Morphological, Morphometrical and Molecular Characterization of Oscheius siddiqii Tabassum and Shahina, 2010 (Rhabditida, Rhabditidae) from India with Its Taxonomic Consequences for the Subgenus Oscheius Andrássy, 1976
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematode Source
2.2. Morphological and Morphometrical Characterization
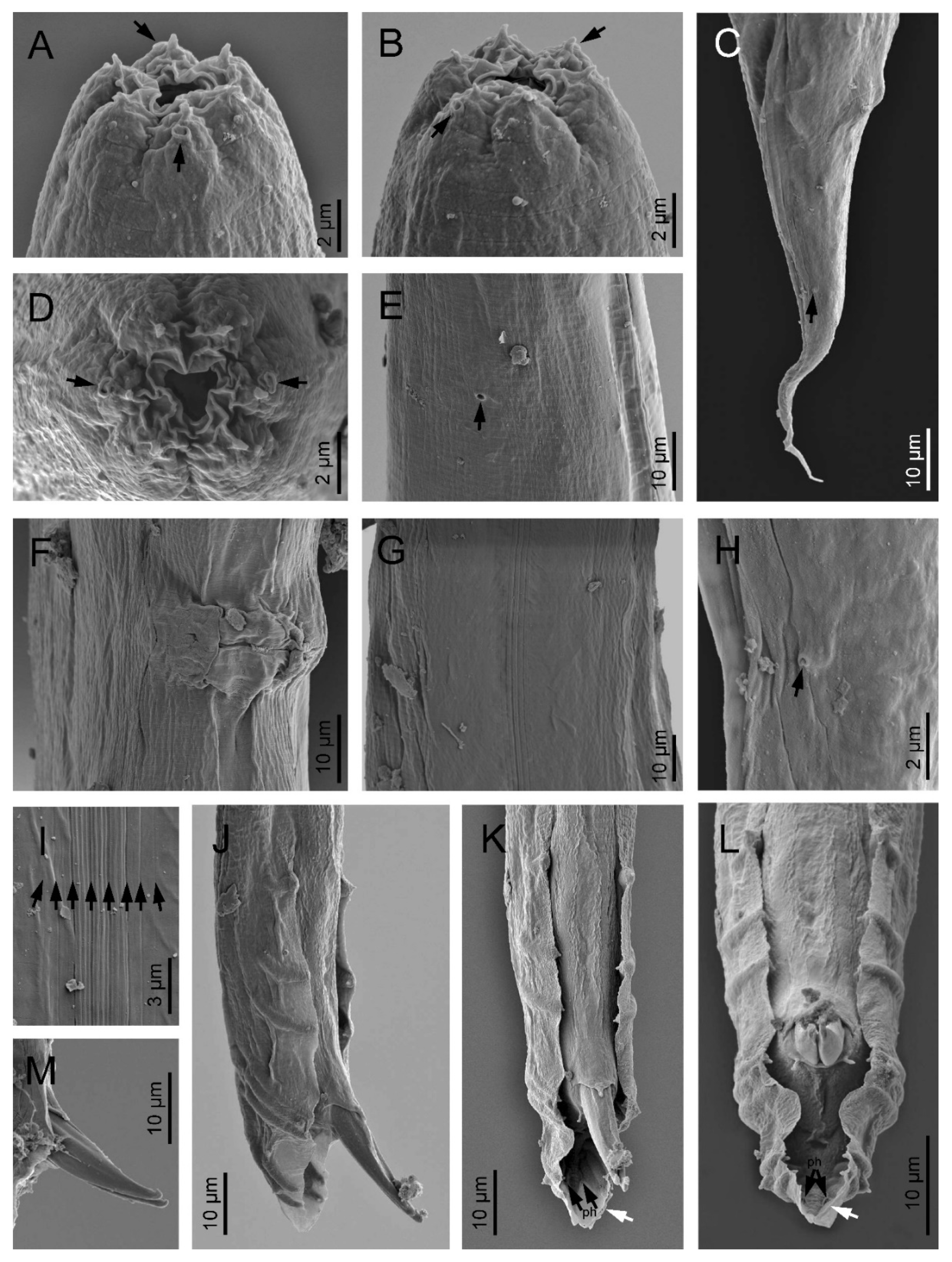
2.3. Scanning Electron Microscopy
2.4. Molecular Analyses
2.5. Sequence Alignment and Phylogenetic Analyses
2.6. Analysis of the Cytochrome Oxidase I Gene
3. Results
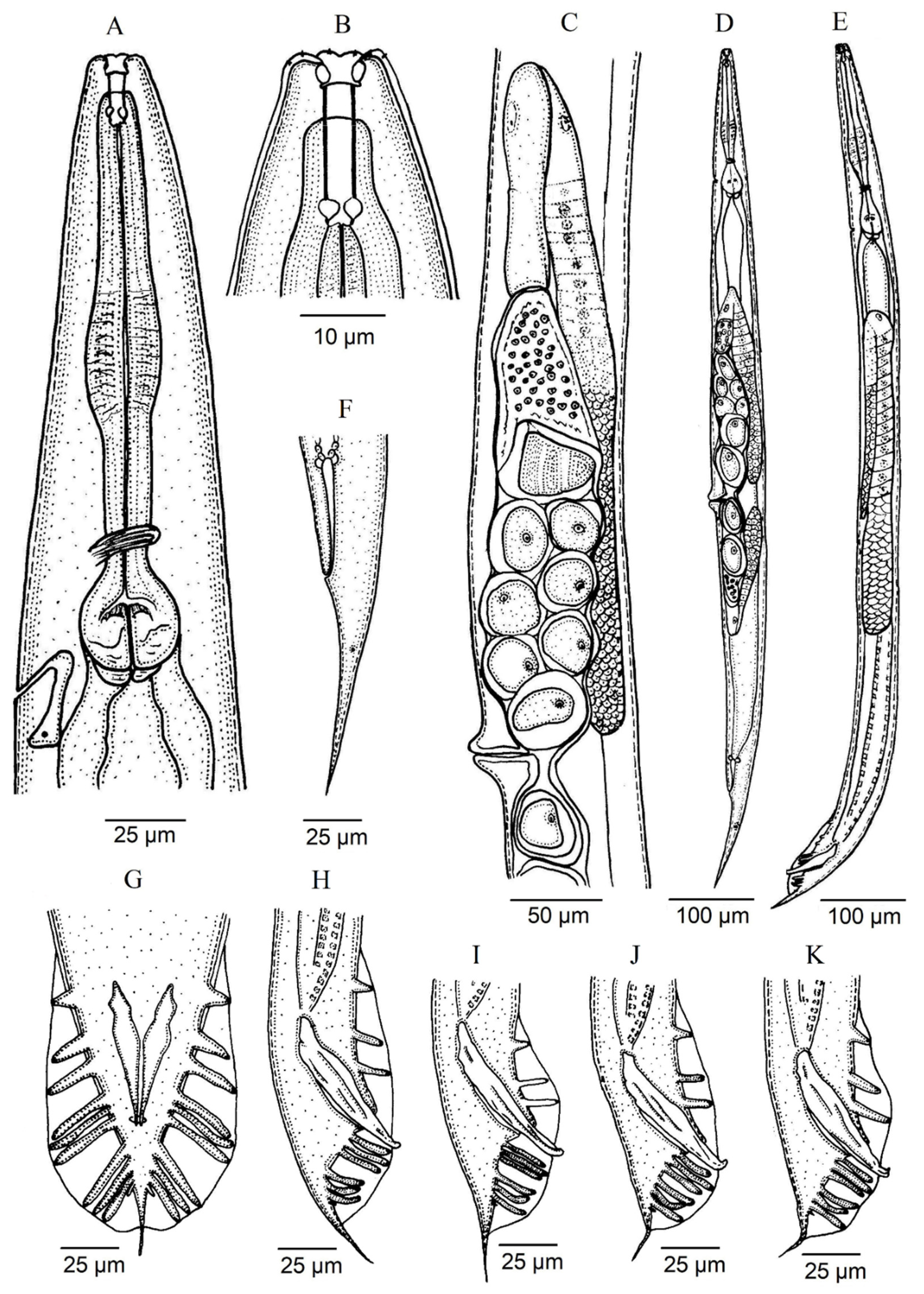
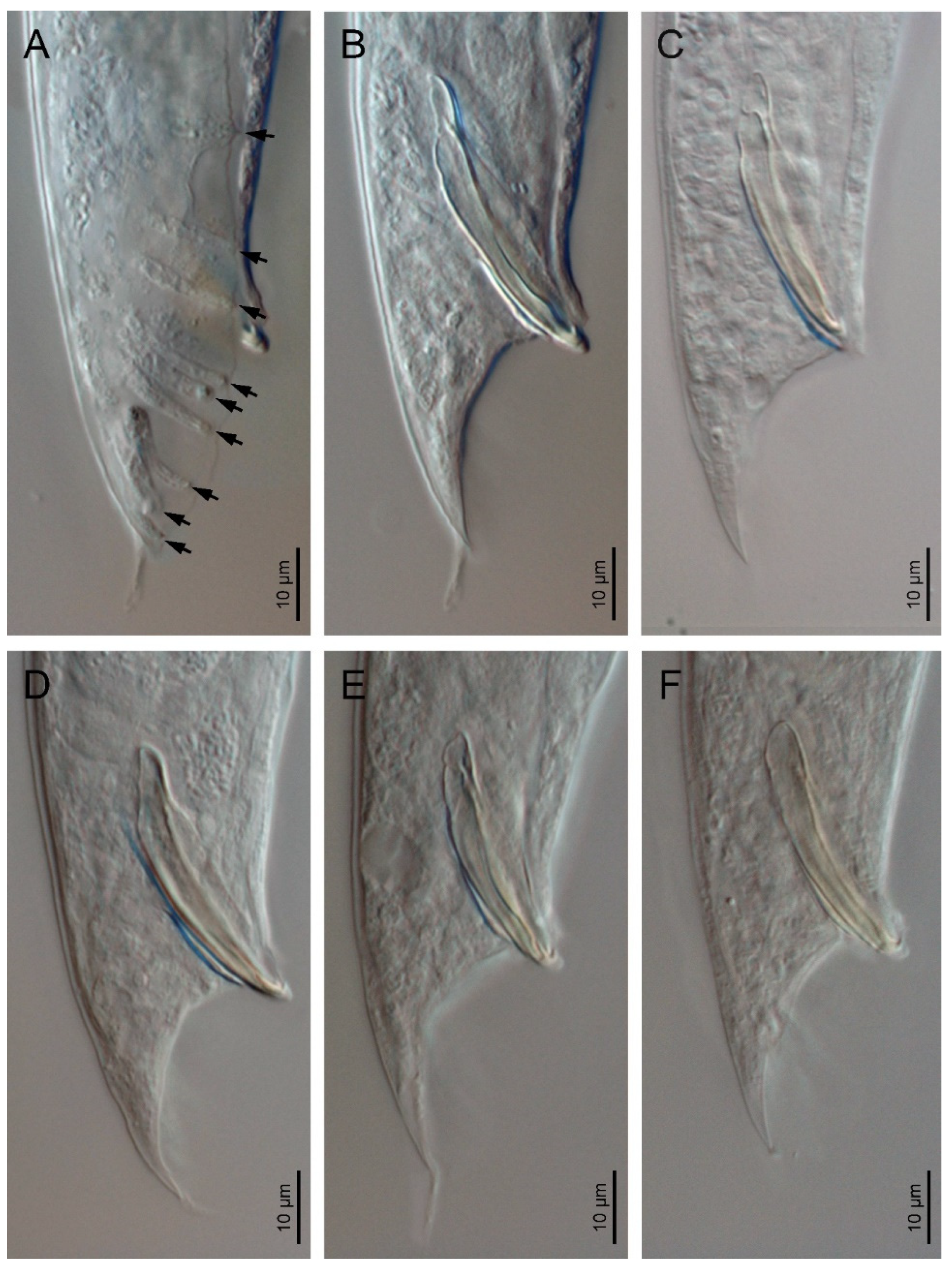
3.1. Description of Oscheius (Oscheius) siddiqii Tabassum & Shahina, 2010
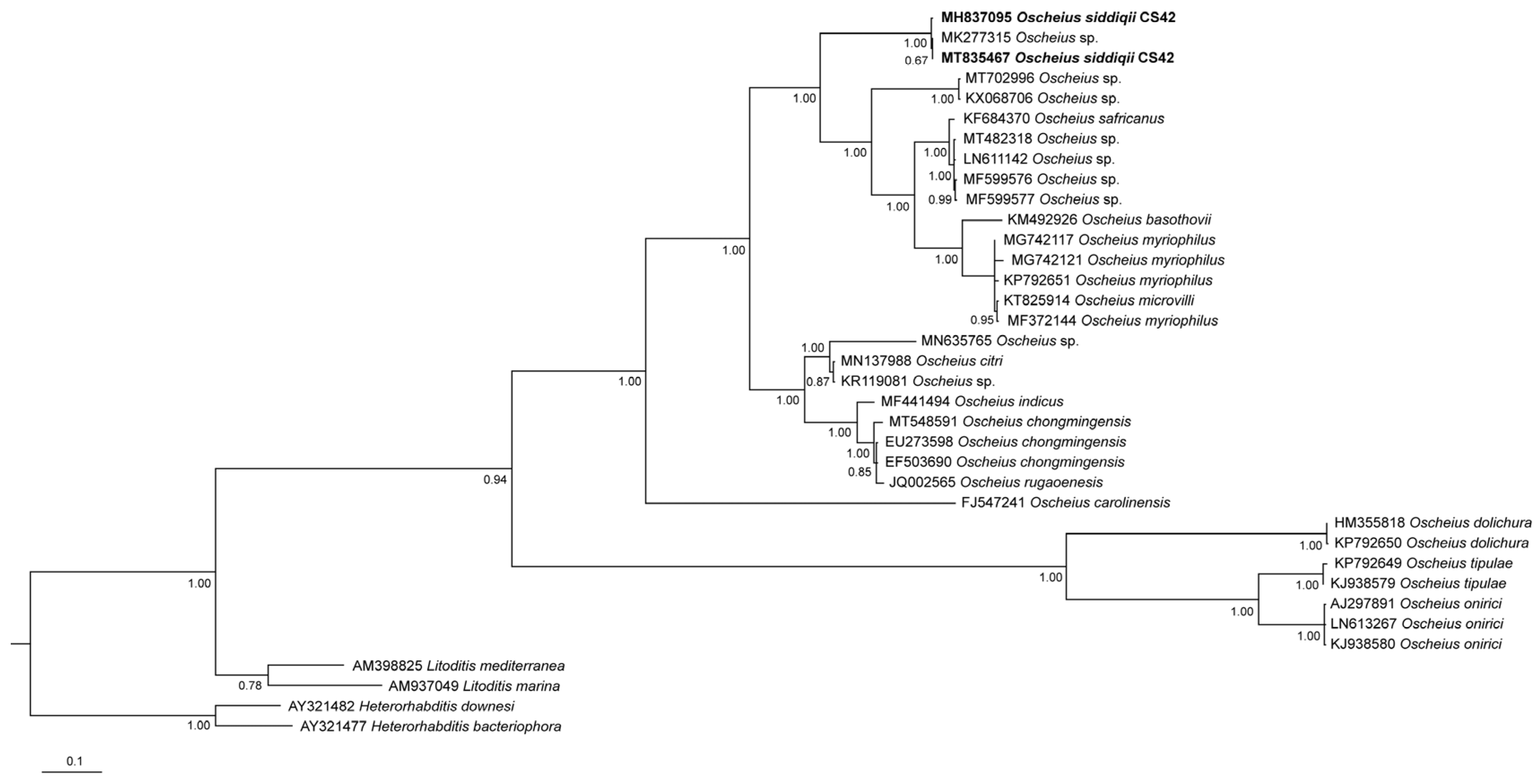
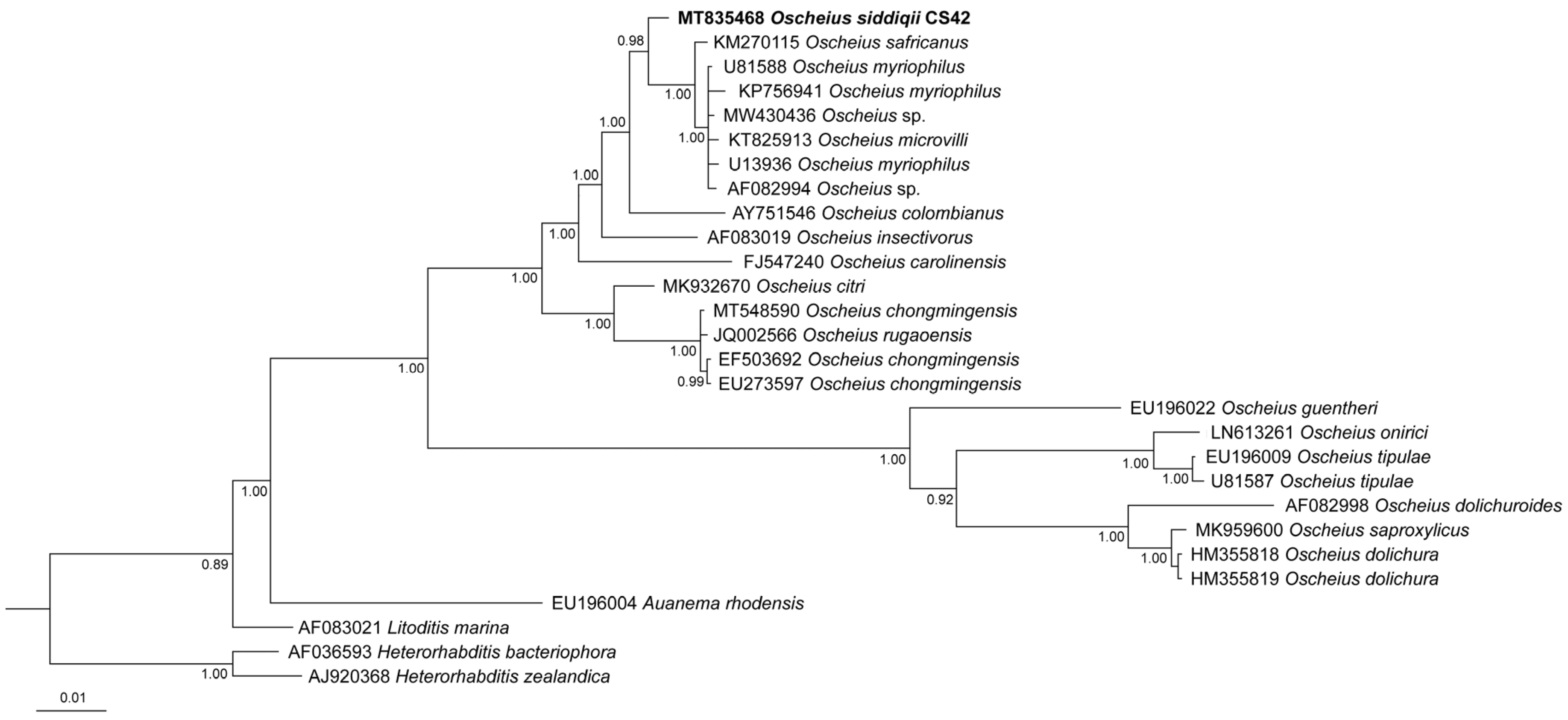
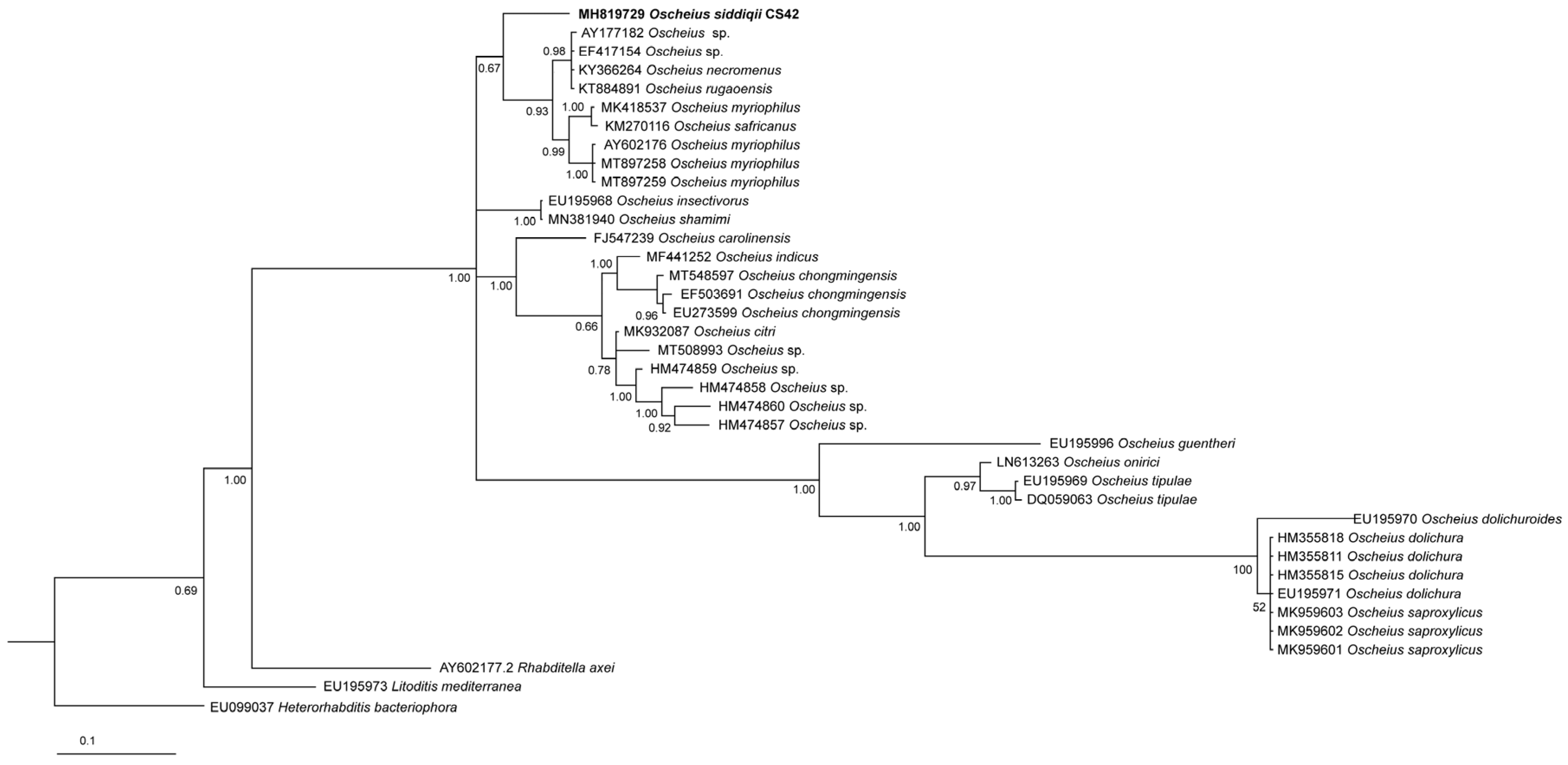
3.2. Molecular Characterization
4. Discussion
4.1. Remarks
4.2. Diagnosis (Based on Indian Populations)
4.3. Phylogenetic Relationships of the Species of the Subgenus Oscheius
4.3.1. Oscheius basothovii and O. safricanus
4.3.2. Oscheius microvilli
4.3.3. Oscheius rugaoensis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrássy, I. Evolution as a Basis for the Systematization of Nematodes; Pitman Publishing Ltd.: London, UK, 1976. [Google Scholar]
- Sudhaus, W. Vergleichende Untersuchungen zur Phylogenie, Systematik, Ökologie, Biologie und Ethologie der Rhabditidae (Nematoda); Schweizerbart Science Publishers: Stuttgart, Germany, 1976. [Google Scholar]
- Sudhaus, W. Phylogenetic systematisation and catalogue of paraphyletic “Rhabditidae” (Secernentea, Nematoda). J. Nematode Morphol. Syst. 2011, 14, 113–178. [Google Scholar]
- Abolafia, J.; Peña-Santiago, R. Morphological and molecular characterization of Oscheius saproxylicus sp. n. (Rhabditida, Rhabditidae) from decaying wood in Spain, with new insights into the phylogeny of the genus and a revision of its taxonomy. J. Nematol. 2019, 51, e2019-53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrássy, I. A Taxonomic Review of the Suborder Rhabditina (Nematoda: Secernentia); ORSTOM: Paris, France, 1983. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary: Nematoda Errantia; Hungarian Natural History Museum: Budapest, Hungary, 2005; Volume 1. [Google Scholar]
- Rana, A.; Bhat, A.; Chaubey, A.; Půža, V.; Abolafia, J. Redescription and synonymization of Oscheius citri Tabassum, Shahina, Nasira and Erum, 2016 (Rhabditida, Rhabditidae) from India and its taxonomical consequences. J. Helminthol. 2021, 95, e24. [Google Scholar] [CrossRef]
- Adams, B.J.; Dillman, A.R.; Finlinson, C. Molecular taxonomy and phylogeny. In Root-Knot Nematodes; CABI: Egham, UK, 2009; pp. 119–138. [Google Scholar]
- Landa, B.B.; Rius, J.E.P.; Vovlas, N.; Carneiro, R.M.; Maleita, C.M.; de Abrantes, I.M.; Castillo, P. Molecular characterization of Meloidogyne hispanica (Nematoda, Meloidogynidae) by Phylogenetic analysis of genes within the rDNA in Meloidogyne spp. Plant Dis. 2008, 92, 1104–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugall, A.; Stanton, J.; Moritz, C. Reticulate Evolution and the origins of ribosomal internal transcribed spacer diversity in apomictic Meloidogyne. Mol. Biol. Evol. 1999, 16, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Půža, V.; Chundelová, D.; Nermuť, J.; Žurovcová, M.; Mráček, Z. Intra-individual variability of its regions in entomopathogenic nematodes (Steinernematidae: Nematoda): Implications for their taxonomy. BioControl 2015, 60, 547–554. [Google Scholar] [CrossRef]
- Kiewnick, S.; Holterman, M.; van den Elsen, S.; van Megen, H.; Frey, J.E.; Helder, J. Comparison of two short DNA barcoding loci (COI and COII) and two longer ribosomal DNA genes (SSU & LSU rRNA) for specimen identification among quarantine root-knot nematodes (Meloidogyne spp.) and their close relatives. Eur. J. Plant Pathol. 2014, 140, 97–110. [Google Scholar]
- Torrini, G.; Mazza, G.; Carletti, B.; Benvenuti, C.; Roversi, P.F.; Fanelli, E.; De Luca, F.; Troccoli, A.; Tarasco, E. Oscheius onirici sp. n. (Nematoda: Rhabditidae): A new entomopathogenic nematode from an italian cave. Zootaxa 2015, 3937, 533–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foye, S.; Steffan, S.A. A rare, recently discovered nematode, Oscheius onirici (Rhabditida: Rhabditidae), kills Drosophila suzukii (Diptera: Drosophilidae) within fruit. J. Econ. Entomol. 2020, 113, 1047–1051. [Google Scholar] [CrossRef]
- Bedding, R.; Akhurst, R. A Simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 1975, 21, 109–110. [Google Scholar] [CrossRef]
- Tabassum, K.; Shahina, F. Oscheius siddiqii and O. niazii, Two new entomopathogenic nematode species from pakistan, with observations on O. shamimi. Int. J. Nematol. 2010, 20, 75–84. [Google Scholar]
- Bhat, A.H.; Chaubey, A.K.; Hartmann, J.; Půža, V. Notes on the morphology, bionomics, distribution and efficacy of Steinernema siamkayai (Rhabditida: Steinernematidae) from Western Uttar Pradesh, India. Nematology 2021, 1, 817–836. [Google Scholar] [CrossRef]
- Bhat, A.H.; Chaubey, A.K.; Abolafia, J. Morphological and molecular characterisation of Distolabrellus veechi (Rhabditida: Mesorhabditidae) from India. Nematology 2020, 22, 439–452. [Google Scholar]
- Bhat, A.H.; Istkhar, A.K.C.; Půža, V.; San-Blas, E. First report and comparative study of Steinernema surkhetense (Rhabditida: Steinernematidae) and its symbiont bacteria from subcontinental india. J. Nematol. 2017, 49, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney, W.D.; Polley, D.; Miller, V. TAF, an improved fixative in nematode technique. Plant Dis. Rep. 1955, 39, 570–571. [Google Scholar]
- Seinhorst, J. A Rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef] [Green Version]
- Poinar, G.O. Entomogenous Nematodes: A Manual and Host List of Insect-Nematode Associations; Brill Archive: Leiden, The Nertherlands, 1975; ISBN 90-04-04240-7. [Google Scholar]
- Bhat, A.; Chaubey, A.; Půža, V. The first report of Xenorhabdus indica from Steinernema pakistanense: Co-phylogenetic study suggests co-speciation between X. indica and its steinernematid nematodes. J. Helminthol. 2019, 93, 81–90. [Google Scholar] [CrossRef]
- Abolafia, J. A low-cost technique to manufacture a container to process meiofauna for scanning electron microscopy. Microsc. Res. Tech. 2015, 78, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Vrain, T.; Wakarchuk, D.; Levesque, A.; Hamilton, R. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Nguyen, K.B. Methodology, morphology and identification. In Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts; Brill: Leiden, The Nertherlands, 2007; pp. 59–119. ISBN 90-474-2239-2. [Google Scholar]
- Liu, J.; Berry, R.E.; Moldenke, A.F. Phylogenetic relationships of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) inferred from partial 18s rRNA gene sequences. J. Invertebr. Pathol. 1997, 69, 246–252. [Google Scholar] [CrossRef]
- Blaxter, M.L.; De Ley, P.; Garey, J.R.; Liu, L.X.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; Frisse, L.M. A molecular evolutionary framework for the phylum nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef]
- Mráček, Z.; Půža, V.; Nermut, J. Steinernema poinari sp. n.(Nematoda: Steinernematidae) a new entomopathogenic nematode from the Czech Republic. Zootaxa 2014, 3760, 336–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ley, P.; Blaxter, M. Systematic position and phylogeny. In The Biology of Nematodes; Taylor & Francis: Singapore, 2002; pp. 1–30. [Google Scholar]
- Bhat, A.H.; Askary, T.H.; Ahmad, M.J.; Chaubey, A.K. Description of Heterorhabditis bacteriophora (Nematoda: Heterorhabditidae) Isolated from hilly areas of Kashmir valley. Egypt. J. Biol. Pest Control 2019, 29, 96. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT; ScienceOpen: Berlin, Germany, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar]
- Nylander, J. MrModeltest 2.0; Program Distributed by the Author; Department of Systematic Zoology, Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Maddison, W.; Maddison, D. Mesquite, version 3.51; A Modular System for Evolutionary Analysis. 2018; Chelsea Green Publishing: Chelsea, VT, USA, 2019. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit i from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Sudhaus, W.; Kiontke, K. Comparison of the cryptic nematode species Caenorhabditis brenneri sp. n. and C. remanei (Nematoda: Rhabditidae) with the stem species pattern of the Caenorhabditis elegans group. Zootaxa 2007, 1456, 45–62. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, N.; Ragsdale, E.J.; Herrmann, M.; Mayer, W.E.; Sommer, R.J. Description of three Pristionchus species (Nematoda: Diplogastridae) from Japan that form a cryptic species complex with the model organism P. pacificus. Zoolog. Sci. 2012, 29, 403–417. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Cantalapiedra-Navarrete, C.; Castillo, P. Cryptic species in plant-parasitic nematodes. Nematology 2014, 16, 1105–1118. [Google Scholar] [CrossRef]
- Carstens, B.C.; Pelletier, T.A.; Reid, N.M.; Satler, J.D. How to fail at species delimitation. Mol. Ecol. 2013, 22, 4369–4383. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, M.; Nguyen, K.B.; Hunt, D.J.; Ehlers, R.-U.; Spiridonov, S.E.; Subbotin, S.A. Molecular identification, phylogeny and phylogeography of the entomopathogenic nematodes of the genus Heterorhabditis Poinar, 1976: A multigene approach. Nematology 2020, 1, 451–466. [Google Scholar] [CrossRef]
- Wu, S.G.; Wang, G.T.; Xi, B.W.; Xiong, F.; Liu, T.; Nie, P. Population genetic structure of the parasitic nematode Camallanus cotti inferred from DNA sequences of ITS1 rDNA and the mitochondrial COI gene. Vet. Parasitol. 2009, 164, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Rashidifard, M.; Marais, M.; Daneel, M.S.; Mienie, C.M.; Fourie, H. Molecular characterisation of Meloidogyne enterolobii and other Meloidogyne spp. from South Africa. Trop. Plant Pathol. 2019, 44, 213–224. [Google Scholar] [CrossRef]
- Serepa-Dlamini, M.H.; Gray, V.M. A new species of entomopathogenic nematode Oscheius safricana n. sp. (Nematoda: Rhabditidae) from South Africa. Arch. Phytopathol. Plant Prot. 2018, 51, 309–321. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Liu, X.H.; Tan, J.; Wang, Y.; Qiao, L.; Yedid, G.; Dai, C.S.; Qiu, R.L.; Yan, X.W.; Tan, H.W. Heterorhabditidoides rugaoensis n. sp. (Rhabditida: Rhabditidae), a novel highly pathogenic entomopathogenic nematode member of Rhabditidae. J. Nematol. 2012, 44, 348. [Google Scholar]
- Lephoto, T.E.; Gray, V.M. Oscheius basothovii n. sp. (Nematoda: Rhabditidae), a new entomopathogenic ematode isolated from an uncultivated grassland in South Africa. Arch. Phytopathol. Plant Prot. 2019, 52, 125–140. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, H.; Wang, F.; Bao, H.; Wang, G.; Hou, X.; Lin, J.; Yedid, G.; Zhang, K. Oscheius microvilli n. sp. (Nematoda: Rhabditidae): A facultatively pathogenic nematode from Chongming Island, China. J. Nematol. 2017, 49, 33. [Google Scholar] [CrossRef] [Green Version]
- Carta, L.; Thomas, W.; Meyer-Rochow, V. Two nematodes (Nematoda: Diplogastridae, Rhabditidae) from the invasive millipede Chamberlinius hualienensis Wang, 1956 (Diplopoda, Paradoxosomatidae) on Hachijojima Island in Japan. J. Nematol. 2018, 50, 479. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, A.; Goldasteh, S.; Rafiei-Karahroodi, Z.; Pedram, M. The occurrence of three species of the genus Oscheius Andrássy, 1976 (Nematoda: Rhabditida) in Iran. J. Plant Prot. Res. 2017, 57, 248–255. [Google Scholar] [CrossRef] [Green Version]

| Characters | Female | Male | J3 Juvenile |
|---|---|---|---|
| n | 20 | 20 | 20 |
| Body length (L) | 1465 ± 135 (1204–1697) | 1135 ± 126 (916–1441) | 534 ± 21 (502–574) |
| a (L/MBD) | 16.7 ± 1.2 (14.2–19.4) | 24 ± 4.7 (14.7–25.8) | 21 ± 1.0 (19.3–22.7) |
| b (L/NL) | 7.7 ± 0.9 (6.1–8.9) | 6.7 ± 0.6 (5.3–7.7) | 4.3 ± 0.3 (3.7–4.9) |
| c (L/T) | 10.4 ± 1.5 (7.8–15.4) | 28 ± 3.1 (20.1–32.9) | 7.4 ± 0.6 (7.0–8.2) |
| c’ (T/ABW) | 4.8 ± 0.8 (3.5–6.3) | 1.8 ± 0.2 (1.5–2.5) | 6.0 ± 0.7 (4.9–7.2) |
| V% (AV/L × 100) | 50 ± 3.1 (45–57) | – | – |
| Lip region width | 7.1 ± 0.6 (6–8) | 7.1 ± 0.8 (6–9) | 4.1 ± 0.8 (3–6) |
| Stoma length | 17.1 ± 1.1 (15–20) | 17.1 ± 2.1 (13–20) | 13.9 ± 1.6 (10–17) |
| Stomatal tube width | 3.1 ± 0.3 (2.5–3.8) | 4.0 ± 0.7 (3.2–5.4) | – |
| Procorpus length | 64 ± 3.4 (58–69) | 58 ± 5.6 (47–66) | 42 ± 2.6 (37–46) |
| Metacorpus length | 38 ± 2.9 (32–42) | 33 ± 1.6 (31–36) | 21 ± 2.1 (18–23) |
| Isthmus length | 64 ± 3.4 (58–69) | 45 ± 3.3.6 (37–50) | 29 ± 2.2 (37–46) |
| Bulb length | 35 ± 3.5 (24–34) | 31 ± 3.0 (27–37) | 21 ± 2.1 (18–23) |
| Pharynx length | 183 ± 6.6 (169–198) | 165 ± 7.3 (139–173) | 106 ± 7.5 (95–127) |
| Nerve ring—ant. end (NR) | 153 ± 10.6 (133–175) | 138 ± 10.6 (119–178) | 73 ± 6.9 (61–81) |
| Excretory pore– ant. end (EP) | 191 ± 17 (171–224) | 189 ± 22.4 (162–212) | 120 ± 6.6 (100–132) |
| Deirid–ant. end | 163 ± 13.0 (141–196) | 135 ± 13.6 (106–156) | – |
| Neck length (stoma + pharynx, NL) | 221 ± 8.0 (205–239) | 183± 7.2 (172–192) | 125 ± 4.8 (116–136) |
| Body width at neck base | 54 ± 6.3 (42–66) | 42 ± 3.8 (36–51) | 28 ± 2.1 (25–31) |
| Mid-body diameter (MBD) | 94 ±7.5 (81–114) | 49 ± 7.7 (38–64) | 25 ± 1.4 (23–28) |
| Uterus or testis length | 69 ± 15.5 (53–87) | 557 ± 36 (464–596) | – |
| Anterior spermatheca length | 44 ± 8.0 (31–63) | – | – |
| Anterior genital branch | 313 ± 50 (213–392) | – | – |
| Posterior spermatheca length | 39 ± 7.4 (31–54) | – | – |
| Posterior genital branch | 257 ± 40 (202–329) | – | – |
| Vagina length | 24 ± 4.1 (18–31) | – | – |
| Vulva—ant. end (AV) | 732 ± 57 (610–860) | – | – |
| Rectum length | 70 ± 9.8 (57–81) | – | 31 ± 2.6 (26–39) |
| Anal body diam. (ABD) | 32 ± 2.9 (26–41) | 21 ± 2.9 (19–26) | 12.8 ± 1.3 (11–16) |
| Tail length (T) | 147 ± 20 (123–169) | 41 ± 3.3 (38–48) | 76 ± 10.1 (59–98) |
| Phasmid to anus distance | 42 ± 6.3 (33–57) | 24 ± 1.2 (21–26) | – |
| Spicule length (SL) | – | 44 ± 5.1 (39–53) | – |
| Gubernaculum length (GL) | – | 21 ± 3.1 (22–26) | – |
| Hyaline part of tail (H) | – | – | 41 ± 6.3 (36–49) |
| Characters | Female | Male | J3 Juvenile |
|---|---|---|---|
| n | 20 | 20 | 20 |
| Body length (L) | 1439 ± 139 (1121–1586) | 1021 ± 77 (920–1180) | 551 ± 28 (500–598) |
| a (L/MBD) | 16.9 ± 1.8 (13.6–19.2) | 23 ± 2.1 (19.9–26.7) | 19.9 ± 1.3 (17.7–22.4) |
| b (L/NL) | 72 ± 0.9 (5.6–8.2) | 6.0 ± 0.6 (5.1–7.2) | 4.2 ± 0.2 (3.8–4.8) |
| c (L/T) | 8.3 ± 0.8 (6.3–9.5) | 25 ± 2.5 (19.7–30.5) | 6.9 ± 0.7 (5.7–8.3) |
| c’ (T/ABD) | 6.6 ± 0.7 (5.2–7.5) | 1.8 ± 0.3 (1.4–2.5) | 5.9 ± 0.7 (4.8–7.7) |
| V% (AV/L × 100) | 52 ± 11.2 (48–61) | – | – |
| Lip region width | 6.1 ± 0.6 (6–7) | 5.8 ± 0.8 (5–7) | 3.5 ± 0.6 (2–5) |
| Stoma length | 15.9 ± 1.6 (13–19) | 14.9 ± 1.6 (13–20) | 13.8 ± 2.0 (10–17) |
| Stomatal tube width | 3.2 ± 0.8 (2–5) | 3.1 ± 0.5 (2–4) | – |
| Procorpus length | 61 ± 6.0 (53–68) | 54 ± 6.1 (45–69) | 41 ± 1.4 (37–48) |
| Metacorpus length | 36 ± 3.3 (31–41) | 32 ± 3.4 (27–39) | 24 ± 1.6 (21–27) |
| Isthmus length | 50 ± 2.3 (46–53) | 38 ± 5.0 (44–48) | 33 ± 3.1 (30–41) |
| Bulb length | 30 ± 2.0 (28–34) | 29 ± 3.0 (24–35) | 18.9 ± 1.7 (17–24) |
| Pharynx length | 177 ± 4.7 (169–183) | 153 ± 9.8 (139–173) | 117 ± 4.9 (108–129) |
| Nerve ring—ant. end (NR) | 154 ± 12.9 (138–181) | 149 ± 8.0 (130–162) | 82 ± 4.9 (70–89) |
| Excretory pore—ant. end (EP) | 197 ± 13 (172–216) | 195 ± 14.1 (162–220) | 118 ± 5.6 (108–129) |
| Deirid—ant. end | 159 ±10.0 (139–170) | 133 ± 8.4 (118–149) | – |
| Neck length (stoma + pharynx, NL) | 196 ± 4.4 (189–201) | 171 ± 10.1 (158–190) | 131 ± 4.7 (123–143) |
| Body width at neck base | 34 ± 2.7 (30–38) | 35 ± 4.8 (26–42) | 27 ± 2.6 (23–34) |
| Mid-body diameter (MBD) | 81 ± 12 (72–98) | 44 ± 5.6 (38–58) | 28 ± 1.4 (25–30) |
| Uterus or testis length | 74.1 ± 15.3 (56–98) | 539 ± 40 (545–590) | – |
| Anterior spermatheca length | 38 ± 5.1 (32–49) | – | – |
| Anterior genital branch | 275 ± 41 (210–343) | – | – |
| Posterior spermatheca length | 37 ± 5.0 (28–44) | – | – |
| Posterior genital branch | 240 ± 30 (204–290) | – | – |
| Vagina length | 23 ± 2.6 (19–28) | – | – |
| Body width at vulva | 77 ± 11.2 (59–109) | – | – |
| Vulva—ant. end (AV) | 741 ± 74 (568–829) | – | – |
| Rectum length | 67 ± 4.6 (61–75) | – | 35 ± 2.8 (28–39) |
| Anal body diam. (ABD) | 27 ± 3.0 (22–34) | 23 ± 1.9 (20–27) | 14 ± 1.5 (11–16) |
| Tail length (T) | 173 ± 6.2 (154–180) | 42 ± 4.7 (36–49) | 81 ± 7.7 (70–96) |
| Phasmid to anus distance | 39 ± 4.8 (34–50) | 22 ± 3.6 (17–26) | – |
| Spicule length (SL) | – | 51 ± 4.9 (37–55) | – |
| Gubernaculum length (GL) | – | 25 ± 2.7 (21–26) | – |
| Hyaline part of tail (H) | – | – | 42 ± 3.2 (36–47) |
| S. No. | COI Region | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OK142792 O. siddiqii CS42 | 92.9 | 92.9 | 91.0 | 97.3 | 89.1 | 89.4 | 88.5 | 89.9 | 88.1 | 88.1 | |
| 2 | OK142795 O. myriophilus 1b | 44 | 99.9 | 89.7 | 92.5 | 89.2 | 89.8 | 89.7 | 90.2 | 88.7 | 88.7 | |
| 3 | OK142794 O. myriophilus JU1386 | 44 | 1 | 89.6 | 92.6 | 89.4 | 89.4 | 89.2 | 89.7 | 88.3 | 88.3 | |
| 4 | OK142796 O. citri WGN | 56 | 64 | 65 | 90.4 | 86.8 | 89.4 | 88.1 | 88.9 | 87.3 | 87.3 | |
| 5 | OK142793 O. chongmingensis | 17 | 51 | 57 | 60 | 88.9 | 89.2 | 88.4 | 89.5 | 88.1 | 88.1 | |
| 6 | OK142797 O. guentheri | 68 | 73 | 81 | 82 | 85 | 88.0 | 87.4 | 88.3 | 87.4 | 87.4 | |
| 7 | MF196100.1 O. onirici 16-33834 | 46 | 50 | 55 | 46 | 56 | 62 | 99.2 | 99.7 | 98.6 | 98.6 | |
| 8 | MK754223.1 O. onirici N6691 | 48 | 49 | 54 | 50 | 58 | 63 | 5 | 99.7 | 98.4 | 98.4 | |
| 9 | KY582595.1 O. onirici Wisconsin 6 | 40 | 44 | 49 | 44 | 50 | 56 | 2 | 2 | 98.3 | 98.3 | |
| 10 | LN613269.1 O. onirici FDL-2014 | 44 | 48 | 53 | 47 | 54 | 57 | 8 | 10 | 10 | 100 | |
| 11 | LN613268.1 O. onirici FDL-2014 | 44 | 48 | 53 | 47 | 54 | 57 | 8 | 10 | 10 | 0 |
| S. No. | SSU Region | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | U81588 O. myriophilus | 99.8 | 100 | 99.8 | 96.0 | 99.9 | 99.6 | 96.9 | 97.1 | 96.8 | 96.7 | 98.9 | 97.5 | 97.7 | 97.0 | 96.4 | |
| 2 | KP756941 O. myriophilus | 3 | 99.7 | 99.6 | 95.8 | 99.8 | 99.3 | 96.7 | 96.9 | 96.7 | 96.5 | 98.7 | 97.3 | 97.5 | 96.8 | 96.2 | |
| 3 | MW430436.1 O. myriophilus | 0 | 3 | 100 | 98.2 | 99.9 | 99.7 | 96.8 | 96.8 | 96.8 | 96.7 | 99.0 | 97.2 | 98.5 | 97.1 | 97.9 | |
| 4 | U13936 O. myriophilus | 3 | 6 | 0 | 95.9 | 99.8 | 99.6 | 96.8 | 96.9 | 96.7 | 96.6 | 98.8 | 97.4 | 97.6 | 96.9 | 96.3 | |
| 5 | KT825913 O. microvilli | 63 | 66 | 17 | 65 | 95.9 | 97.8 | 93.7 | 94.7 | 93.7 | 93.7 | 96.0 | 93.9 | 93.9 | 94.7 | 93.1 | |
| 6 | AF082994 O. myriophilus | 1 | 4 | 1 | 4 | 64 | 99.6 | 96.8 | 97.0 | 96.8 | 96.7 | 98.9 | 97.5 | 97.7 | 97.0 | 96.4 | |
| 7 | KM270115 O. safricanus | 4 | 7 | 2 | 4 | 22 | 4 | 96.3 | 96.3 | 96.2 | 96.2 | 98.7 | 96.9 | 97.0 | 96.6 | 96.9 | |
| 8 | MT548590 O. chongmingensis | 51 | 54 | 30 | 53 | 98 | 52 | 37 | 99.9 | 99.9 | 99.9 | 96.4 | 96.5 | 96.2 | 98.3 | 95.9 | |
| 9 | EF503692 O. chongmingensis | 45 | 48 | 30 | 47 | 82 | 46 | 37 | 1 | 100 | 99.9 | 96.7 | 96.5 | 96.3 | 98.4 | 95.9 | |
| 10 | EU273597 O. chongmingensis | 53 | 56 | 30 | 55 | 99 | 54 | 38 | 1 | 0 | 99.9 | 96.3 | 96.3 | 95.9 | 98.3 | 95.8 | |
| 11 | JQ002566 O. rugaoensis | 52 | 55 | 31 | 54 | 99 | 53 | 38 | 1 | 2 | 2 | 96.3 | 96.3 | 96.0 | 98.3 | 95.7 | |
| 12 | MT835468 O. siddiqii CS42 | 16 | 19 | 9 | 18 | 60 | 17 | 12 | 55 | 49 | 56 | 56 | 98.0 | 98.3 | 97.1 | 97.0 | |
| 13 | AF083019 O. insectivorus | 42 | 45 | 26 | 43 | 96 | 43 | 31 | 58 | 54 | 64 | 59 | 30 | 97.0 | 96.8 | 96.4 | |
| 14 | AY751546 O. colombianus | 38 | 41 | 14 | 40 | 95 | 39 | 30 | 63 | 57 | 69 | 64 | 26 | 50 | 96.6 | 96.8 | |
| 15 | MK932670 O. citri WGN | 46 | 49 | 27 | 48 | 81 | 47 | 33 | 26 | 24 | 27 | 27 | 44 | 50 | 52 | 96.1 | |
| 16 | FJ547240 O. carolinensis | 60 | 63 | 20 | 62 | 108 | 61 | 31 | 68 | 63 | 71 | 69 | 46 | 60 | 53 | 60 |
| S. No. | ITS Region | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MG742117 O. myriophilus | 97.6 | 99.4 | 99.3 | 99.4 | 85.7 | 89.2 | 73.2 | 74.6 | 75.4 | 73.8 | 64.9 | 75.6 | 76.7 | 79.7 | 78.8 | 73.1 | 86.5 | 70.0 | |
| 2 | MG742121 O. myriophilus | 23 | 97.6 | 97.6 | 98.2 | 84.4 | 88.6 | 73.9 | 74.1 | 74.9 | 74.1 | 64.1 | 75.7 | 76.1 | 79.9 | 79.7 | 72.3 | 85.3 | 69.4 | |
| 3 | KT825914 O. microvilli | 6 | 23 | 99.3 | 99.5 | 85.7 | 89.3 | 74.2 | 74.8 | 75.6 | 74.1 | 64.9 | 76.1 | 76.7 | 80.1 | 79.5 | 72.8 | 86.5 | 69.8 | |
| 4 | KP792651 O. myriophilus | 7 | 24 | 7 | 98.9 | 85.4 | 88.8 | 74.1 | 74.8 | 75.6 | 74.5 | 64.6 | 76.4 | 76.5 | 80.4 | 79.6 | 72.8 | 86.2 | 69.9 | |
| 5 | MF372144 O. myriophilus | 5 | 15 | 4 | 9 | 85.8 | 90.6 | 72.7 | 72.8 | 72.9 | 72.6 | 65.6 | 72.8 | 76.9 | 77.6 | 77.7 | 72.6 | 86.3 | 68.7 | |
| 6 | KF684370 O. safricanus | 115 | 125 | 115 | 117 | 112 | 83.8 | 72.4 | 72.6 | 72.7 | 72.4 | 67.3 | 73.3 | 76.9 | 77.7 | 77.7 | 74.1 | 98.5 | 67.6 | |
| 7 | KM492926 O. basothovii | 86 | 91 | 85 | 89 | 73 | 122 | 72.2 | 72.3 | 72.4 | 72.2 | 66.7 | 72.9 | 75.5 | 74.7 | 74.8 | 72.5 | 84.1 | 69.7 | |
| 8 | MT548591 O. chongmingensis | 260 | 247 | 245 | 250 | 216 | 217 | 214 | 98.2 | 98.6 | 98.1 | 76.0 | 88.8 | 77.4 | 79.5 | 79.9 | 93.3 | 74.1 | 69.1 | |
| 9 | EU273598 O. chongmingensis | 238 | 244 | 237 | 237 | 215 | 215 | 213 | 18 | 99.9 | 98.7 | 76.3 | 89.4 | 77.1 | 79.0 | 79.0 | 93.7 | 74.3 | 68.5 | |
| 10 | EF503690 O. chongmingensis | 228 | 234 | 227 | 227 | 214 | 214 | 212 | 14 | 1 | 99.1 | 76.4 | 89.5 | 77.2 | 79.2 | 79.2 | 93.8 | 74.4 | 68.9 | |
| 11 | JQ002565 O. rugaoensis | 248 | 248 | 246 | 247 | 217 | 217 | 214 | 20 | 13 | 9 | 76.2 | 89.2 | 76.9 | 79.3 | 79.5 | 93.6 | 74.0 | 68.8 | |
| 12 | MN635765 Oscheius sp. | 285 | 291 | 285 | 287 | 273 | 262 | 251 | 205 | 202 | 201 | 202 | 84.6 | 70.6 | 69.4 | 69.5 | 78.3 | 67.5 | 62.3 | |
| 13 | MN137988.2 O. citri | 229 | 236 | 226 | 228 | 220 | 214 | 213 | 111 | 105 | 104 | 108 | 135 | 79.1 | 81.4 | 81.5 | 89.9 | 74.9 | 70.0 | |
| 14 | MK277315 Oscheius sp. | 175 | 179 | 175 | 176 | 170 | 165 | 181 | 176 | 178 | 177 | 180 | 222 | 164 | 99.6 | 99.7 | 77.9 | 77.9 | 69.3 | |
| 15 | MT835467 O. siddiqii CS42 | 187 | 191 | 184 | 185 | 173 | 171 | 190 | 196 | 200 | 196 | 200 | 248 | 180 | 3 | 99.9 | 77.5 | 78.8 | 72.9 | |
| 16 | MH837095.2 O. siddiqii CS42 | 199 | 193 | 191 | 197 | 172 | 171 | 189 | 196 | 200 | 196 | 200 | 247 | 179 | 2 | 1 | 77.6 | 78.8 | 73.4 | |
| 17 | MF441494 O. indicus | 217 | 223 | 219 | 219 | 214 | 200 | 207 | 57 | 53 | 52 | 54 | 178 | 86 | 171 | 185 | 184 | 75.4 | 66.4 | |
| 18 | LN611142 Oscheius sp. | 113 | 123 | 113 | 115 | 111 | 12 | 125 | 212 | 210 | 209 | 212 | 262 | 209 | 165 | 169 | 169 | 198 | 69.1 | |
| 19 | FJ547241 O. carolinensis | 290 | 293 | 290 | 291 | 256 | 263 | 237 | 309 | 309 | 302 | 309 | 325 | 294 | 238 | 254 | 253 | 282 | 259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, A.H.; Gautum, S.; Rana, A.; Chaubey, A.K.; Abolafia, J.; Půža, V. Morphological, Morphometrical and Molecular Characterization of Oscheius siddiqii Tabassum and Shahina, 2010 (Rhabditida, Rhabditidae) from India with Its Taxonomic Consequences for the Subgenus Oscheius Andrássy, 1976. Biology 2021, 10, 1239. https://doi.org/10.3390/biology10121239
Bhat AH, Gautum S, Rana A, Chaubey AK, Abolafia J, Půža V. Morphological, Morphometrical and Molecular Characterization of Oscheius siddiqii Tabassum and Shahina, 2010 (Rhabditida, Rhabditidae) from India with Its Taxonomic Consequences for the Subgenus Oscheius Andrássy, 1976. Biology. 2021; 10(12):1239. https://doi.org/10.3390/biology10121239
Chicago/Turabian StyleBhat, Aashaq Hussain, Swati Gautum, Aasha Rana, Ashok Kumar Chaubey, Joaquín Abolafia, and Vladimír Půža. 2021. "Morphological, Morphometrical and Molecular Characterization of Oscheius siddiqii Tabassum and Shahina, 2010 (Rhabditida, Rhabditidae) from India with Its Taxonomic Consequences for the Subgenus Oscheius Andrássy, 1976" Biology 10, no. 12: 1239. https://doi.org/10.3390/biology10121239






