Characterization of Enlarged Kidneys and Their Potential for Inducing Diabetes in DEK Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Determination of Glomerular Number (GN)
2.3. Measurement of Glomerular Size
2.4. Nephrectomy
2.5. Immunofluorescence and Pathological Analysis of Kidney
2.6. Biochemical Analysis
2.7. Oral Glucose Tolerance Test (OGTT)
2.8. Statistical Analysis
3. Results
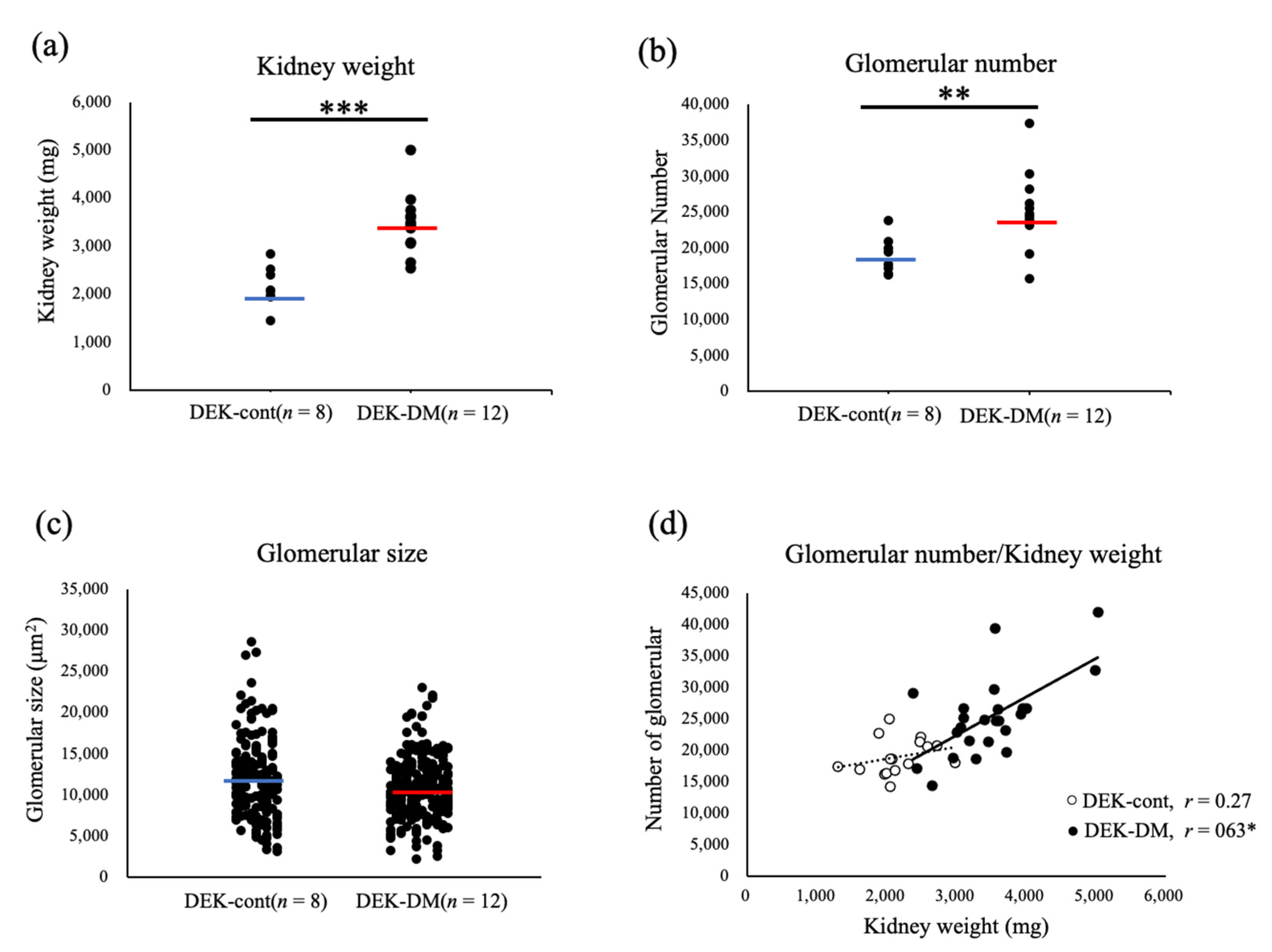
3.1. Characterization of Enlarged Kidneys in DEK-DM Rats
3.2. Relationship between Kidney Weight and Hyperglycemia
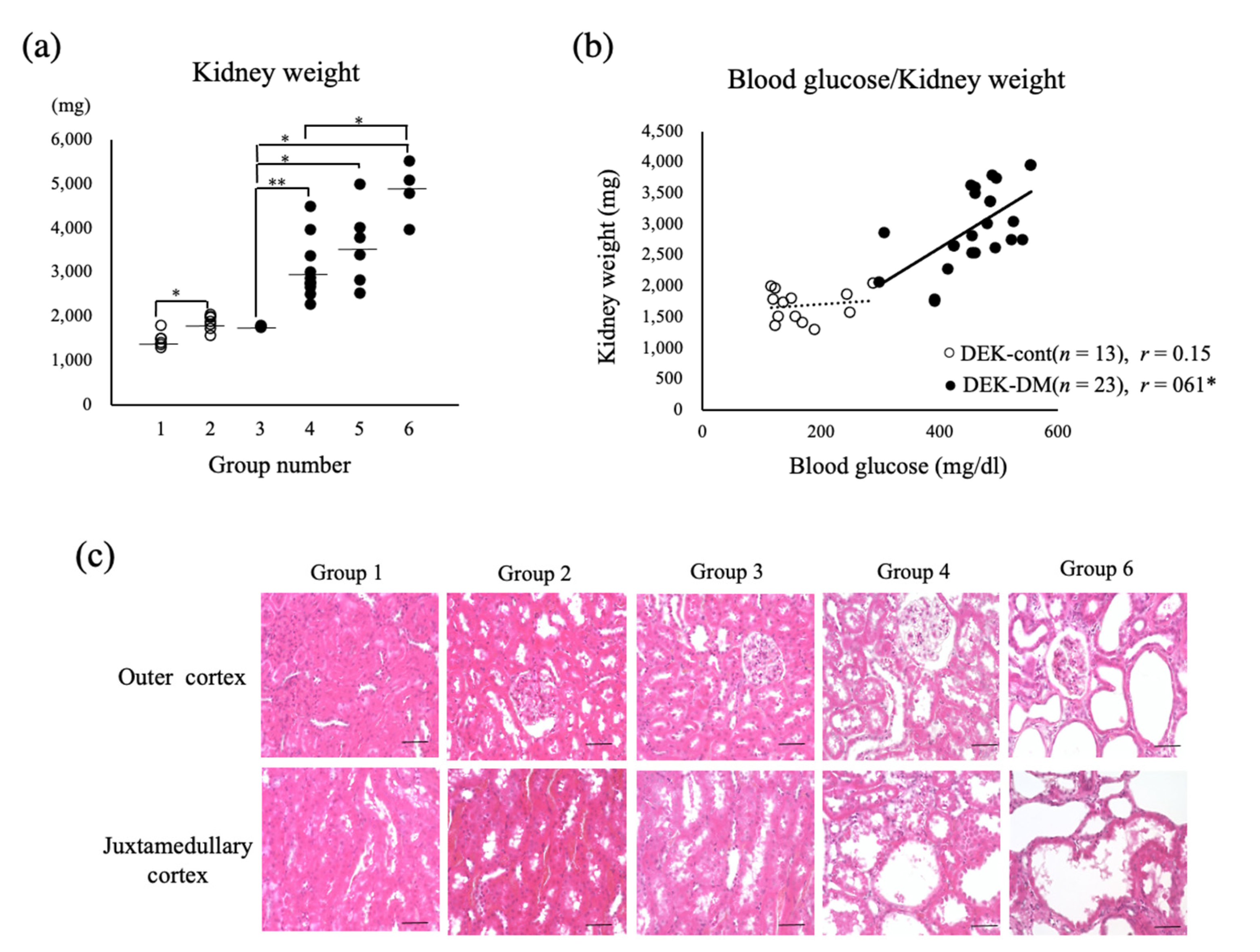
3.3. Effect of Uninephrectomy (1/2Nx) on General Conditions Related to Diabetes
3.4. Effect of Uninephrectomy on Biochemical Parameters
3.5. Histology of Nephrectomized Kidneys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [Green Version]
- Wilding, J.P. The role of the kidneys in glucose homeostasis in type 2 diabetes: Clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 2014, 63, 1228–1237. [Google Scholar] [CrossRef]
- Hinnen, D. The Role of the Kidney in Hyperglycemia. J. Cardiovasc. Nurs. 2013, 28, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefansson, B.V.; Chertow, G.M.; Correa-Rotter, R.; Greene, T.; Hou, F.-F.; Lindberg, M.; McMurray, J.; Rossing, P.; Toto, R.; et al. Rationale and protocol of the Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol. Dial. Transplant. 2020, 35, 274–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsahli, M.; Gerich, J.E. Renal glucose metabolism in normal physiological conditions and in diabetes. Diabetes Res. Clin. Pract. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- Rabkin, R.; Ryan, M.P.; Duckworth, W.C. The renal metabolism of insulin. Diabetologia 1984, 27, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Gerich, J.E.; Meyer, C.; Woerle, H.J.; Stumvoll, M. Renal Gluconeogenesis: Its importance in human glucose homeostasis. Diabetes Care 2001, 24, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Meyer, C.; Dostou, J.M.; Welle, S.L.; Gerich, J.E. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am. J. Physiol. Metab. 2002, 282, E419–E427. [Google Scholar] [CrossRef] [Green Version]
- Meyer, C.; Stumvoll, M.; Nadkarni, V.; Dostou, J.; Mitrakou, A.; Gerich, J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J. Clin. Investig. 1998, 102, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Sochor, M.; Kunjara, S.; Greenbaum, A.L.; McLean, P. Renal hypertrophy in experimental diabetes. Effect of diabetes on the pathways of glucose metabolism: Differential response in adult and immature rats. Biochem. J. 1986, 234, 573–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satriano, J. Kidney growth, hypertrophy and the unifying mechanism of diabetic complications. Amino Acids 2007, 33, 331–339. [Google Scholar] [CrossRef]
- Seyer-Hansen, K. Renal hypertrophy in experimental diabetes mellitus. Kidney Int. 1983, 23, 643–646. [Google Scholar] [CrossRef] [Green Version]
- Douglas-Denton, R.N.; McNamara, B.J.; Hoy, W.E.; Hughson, M.D.; Bertram, J.F. Does Nephron Number Matter in the Development of Kidney Disease? Ethn Dis. 2006, 16 (Suppl. 2), S2-40-5. [Google Scholar] [PubMed]
- Domon, A.; Katayama, K.; Tochigi, Y.; Suzuki, H. Characterization of Novel Nonobese Type 2 Diabetes Rat Model with Enlarged Kidneys. J. Diabetes Res. 2019, 2019, 8153140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Suzuki, K. Pathophysiology and postnatal pathogenesis of hypoplastic kidney (hpk/hpk) in the male hypogonadic mutant rat (hgn/hgn). J. Vet. Med Sci. 1995, 57, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Tokuriki, T.; Saito, K.; Hishida, A.; Suzuki, K. Glomerular hyperfiltration and hypertrophy in the rat hypoplastic kidney as a model of oligomeganephronic disease. Nephrol. Dial. Transplant. 2005, 20, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Katsuda, Y.; Kemmochi, Y.; Maki, M.; Sano, R.; Toriniwa, Y.; Ishii, Y.; Miyajima, K.; Kakimoto, K.; Ohta, T. Effects of Unilateral Nephrectomy on Renal Function in Male Spontaneously Diabetic Torii Fatty Rats: A Novel Obese Type 2 Diabetic Model. J. Diabetes Res. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, H.; Amakasu, K.; Tochigi, Y.; Katayama, K.; Suzuki, H. Renal Function and Hematology in Rats with Congenital Renal Hypoplasia. Comp. Med. 2016, 66, 10–20. [Google Scholar] [PubMed]
- Domon, A.; Katayama, K.; Sato, T.; Tochigi, Y.; Tazaki, H.; Suzuki, H. Empagliflozin ameliorates symptoms of diabetes and renal tubular dysfunction in a rat model of diabetes with enlarged kidney (DEK). PLoS ONE 2021, 16, e0251135. [Google Scholar] [CrossRef]
- Obineche, E.N.; Mensah-Brown, E.; Chandranath, S.I.; Ahmed, I.; Naseer, O.; Adem, A. Morphological Changes in the Rat Kidney Following Long-Term Diabetes. Arch. Physiol. Biochem. 2001, 109, 241–245. [Google Scholar] [CrossRef]
- Schwieger, J.; Fine, L.G. Renal hypertrophy, growth factors, and nephropathy in diabetes mellitus. Semin. Nephrol. 1990, 10, 242–253. [Google Scholar]
- Christiansen, J.S.; Gammelgaard, J.; Frandsen, M.; Parving, H.-H. Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetologia 1981, 20, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Amakasu, K.; Suzuki, K.; Suzuki, H. The Unilateral Urogenital Anomalies (UUA) Rat: A New Mutant Strain Associated with Unilateral Renal Agenesis, Cryptorchidism, and Malformations of Reproductive Organs Restricted to the Left Side. Comp. Med. 2009, 59, 249–256. [Google Scholar]
- Zimanyi, M.A.; Hoy, W.E.; Douglas-Denton, R.N.; Hughson, M.D.; Holden, L.M.; Bertram, J.F. Nephron number and individual glomerular volumes in male Caucasian and African American subjects. Nephrol. Dial. Transplant. 2009, 24, 2428–2433. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, W.C.; Bennett, R.G.; Hamel, F.G. Insulin Degradation: Progress and Potential. Endocr. Rev. 1998, 19, 608–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swe, M.T.; Pongchaidecha, A.; Chatsudthipong, V.; Chattipakorn, N.; Lungkaphin, A. Molecular signaling mechanisms of renal gluconeogenesis in nondiabetic and diabetic conditions. J. Cell. Physiol. 2019, 234, 8134–8151. [Google Scholar] [CrossRef]
- Neumiller, J.J.; Alicic, R.Z.; Tuttle, K. Therapeutic Considerations for Antihyperglycemic Agents in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2017, 28, 2263–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, M.B.; Hornum, M.; Van Hall, G.; Bistrup, C.; Hansen, J.M.; Mathiesen, E.R.; Feldt-Rasmussen, B. The impact of kidney transplantation on insulin sensitivity. Transpl. Int. 2016, 30, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, D.Z.; Iacovitti, M.; Robertson, S.J. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. II. Effects of renal mass reduction. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R1840–R1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y.; Ohno, Y.; Hayashi, M.; Suzawa, T.; Shibagaki, K.; Sasaki, T.; Saruta, T. Divergent Effects of Unilateral and Subtotal Ne-phrectomy on Insulin Sensitivity in Rats. Ren Fail. 2005, 27, 51–57. [Google Scholar] [CrossRef]
- Sugaya, K.; Ogawa, Y.; Hatano, T.; Koyama, Y.; Miyazato, T.; Naito, A.; Yonou, H.; Kagawa, H. Compensatory renal hypertrophy and changes of renal function following nephrectomy. Hinyokika kiyo. Acta Urol. Jpn. 2000, 46, 235–240. [Google Scholar]
- Dicker, S.E.; Shirley, D.G. Mechanism of compensatory renal hypertrophy. J. Physiol. 1971, 219, 507–523. [Google Scholar] [CrossRef]
- Thabet, M.A.E.H.; Salcedo, J.R.; Chan, J.C.M. Hyperlipidemia in childhood nephrotic syndrome. Pediatr. Nephrol. 1993, 7, 559–566. [Google Scholar] [CrossRef]
- Uil, M.; Scantlebery, A.; Butter, L.M.; Larsen, P.W.B.; De Boer, O.J.; Leemans, J.C.; Florquin, S.; Roelofs, J.J.T.H. Combining streptozotocin and unilateral nephrectomy is an effective method for inducing experimental diabetic nephropathy in the ‘resistant’ C57Bl/6J mouse strain. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Nakajima, T.; Miyaji, T.; Kato, A.; Ikegaya, N.; Yamamoto, T.; Hishida, A. Uninephrectomy reduces apoptotic cell death and enhances renal tubular cell regeneration in ischemic ARF in rats. Am. J. Physiol. Physiol. 1996, 271, F846–F853. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Kasahara, K.; Ikezaki, S.; Yamaguchi, Y.; Edamoto, H.; Nishimura, N.; Yahata, M.; Tamura, K.; Kamata, E.; Ema, M.; et al. Renal Tubular Cyst Formation in Newborn Rats Treated with p-Cumylphenol. J. Toxicol. Pathol. 2009, 22, 125–131. [Google Scholar] [CrossRef] [Green Version]




| Kidney Weight (g) | Relative Kidney Weight (g/kg) | |||||
|---|---|---|---|---|---|---|
| Pre/L Side | 28 Days/R Side | 84 Days/R Side | Pre/L Side | 28 Days/R Side | 84 Days/R Side | |
| Sham | - | 2.1 ± 0.1 | 2.9 ± 0.2 | - | 4.7 ± 0.2 | 7.6 ± 0.4 |
| 1/2Nx | 2.1 ± 0.1 | 2.6 ± 0.1 *** | 5.5 ± 0.4 *** | 5.0 ± 0.2 | 6.0 ± 0.5 | 12.1 ± 0.7 ** |
| 28 Days | 84 Days | |||
|---|---|---|---|---|
| Sham (n = 3) | 1/2Nx (n = 5) | Sham (n = 3) | 1/2Nx (n = 5) | |
| Food intake (g/kg) | 107.6 ± 13.8 | 46.8 ± 6.7 ** | 104.0 ± 14.1 | 62.1 ± 10.3 * |
| Water intake (g/kg) | 371.5 ± 68.9 | 74.2 ± 8.4 ** | 397.5 ± 94.7 | 185.0 ± 60.1 |
| Urinary volume (mL/kg) | 281.0 ± 76.9 | 44.3 ± 5.3 ** | 362.6 ± 99.0 | 150.2 ± 51.3 |
| 28 Days | 84 Days | |||
|---|---|---|---|---|
| Sham (n = 3) | 1/2Nx (n = 5) | Sham (n = 3) | 1/2Nx (n = 5) | |
| Tcho (mg/dL) | 77.7 ± 3.1 | 103.0 ± 4.4 ** | 76.7 ± 2.1 | 109.7 ± 5.1 ** |
| Cre (mg/dL) | 0.22 ± 0.03 | 0.35 ± 0.03 * | 0.30 ± 0.02 | 0.55 ± 0.06 * |
| BUN (mg/dL) | 16.5 ± 1.0 | 18.5 ± 0.5 | 22.3 ± 0.7 | 26.3 ± 2.4 |
| Na (mEq/L) | 136.7 ± 0.3 | 140.2 ± 0.8 * | 139.0 ± 0.6 | 140.7 ± 0.6 |
| K (mEq/L) | 4.5 ± 0.2 | 4.5 ± 0.3 | 4.3 ± 0.1 | 4.9 ± 0.2 |
| Cl (mEq/L) | 95.7 ± 0.8 | 99.6 ± 1.2 | 95.0 ± 0.0 | 98.0 ± 1.5 |
| Alb (g/dL) | 3.6 ± 0.1 | 3.7 ± 0.1 | 2.9 ± 0.1 | 3.4 ± 0.1 * |
| TP (g/dL) | 5.00 ± 0.25 | 5.54 ± 0.23 | 5.17 ± 0.09 | 5.38 ± 0.05 * |
| 28 Days | 84 Days | |||
|---|---|---|---|---|
| Sham (n = 3) | 1/2Nx (n = 5) | Sham (n = 3) | 1/2Nx (n = 5 or 6) | |
| Cre (mg/kg) | 49.1 ± 6.1 | 56.0 ± 7.2 | 78.1 ± 24.2 | 47.6 ± 1.5 |
| UN (mg/kg) | 1135.1 ± 235.9 | 658.1 ± 139.0 | 2034.6 ± 910.8 | 788.1 ± 123.5 * |
| Na (mEq/kg) | 8.6 ± 2.0 | 4.4 ± 0.8 | 9.8 ± 1.3 | 6.0 ± 1.3 |
| K (mEq/kg) | 14.2 ± 3.2 | 7.6 ± 1.4 | 16.5 ± 2.3 | 10.1 ± 1.8 |
| Cl (mEq/kg) | 10.5 ± 2.2 | 6.9 ± 1.6 | 12.6 ± 1.5 | 6.1 ± 1.5 * |
| Protein (mg/kg) | 132.5 ± 34.3 | 29.0 ± 11.3 * | 146.9 ± 33.1 | 101.6 ± 42.0 |
| Glu (mg/kg) | 23,143.4 ± 7730.6 | 39.7 ± 13.1 * | 31,603.7 ± 7983.1 | 10,918.6 ± 6777.3 |
| 28 Days | 84 Days | |||
|---|---|---|---|---|
| Sham (n = 3) | 1/2Nx (n = 5) | Sham (n = 3) | 1/2Nx (n = 5 or 6) | |
| Ccre | 15.74 ± 2.69 | 11.60 ± 2.11 | 19.10 ± 7.33 | 6.33 ± 0.68 * |
| Cna (×102) | 4.28 ± 0.98 | 2.16 ± 0.39 | 4.88 ± 0.65 | 2.95 ± 0.65 |
| Cglu | 4.28 ± 1.57 | 0.19 ± 0.06 * | 3.93 ± 0.66 | 1.48 ± 0.76 |
| FEna | 0.27 ± 0.02 | 0.19 ± 0.02 * | 0.31 ± 0.07 | 0.49 ± 0.09 |
| FEglu | 25.57 ± 4.80 | 1.70 ± 0.45 * | 24.91 ± 6.55 | 20.93 ± 9.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domon, A.; Katayama, K.; Yamada, T.; Tochigi, Y.; Suzuki, H. Characterization of Enlarged Kidneys and Their Potential for Inducing Diabetes in DEK Rats. Biology 2021, 10, 633. https://doi.org/10.3390/biology10070633
Domon A, Katayama K, Yamada T, Tochigi Y, Suzuki H. Characterization of Enlarged Kidneys and Their Potential for Inducing Diabetes in DEK Rats. Biology. 2021; 10(7):633. https://doi.org/10.3390/biology10070633
Chicago/Turabian StyleDomon, Ayaka, Kentaro Katayama, Takashi Yamada, Yuki Tochigi, and Hiroetsu Suzuki. 2021. "Characterization of Enlarged Kidneys and Their Potential for Inducing Diabetes in DEK Rats" Biology 10, no. 7: 633. https://doi.org/10.3390/biology10070633






