 | 5 | 5 | 1 |
| 4 | 4–5 | 4–5 | 5 | 4–5 | 3–4 | 2–3 | 5 | 1 |
| 5 | 5 | 4–5 | 5 | 5 | 4 | 2–3 | 4 | 1–2 |
| Dye | ZI of the Dyed Nylon in scCO2 | ZI of the Dyed Nylon in Water | ||||||
|---|---|---|---|---|---|---|---|---|
| G-ve | G+ve | G-ve | G+ve | |||||
| Pseudomonas Aeruginosa | Escherichia coli | Bacillussubtilis | Staphylococcus Aureus | Pseudomonasaeruginosa | Escherichia coli | Bacillussubtilis | Staphylococcus S. aureus | |
| 1 | 12 | 13 | 13 | 13 | 12 | 13 | 12 | 13 |
| 2 | 13 | 12 | 14 | 13 | 12 | 13 | 14 | 13 |
| 3 | 14 | 13 | 14 | 13 | 13 | 13 | 13 | 13 |
| 4 | 13 | 12 | 14 | 13 | 12 | 12 | 12 | 13 |
| 5 | 12 | 11 | 12 | 11 | 11 | 11 | 12 | 11 |
3.3. Antimicrobial Activity
3.4. Color Assessment
| Dye | L* | C* | H | a* | b* |
|---|---|---|---|---|---|
| 1 | 88.11 | 79.1 | 97.84 | −10.79 | 78.36 |
| 2 | 90.75 | 90.34 | 100.11 | −12.4 | 88.94 |
| 3 | 92.12 | 76.33 | 102.71 | −16.79 | 74.46 |
| 4 | 90.04 | 95.11 | 99.76 | −14.31 | 83.16 |
| 5 | 92.73 | 81.86 | 102.51 | −17.69 | 79.75 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bach, E.; Cleve, E.; Schollmeyer, E. Past, present and future of supercritical fluid dyeing technology—An overview. Rev. Prog. Color. 2002, 32, 88–102. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical fluid dyeing of synthetic and natural textiles—A review. Color. Technol. 2012, 129, 2–17. [Google Scholar] [CrossRef]
- Montero, G.A.; Smith, C.B.; Hendrix, W.A.; Butcher, D.L. Supercritical Fluid Technology in Textile Processing: An Overview. Ind. Eng. Chem. Res. 2000, 39, 4806–4812. [Google Scholar] [CrossRef]
- Miah, L.; Ferdous, N.; Azad, M.M. Textiles Material Dyeing with Supercritical Carbon Dioxide (CO2) without Using Water. Chem. Mater. Res. 2013, 3, 38–40. [Google Scholar]
- Makhlouf, C.; Ladhari, N.; Roudesli, S.; Sakly, F. Influence of grafting with acrylic acid on the dyeing properties of polyamide 6.6 fibres. Color. Technol. 2012, 128, 176–183. [Google Scholar] [CrossRef]
- Bahtiyari, M.I. Laser modification of polyamide fabrics. Opt. Laser Technol. 2011, 43, 114–118. [Google Scholar] [CrossRef]
- Giorgi, M.R.D.; Cadoni, E.; Maricca, D.; Piras, A. Dyeing polyester fibres with disperse dyes in supercritical CO2. Dye. Pigment. 2000, 45, 75–79. [Google Scholar] [CrossRef]
- Gharanjig, K.; Arami, M.; Bahrami, H.; Movassagh, B.; Mahmoodi, N.M.; Rouhani, S. Synthesis, spectral properties and application of novel monoazo disperse dyes derived from N-ester-1,8-naphthalimide to polyester. Dye. Pigment. 2008, 76, 684–689. [Google Scholar] [CrossRef]
- Miyazaki, K.; Tabatab, I.; Horia, T. Relationship between colour fastness and colour strength of polypropylene fabrics dyed in supercritical carbon dioxide: Effect of chemical structure in 1, 4-bis (alkylamino)anthraquinone dyestuffs on dyeing performance. Color. Technol. 2011, 128, 60–67. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Ma, J.; El-Taweel, F.; Abd El-Aziz, E.; Okubayashi, S. Facile Bifunctional Dyeing of Polyester under Supercritical Carbon Dioxide Medium with New Antibacterial Hydrazono Propanenitrile Dyes. Ind. Eng. Chem. Res. 2014, 53, 15566–15570. [Google Scholar] [CrossRef]
- Miyazaki, K.; Tabatab, I.; Horia, T. Effects of molecular structure on dyeing performance and colour fastness of yellow dyestuffs applied to polypropylene fibres in supercritical carbon dioxide. Color. Technol. 2011, 128, 51–59. [Google Scholar] [CrossRef]
- Ehrhardt, A.; Tabata, I.; Hisada, K.; Hori, T. Impregnation of hydroxy-anthraquinone compounds into polypropylene fabrics by scCO2 and the sorption ability for metal ions. Sen’i Gakkaishi 2005, 61, 201–203. [Google Scholar] [CrossRef]
- Kima, T.K.; Sonb, Y.A.; Limc, Y.J. Affinity of disperse dyes on poly (ethylene terephthalate) in non-aqueous media: Part 1. Adsorption and solubility properties. Dye. Pigment. 2005, 64, 73–78. [Google Scholar] [CrossRef]
- Kima, T.K.; Son, Y.A. Affinity of disperse dyes on poly (ethylene terephthalate) in non-aqueous media. Part 2: Effect of substituents. Dye. Pigment. 2005, 66, 19–25. [Google Scholar] [CrossRef]
- Özcan, A.S.; Özcan, A. Adsorption behavior of a disperse dye on polyester in supercritical carbon dioxide. J. Supercrit. Fluid. 2005, 35, 133–139. [Google Scholar] [CrossRef]
- Banchero, M.; Ferri, A.; Manna, L. The phase partition of disperse dyes in the dyeing of polyethylene terephthalate with a supercritical CO2/methanol mixture. J. Supercrit. Fluid. 2009, 48, 72–78. [Google Scholar] [CrossRef]
- Banchero, M.; Ferri, A.; Manna, L.; Sicardi, S. Dye uptake and partition ratio of disperse dyes between a PET yarn and supercritical carbon dioxide. J. Supercrit. Fluid. 2006, 37, 107–114. [Google Scholar]
- Tabata, I.; Lyu, J.; Cho, S.; Tominaga, T.; Hori, T. Relationship between the solubility of disperse dyes and the equilibrium dye adsorption in supercritical fluid dyeing. Color. Technol. 2001, 117, 346–351. [Google Scholar] [CrossRef]
- Kawahara, Y.; Yoshioka, T.; Sugiura, K.; Ogawa, S.; Kikutani, T. Dyeing behavior of high-speed spun poly (ethylene terephthalate) fibers in supercritical carbon dioxide. J. Macromol. Sci. Part B Phys. 2001, 40, 189–197. [Google Scholar] [CrossRef]
- Bao, P.; Dai, J. Relationships between the Solubility of C.I. Disperse Red 60 and Uptake on PET in Supercritical CO2. J. Chem. Eng. Data 2005, 50, 838–842. [Google Scholar] [CrossRef]
- Hou, A.; Xie, K.; Dai, J. Effect of Supercritical Carbon Dioxide Dyeing Conditions on the Chemical and Morphological Changes of Poly (ethylene terephthalate) Fibers. J. Appl. Polym. Sci. 2004, 92, 2008–2012. [Google Scholar] [CrossRef]
- Filho, L.C.; Mazzer, H.R.; Santos, J.C.; Andreaus, J.; Feihrmann, A.C.; Beninca, C.; Cabral, V.F.; Zanoelo, E.F. Dyeing of polyethylene terephthalate fibers with a disperse dye in supercritical carbon dioxide. Text. Res. J. 2014, 84, 1279–1287. [Google Scholar] [CrossRef]
- Kraan, M.V.D.; Cid, M.V.F.; Woerlee, G.F.; Veugelers, W.J.T.; Witkamp, G.J. Equilibrium Study on the Disperse Dyeing of Polyester Textile in Supercritical Carbon Dioxide. Text. Res. J. 2007, 7, 550–558. [Google Scholar] [CrossRef]
- Liao, S.K.; Chang, P.S.; Lin, Y.C. Analysis on the Dyeing of Polypropylene Fibers in Supercritical Carbon Dioxide. J. Polym. Res. 2000, 7, 155–159. [Google Scholar] [CrossRef]
- Kim, T.; Kim, G.; Park, J.Y.; Lim, J.S.; Yoo, K.P. Solubility Measurement and Dyeing Performance Evaluation of Aramid NOMEX Yarn by Dispersed Dyes in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2006, 45, 3425–3433. [Google Scholar] [CrossRef]
- Gao, D.; Yang, D.F.; Cui, H.S.; Huang, T.T.; Lin, J.X. Synthesis and Measurement of Solubilities of Reactive Disperse Dyes for Dyeing Cotton Fabrics in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2014, 53, 13862–13870. [Google Scholar] [CrossRef]
- 0zcan, A.S.; Clifford, A.A.; Bartlea, K.D.; Lewis, D.M. Dyeing of Cotton Fibres with Disperse Dyes in Supercritical Carbon Dioxide. Dye. Pigment. 1998, 36, 103–110. [Google Scholar]
- Beltrame, P.L.; Castelli, A.; Selli, E.; Mossa, A.; Testa, G.; Bonfattic, A.M.; Seves, A. Dyeing of Cotton in Supercritical Carbon Dioxide. Dye. Pigment. 1998, 39, 335–340. [Google Scholar] [CrossRef]
- Liao, S.K.; Ho, Y.C.; Chang, P.S. Dyeing of nylon 66 with a disperse-reactive dye using supercritical carbon dioxide as the transport medium. JSDC 2000, 116, 403–407. [Google Scholar] [CrossRef]
- Liao, S.K. Dyeing Nylon-6,6 with Some Hydrophobic Reactive Dyes by Supercritical Processing. J. Polym. Res. 2004, 11, 285–291. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; El-Taweel, F.; Abd El-Aziz, E.; Yuesf, M.; Okubayashi, S. Facile bifunctional dyeing of polyester fabrics with new antibacterial β-oxoalkanenitriles disperse dyes. Int. J. Sci. Eng. Res. 2014, 5, 703–706. [Google Scholar]
- JIS L 0844: Test Methods for Color Fastness to Washing and Laundering; Japanese Standards Association: Minato-ku, Tokyo; Suga Weathering Technology Foundation: Tokyo, Japan, 2011.
- JIS L 0849: Test Methods for Color Fastness to Rubbing; Japanese Standards Association: Minato-ku, Tokyo; Suga Weathering Technology Foundation: Tokyo, Japan, 2013.
- JIS L 0842: Test Methods for Colour Fastness to Enclosed Carbon Arc Lamp Light; Japanese Standards Association: Minato-ku, Tokyo; Suga Weathering Technology Foundation: Tokyo, Japan, 2004.
- Hou, A.; Chen, B.; Dai, J.; Zhang, K. Using supercritical carbon dioxide as solvent to replace water in polyethylene terephthalate (PET) fabric dyeing procedures. J. Clean. Prod. 2010, 18, 1009–1014. [Google Scholar] [CrossRef]
- Long, J.J.; Ma, Y.Q.; Zhao, J.P. Investigations on the level dyeing of fabrics in supercritical carbon dioxide. J. Supercrit. Fluid. 2011, 57, 80–86. [Google Scholar]
- Hou, A.; Dai, J. Kinetics of dyeing of polyester with CI Disperse Blue 79 in supercritical carbon dioxide. Color. Technol. 2005, 121, 18–20. [Google Scholar] [CrossRef]
- Elattar, K.M. Synthesis of Novel Azo Disperse dyes Derived from 4-Aminoantipyrine and their Applications to Polyester Fabrics. Am. J. Org. Chem. 2012, 2, 52–57. [Google Scholar]
- Ibrahim, N.A.; Eid, B.M.; Abou Elmaaty, T.M.; Abd El-Aziz, E. A smart approach to add antibacterial functionality to cellulosic pigment prints. Carbohyd. Polym. 2013, 94, 612–618. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmaaty, T.A.; El-Aziz, E.A.; Ma, J.; El-Taweel, F.; Okubayashi, S. Eco-Friendly Disperse Dyeing and Functional Finishing of Nylon 6 Using Supercritical Carbon Dioxide. Fibers 2015, 3, 309-322. https://doi.org/10.3390/fib3030309
Elmaaty TA, El-Aziz EA, Ma J, El-Taweel F, Okubayashi S. Eco-Friendly Disperse Dyeing and Functional Finishing of Nylon 6 Using Supercritical Carbon Dioxide. Fibers. 2015; 3(3):309-322. https://doi.org/10.3390/fib3030309
Chicago/Turabian StyleElmaaty, Tarek Abou, Eman Abd El-Aziz, Jaehuyk Ma, Fathy El-Taweel, and Satoko Okubayashi. 2015. "Eco-Friendly Disperse Dyeing and Functional Finishing of Nylon 6 Using Supercritical Carbon Dioxide" Fibers 3, no. 3: 309-322. https://doi.org/10.3390/fib3030309







