Identification of Clinical Value and Biological Effects of XIRP2 Mutation in Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Somatic Variant Profile and Somatic Variant Analysis
2.2. Acquisition of Gene Expression Profile and Differentially Expressed Genes (DEGs) Analysis
2.3. Biological Process (BP) Enrichment Analysis
2.4. Drug Sensitivity Calculation
2.5. Cell Culture and Transfection of Plasmids and Small Interfering RNA (siRNA)
2.6. Western Blotting
2.7. Ion Level Detection
2.8. Cell Count Kit-8 (CCK-8) Assays
2.9. Sphere-Forming Experiment
2.10. TUNEL Stain
2.11. Statistical Analysis
3. Results
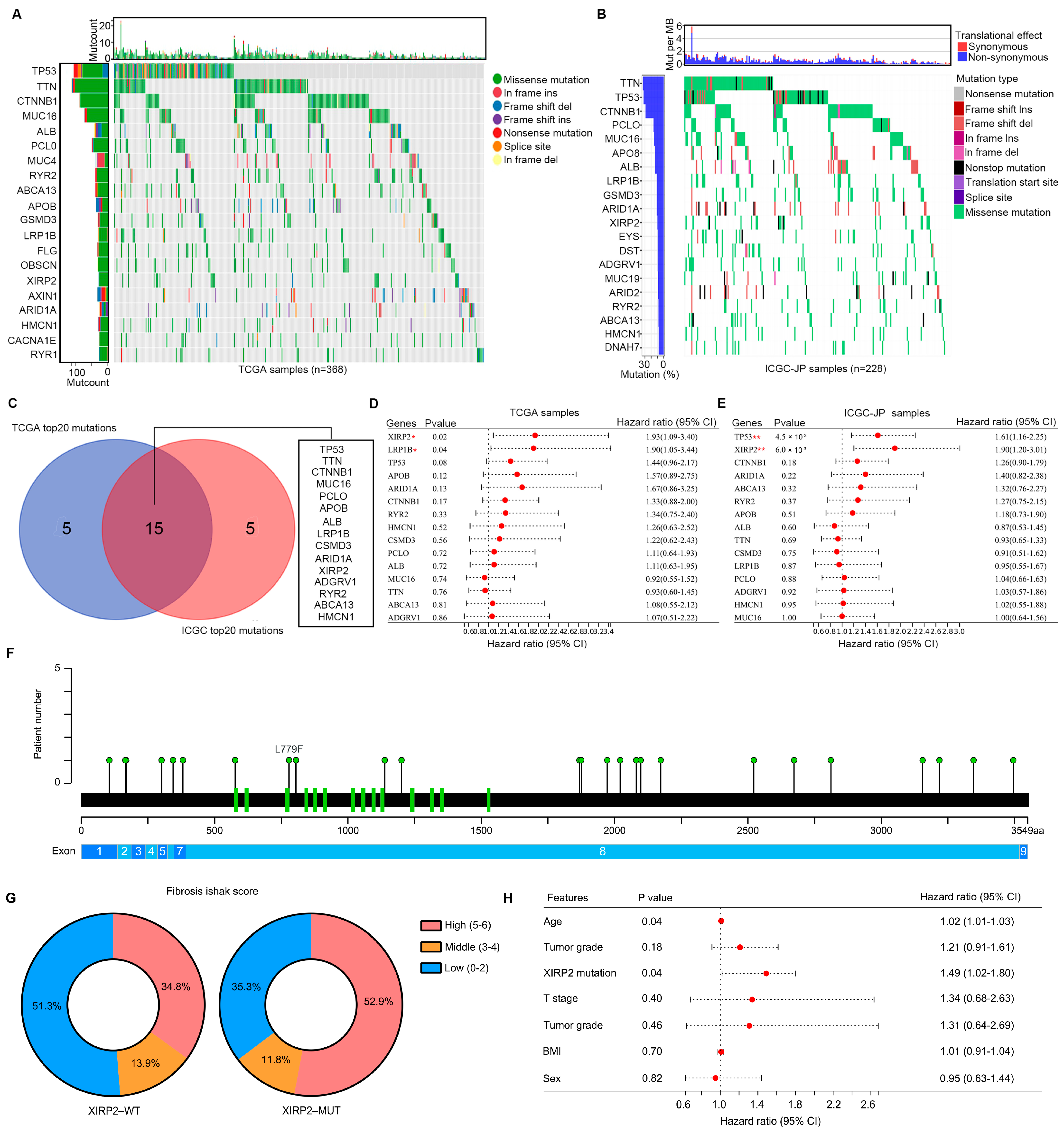
3.1. Aanalysis of Landscape of Somatic Variant in HCC Tissues
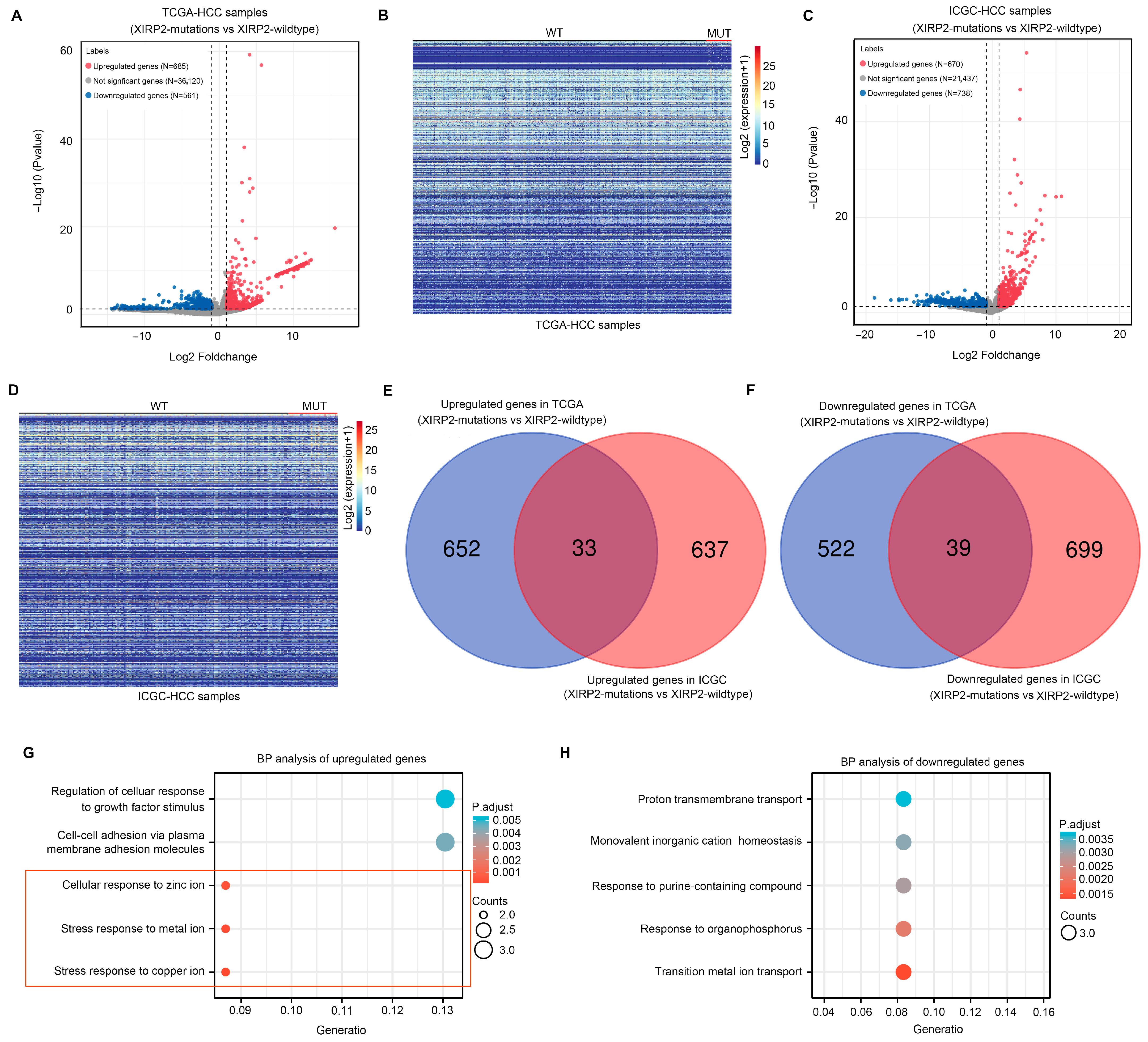
3.2. Identification of Molecular Landscape Affected by XIRP2 Mutation in HCC
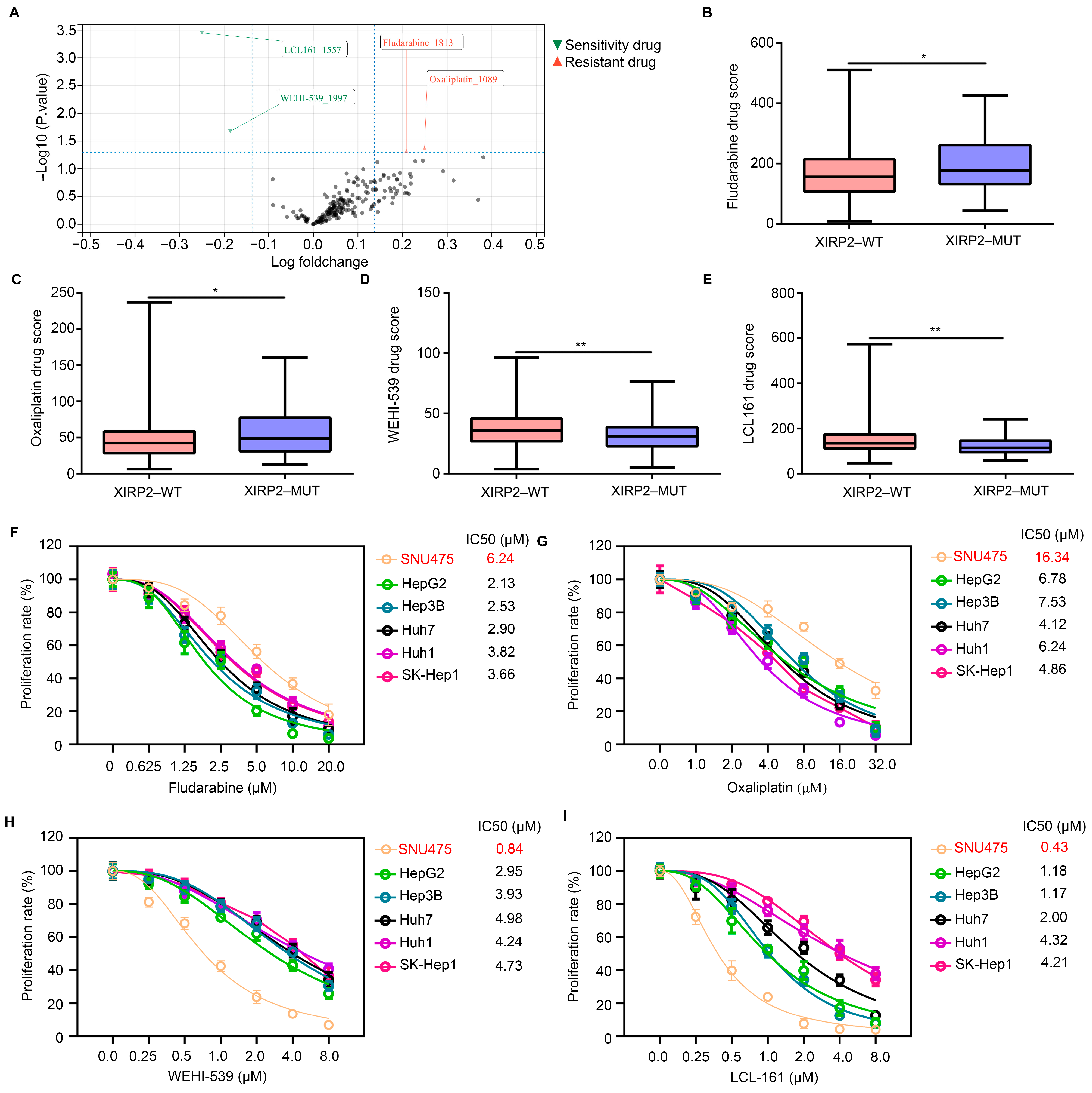
3.3. XIRP2 Mutation Increased the Resistance of HCC Cells to Fludarabine and Oxaliplatin but Increased the Sensitivity to WEHI-539 and LCL 161
3.4. XIRP2 Mutation Increased the Protein Stability of XIRP2 Protein
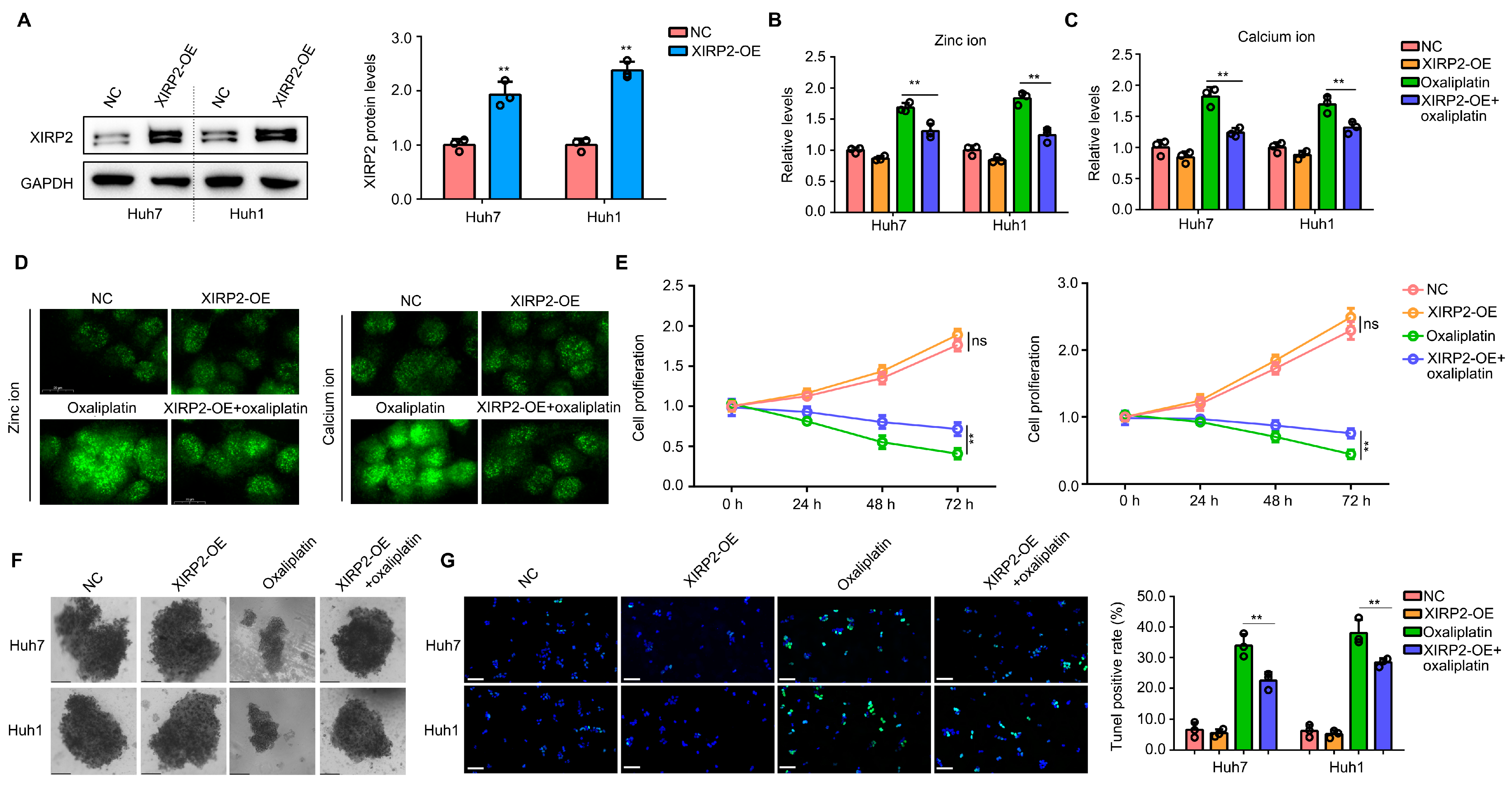
3.5. Increased XIRP2 Protein in HCC Cells with XIRP2 Wildtype Reduced the Sensitivity to Oxaliplatin
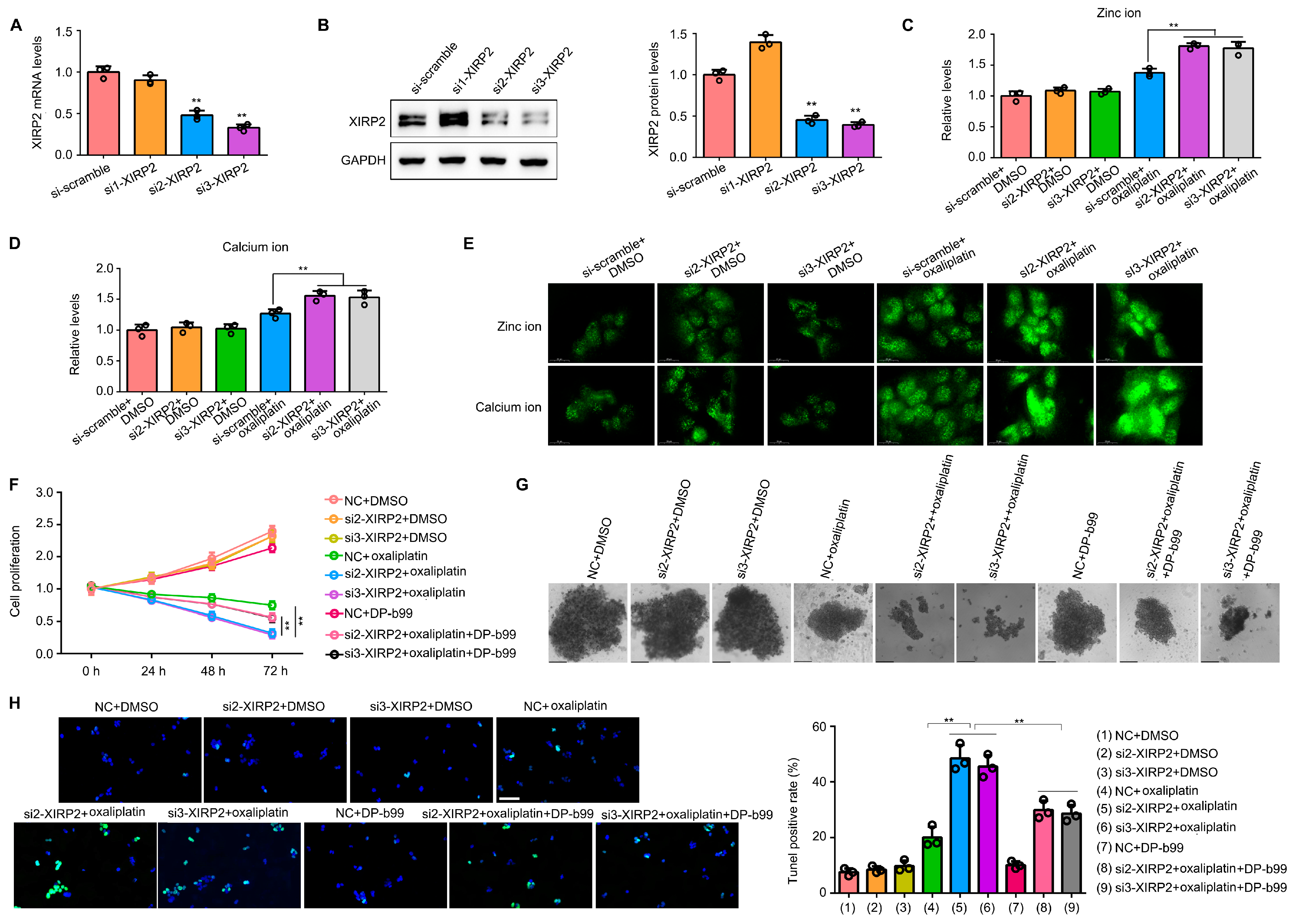
3.6. Knockdown of XIRP2 in HCC Cells with the XIRP2 Mutation Increased the Sensitivity to Oxaliplatin via Inducing the Overload of Zinc and Calcium Ion
4. Discussion
5. Conlusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| DEG | Differentially expressed gene |
| XIRP2 | Xin actin binding repeat containing 2 |
| BP | Biological process |
| siRNA | Small interfering RNA |
| CCK8 | Cell count kit-8 |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
References
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Morizane, C.; Ueno, M.; Okusaka, T.; Ishii, H.; Furuse, J. Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Jpn. J. Clin. Oncol. 2018, 48, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Couri, T.; Pillai, A. Goals and targets for personalized therapy for HCC. Hepatol. Int. 2019, 13, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef]
- Huang, D.; Sun, W.; Zhou, Y.; Li, P.; Chen, F.; Chen, H.; Xia, D.; Xu, E.; Lai, M.; Wu, Y.; et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018, 37, 173–187. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, Q.; Li, Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: Underlying mechanisms and reversal strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328. [Google Scholar] [CrossRef]
- Scheffer, D.I.; Zhang, D.S.; Shen, J.; Indzhykulian, A.; Karavitaki, K.D.; Xu, Y.J.; Wang, Q.; Lin, J.J.; Chen, Z.Y.; Corey, D.P. XIRP2, an actin-binding protein essential for inner ear hair-cell stereocilia. Cell Rep. 2015, 10, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, J.L.; Erives, A.J.; Lin, C.I.; Lin, J.J. New insights into the roles of Xin repeat-containing proteins in cardiac development, function, and disease. Int. Rev. Cell Mol. Biol. 2014, 310, 89–128. [Google Scholar] [PubMed]
- Li, X.; Wu, W.K.; Xing, R.; Wong, S.H.; Liu, Y.; Fang, X.; Zhang, Y.; Wang, M.; Wang, J.; Li, L.; et al. Distinct Subtypes of Gastric Cancer Defined by Molecular Characterization Include Novel Mutational Signatures with Prognostic Capability. Cancer Res. 2016, 76, 1724–1732. [Google Scholar] [CrossRef]
- Mayakonda, A.; Lin, D.C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Maeser, D.; Gruener, R.F.; Huang, R.S. oncoPredict: An R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief. Bioinform. 2021, 22, bbab260. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zeng, Z.; Wang, J.; Cao, W.; Song, C.; Lei, S.; Li, Y.; Ren, Z. Long noncoding RNA LINC00857 promotes pancreatic cancer proliferation and metastasis by regulating the miR-130b/RHOA axis. Cell Death Discov. 2022, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Cui, C.; Luo, Y.; Kim, S.H.; Ko, J.K.; Huo, X.; Ma, J.; Fu, L.W.; Souza, R.F.; Korichneva, I.; et al. Selective inhibitory effects of zinc on cell proliferation in esophageal squamous cell carcinoma through Orai1. FASEB J. 2018, 32, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, B.; Zhao, R.; Feng, L.; Wang, Z.; Dong, S.; Dong, Y.; Gai, S.; Ding, H.; Yang, P. Calcium Peroxide-Based Nanosystem with Cancer Microenvironment-Activated Capabilities for Imaging Guided Combination Therapy via Mitochondrial Ca2+ Overload and Chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 44096–44107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar]
- Luo, Y.D.; Fang, L.; Yu, H.Q.; Zhang, J.; Lin, X.T.; Liu, X.Y.; Wu, D.; Li, G.X.; Huang, D.; Zhang, Y.J.; et al. p53 haploinsufficiency and increased mTOR signalling define a subset of aggressive hepatocellular carcinoma. J. Hepatol. 2021, 74, 96–108. [Google Scholar] [CrossRef]
- Śmiech, M.; Leszczyński, P.; Wardell, C.; Poznański, P.; Pierzchała, M.; Taniguchi, H. Oncogenic Mutation BRAF V600E Changes Phenotypic Behavior of THLE-2 Liver Cells through Alteration of Gene Expression. Int. J. Mol. Sci. 2022, 23, 1548. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.P.; Krey, J.F.; Krystofiak, E.S.; Cui, R.; Nanda, S.; Xu, W.; Kachar, B.; Barr-Gillespie, P.G.; Shin, J.B. A short splice form of Xin-actin binding repeat containing 2 (XIRP2) lacking the Xin repeats is required for maintenance of stereocilia morphology and hearing function. J. Neurosci. 2015, 35, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Przybyła, W.; Gjersvoll Paulsen, K.M.; Mishra, C.K.; Nygård, S.; Engebretsen, S.; Ruud, E.; Trøen, G.; Beiske, K.; Baumbusch, L.O. Whole exome sequencing of high-risk neuroblastoma identifies novel non-synonymous variants. PLoS ONE. 2022, 17, e0273280. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.R.; Pan, T.C.; Pant, D.K.; Shih, N.N.; Chen, Y.; Harvey, K.L.; Solomon, A.; Lieberman, D.; Morrissette, J.J.; Soucier-Ernst, D.; et al. Genomic landscape of metastatic breast cancer identifies preferentially dysregulated pathways and targets. J. Clin. Investig. 2020, 130, 4252–4265. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Kovacevic, Z.; Jansson, P.J.; Sahni, S.; Huang, M.L.; Lane, D.J.R.; Lok, H.; Richardson, D.R. Exploiting Cancer Metal Metabolism using Anti-Cancer Metal- Binding Agents. Curr. Med. Chem. 2019, 26, 302–322. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maret, W. Transient fluctuations of intracellular zinc ions in cell proliferation. Exp. Cell Res. 2009, 315, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- To, P.K.; Do, M.H.; Cho, J.H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int. J. Mol. Sci. 2020, 21, 2991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shao, J.; Tan, S.W.; Ye, H.P.; Shan, X.Y. Association between serum copper/zinc ratio and lung cancer: A systematic review with meta-analysis. J. Trace Elem. Med. Biol. 2022, 74, 127061. [Google Scholar] [CrossRef]
- Gelbard, A. Zinc in Cancer Therapy Revisited. Isr. Med. Assoc. J. 2022, 24, 258–262. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Bao, X.; Lei, S.; Cao, W.; Zeng, Z.; Chen, T. Identification of Clinical Value and Biological Effects of XIRP2 Mutation in Hepatocellular Carcinoma. Biology 2024, 13, 633. https://doi.org/10.3390/biology13080633
Li D, Bao X, Lei S, Cao W, Zeng Z, Chen T. Identification of Clinical Value and Biological Effects of XIRP2 Mutation in Hepatocellular Carcinoma. Biology. 2024; 13(8):633. https://doi.org/10.3390/biology13080633
Chicago/Turabian StyleLi, Dahuan, Xin Bao, Shan Lei, Wenpeng Cao, Zhirui Zeng, and Tengxiang Chen. 2024. "Identification of Clinical Value and Biological Effects of XIRP2 Mutation in Hepatocellular Carcinoma" Biology 13, no. 8: 633. https://doi.org/10.3390/biology13080633




