Assessment of Extracellular Matrix Fibrous Elements in Male Dermal Aging: A Ten-Year Follow-Up Preliminary Case Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Histological Examination of Skin
2.3. Digital Image Acquisition and Segmentation
2.4. Digital Analysis of Dermis
3. Results
3.1. Histological Staining Analysis
3.2. Digital Analysis of Dermis
3.2.1. Analysis of the Area Occupied by the ECM
3.2.2. Collagen Type I Bundle Content in H&E Analysis
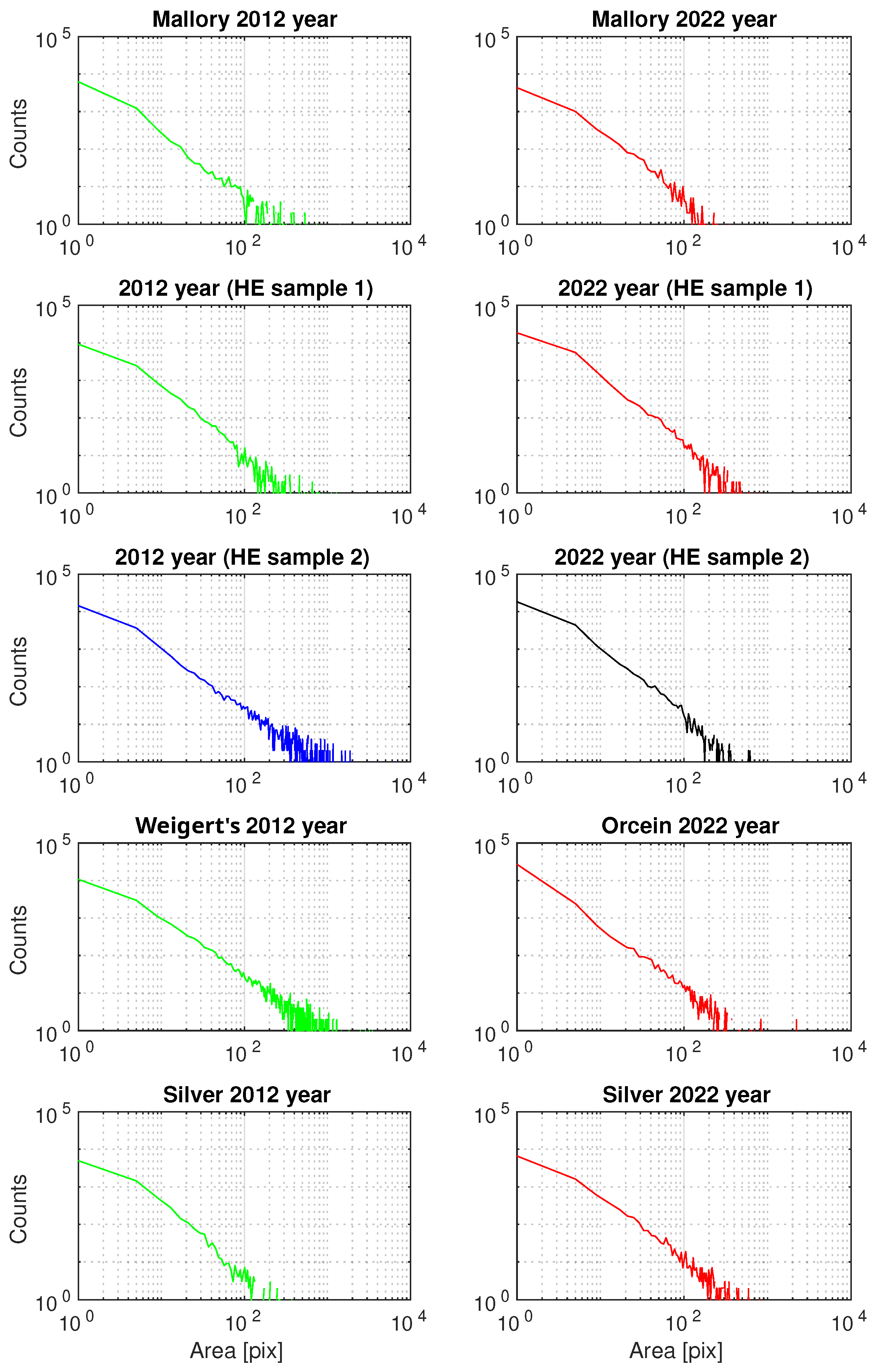
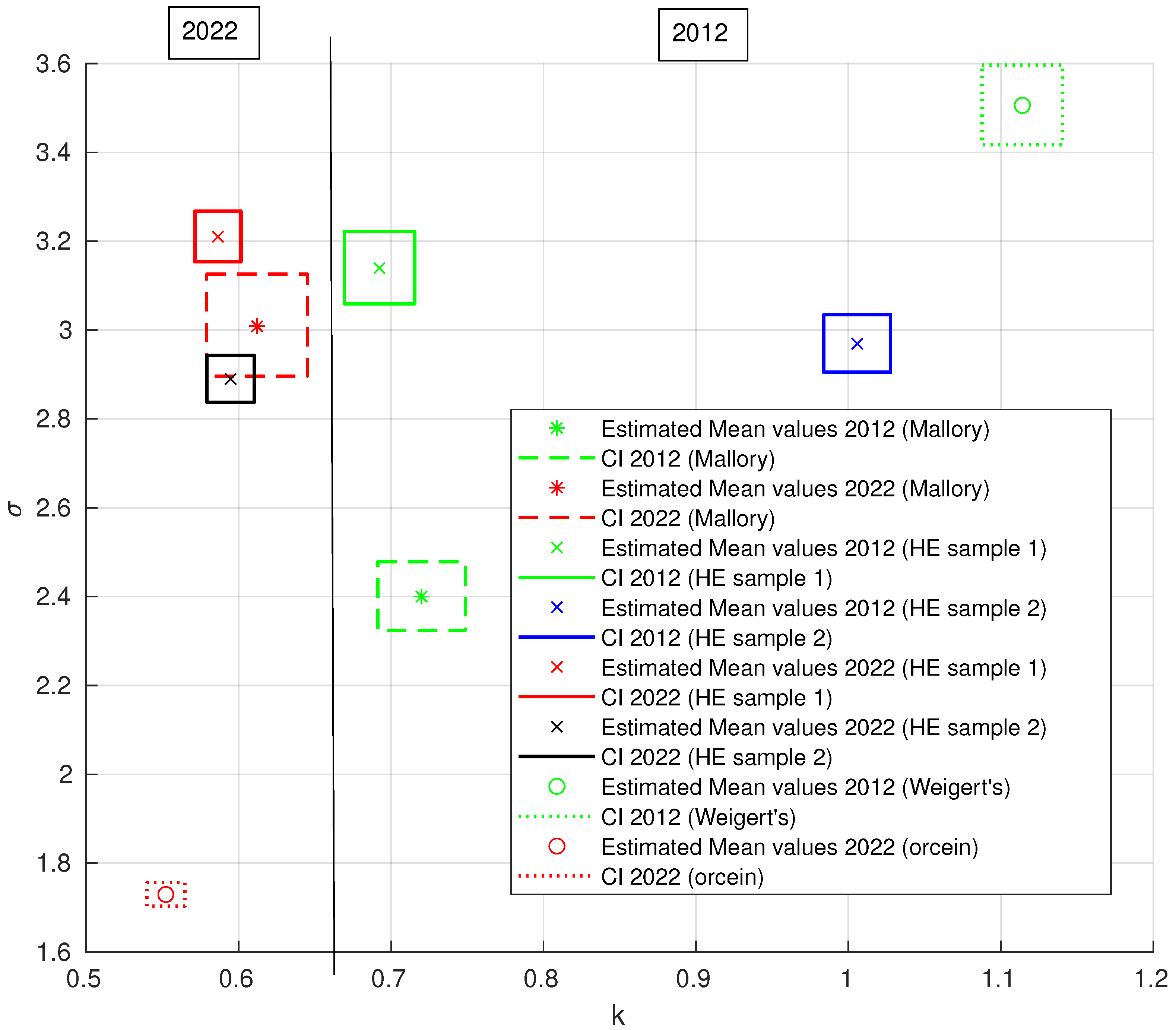
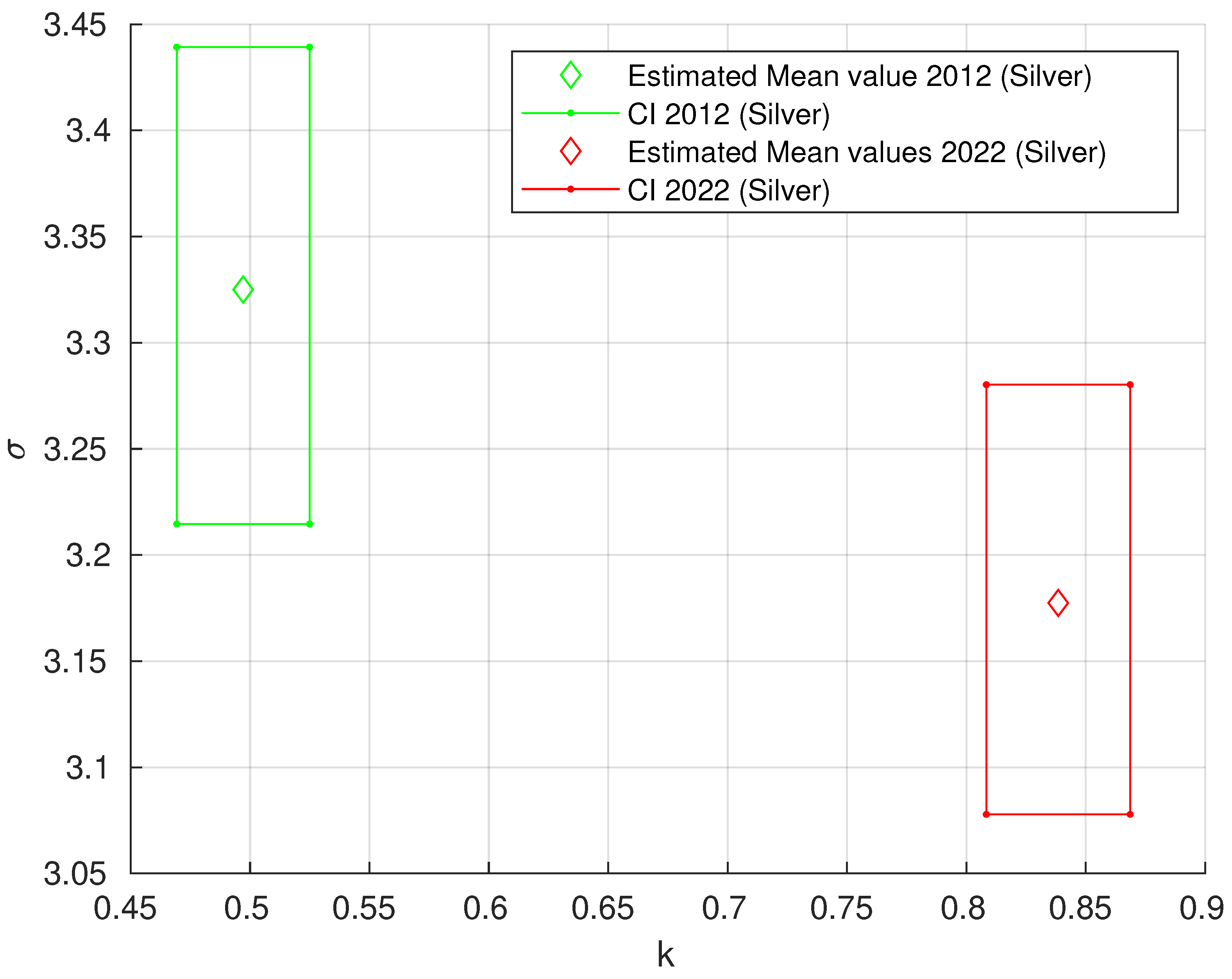
3.2.3. Generalized Pareto Distribution (GPD) Analysis of the Area of ECM Fibrous Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCD | charge-coupled device |
| CI | confidence interval |
| ECM | extracellular matrix |
| GPD | generalized Pareto distribution |
| H&E | hematoxylin and eosin |
| MMPs | matrix metalloproteinases |
References
- Makrantonaki, E.; Zouboulis, C.C. The skin as a mirror of the aging process in the human organism–state of the art and results of the aging research in the German National Genome Research Network 2 (NGFN-2). Exp. Gerontol. 2007, 42, 879–886. [Google Scholar] [CrossRef]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zargaran, D.; Zoller, F.; Zargaran, A.; Weyrich, T.; Mosahebi, A. Facial skin ageing: Key concepts and overview of processes. Int. J. Cosmet. Sci. 2022, 44, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Avelar, J.M. Importance and Behavior of Fascia Superficialis for Body-Couturing Surgery. In Body Contouring: Current Concepts and Best Practices; Avelar, J.M., Cavalcanti Ribeiro, R., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 49–69. [Google Scholar]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Bruckner, P. Suprastructures of extracellular matrices: Paradigms of functions controlled by aggregates rather than molecules. Cell Tissue Res. 2010, 339, 7–18. [Google Scholar] [CrossRef]
- Krieg, T.; Aumailley, M. The extracellular matrix of the dermis: Flexible structures with dynamic functions. Exp. Dermatol. 2011, 20, 689–695. [Google Scholar] [CrossRef]
- Cotta-Pereira, G.; Guerra Rodrigo, F.; Bittencourt-Sampaio, S. Oxytalan, elaunin, and elastic fibers in the human skin. J. Investig. Dermatol. 1976, 66, 143–148. [Google Scholar] [CrossRef]
- Uitto, J.; Li, Q.; Urban, Z. The complexity of elastic fibre biogenesis in the skin–A perspective to the clinical heterogeneity of cutis laxa. Exp. Dermatol. 2013, 22, 88–92. [Google Scholar] [CrossRef]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Janson, D.G.; Saintigny, G.; van Adrichem, A.; Mahé, C.; El Ghalbzouri, A. Different gene expression patterns in human papillary and reticular fibroblasts. J. Investig. Dermatol. 2012, 132, 2565–2572. [Google Scholar] [CrossRef]
- Nyström, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef]
- Huang, J.; Heng, S.; Zhang, W.; Liu, Y.; Xia, T.; Ji, C.; Zhang, L.J. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin. Cell Dev. Biol. 2022, 128, 137–144. [Google Scholar] [CrossRef]
- Leask, A. Integrin beta-1: A Mechanosignaling Sensor Essential for Connective Tissue Deposition by Fibroblasts. Adv. Wound Care 2013, 4, 160–166. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, B.; Cui, Y.; Shi, M.; Zhao, Y.; Quan, T.; Voorhees, J.J. Skin aging from the perspective of dermal fibroblasts: The interplay between the adaptation to the extracellular matrix microenvironment and cell autonomous processes. J. Cell Commun. Signal. 2023, 17, 523–529. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007, 214, 352–360. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Wyles, S.P.; Carruthers, J.D.; Dashti, P.; Yu, G.; Yap, J.Q.; Gingery, A.; Tchkonia, T.; Kirkland, J. Cellular Senescence in Human Skin Aging: Leveraging Senotherapeutics. Gerontology 2024, 70, 7–14. [Google Scholar] [CrossRef]
- Schikowski, T.; Hüls, A. Air Pollution and Skin Aging. Curr. Environ. Health Rep. 2020, 7, 58–64. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Ambient Air Quality Database (Update 2024), Version 6.1; WHO: Geneva, Switzerland, 2024.
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Langton, A.K.; Sherratt, M.J.; Griffiths, C.E.M.; Watson, R.E.B. A new wrinkle on old skin: The role of elastic fibres in skin ageing. Int. J. Cosmet. Sci. 2010, 32, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Flament, F.; Abric, A.; Amar, D. Gender-related differences in the facial aging of Chinese subjects and their relations with perceived ages. Ski. Res. Technol. 2020, 26, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Belkebla, S.; Adam, A.S.; Abric, A.; Amar, D. Gender-related differences in the facial aging of Caucasian French subjects and their relations with perceived ages and tiredness. J. Cosmet. Dermatol. 2021, 20, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Machaliński, B.; Brodkiewicz, A.; Szumilas, K.; Rogińska, D.; Kawa, M.P.; Stecewicz, I.; Trybek, G.; Marchlewicz, M.; Wiszniewska, B. Morphologic Changes in the Dermis After the Single Administration of Autologous Fibroblastic Cells: A Preliminary Study. Transplant. Proc. 2016, 48, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- MathWorks. Statistics and Machine Learning Toolbox User’s Guide; MathWorks: Natick, MA, USA, 2024. [Google Scholar]
- Arnold, B.C. Pareto Distribution. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–10. [Google Scholar]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Haydont, V.; Neiveyans, V.; Fortunel, N.O.; Asselineau, D. Transcriptome profiling of human papillary and reticular fibroblasts from adult interfollicular dermis pinpoints the ‘tissue skeleton’ gene network as a component of skin chrono-ageing. Mech. Ageing Dev. 2019, 179, 60–77. [Google Scholar] [CrossRef]
- He, T.; Fisher, G.J.; Kim, A.J.; Quan, T. Age-related changes in dermal collagen physical properties in human skin. PLoS ONE 2023, 18, e0292791. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Pauschinger, M.; Knopf, D.; Petschauer, S.; Doerner, A.; Poller, W.; Schwimmbeck, P.L.; Kühl, U.; Schultheiss, H.P. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation 1999, 99, 2750–2756. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.; Klinge, U.; Rosch, R.; Mertens, P.R.; Kirch, J.; Klosterhalfen, B.; Lynen, P.; Schumpelick, V. Decreased collagen type I/III ratio in patients with recurring hernia after implantation of alloplastic prostheses. Langenbeck’s Arch. Surg. 2004, 389, 17–22. [Google Scholar]
- Sugita, S.; Suzumura, T.; Nakamura, A.; Tsukiji, S.; Ujihara, Y.; Nakamura, M. Second harmonic generation light quantifies the ratio of type III to total (I + III) collagen in a bundle of collagen fiber. Sci. Rep. 2021, 11, 11874. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Boismal, F.; Serror, K.; Dobos, G.; Zuelgaray, E.; Bensussan, A.; Michel, L. Skin aging: Pathophysiology and innovative therapies. Med. Sci. M/S 2020, 36, 1163–1172. [Google Scholar]
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Duca, L. Elastic fibers: Formation, function, and fate during aging and disease. FEBS J. 2022, 289, 3704–3730. [Google Scholar] [CrossRef] [PubMed]
- Machaliński, B.; Rogińska, D.; Szumilas, K.; Zawiślak, A.; Wilk, A.; Stecewicz, I.; Brodkiewicz, A.; Wiszniewska, B. Transcriptome Profile of Human Fibroblasts in an Ex Vivo Culture. Int. J. Med Sci. 2020, 17, 125–136. [Google Scholar] [CrossRef]
- Machaliński, B.; Rogińska, D.; Wilk, A.; Szumilas, K.; Prowans, P.; Paczkowska, E.; Szumilas, P.; Stecewicz, I.; Zawodny, P.; Ziętek, M.; et al. Global Gene Expression of Cultured Human Dermal Fibroblasts: Focus on Cell Cycle and Proliferation Status in Improving the Condition of Face Skin. Int. J. Med Sci. 2021, 18, 1519–1531. [Google Scholar] [CrossRef]
- Windhager, S.; Mitteroecker, P.; Rupić, I.; Lauc, T.; Polašek, O.; Schaefer, K. Facial aging trajectories: A common shape pattern in male and female faces is disrupted after menopause. Am. J. Phys. Anthropol. 2019, 169, 678–688. [Google Scholar] [CrossRef]

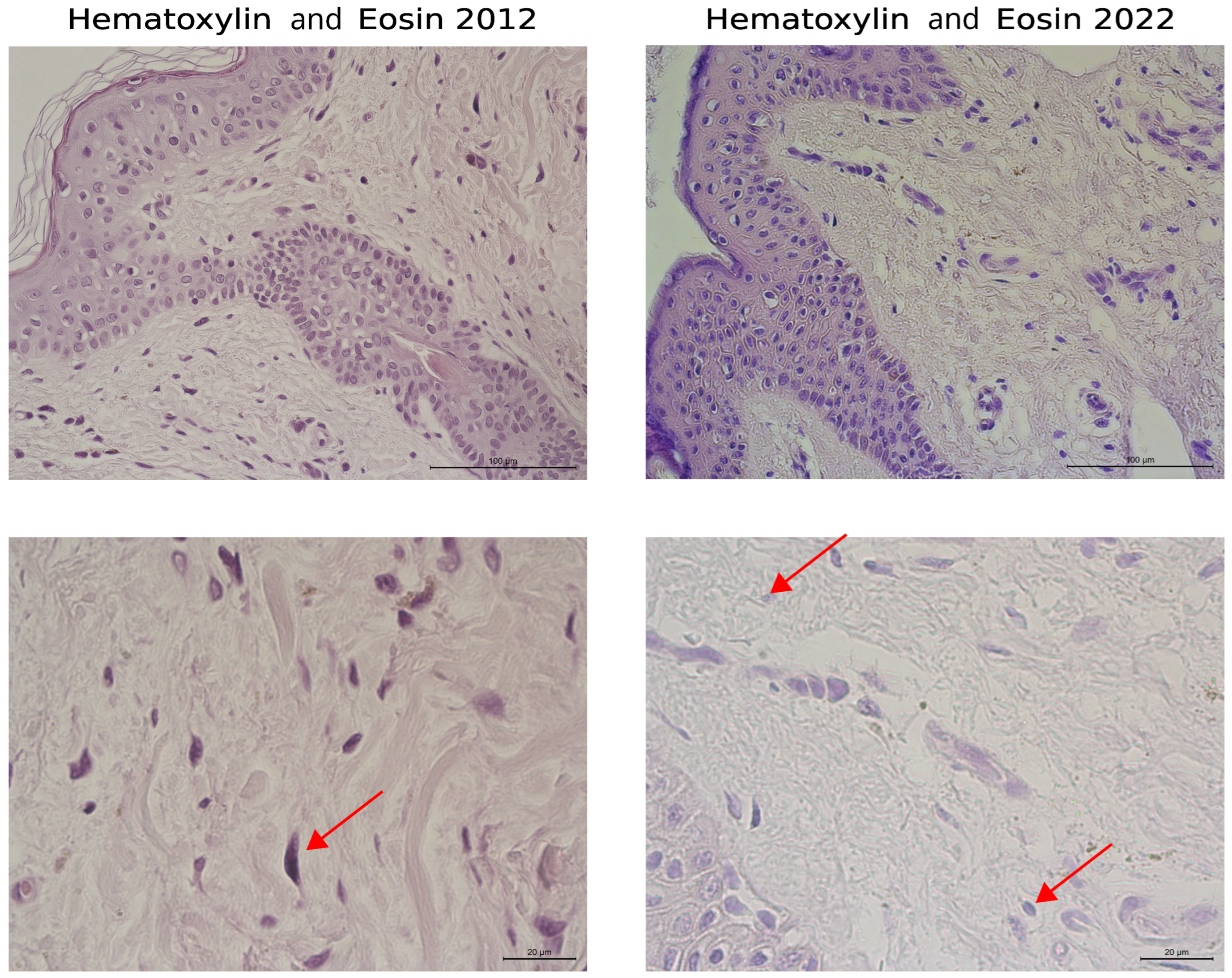
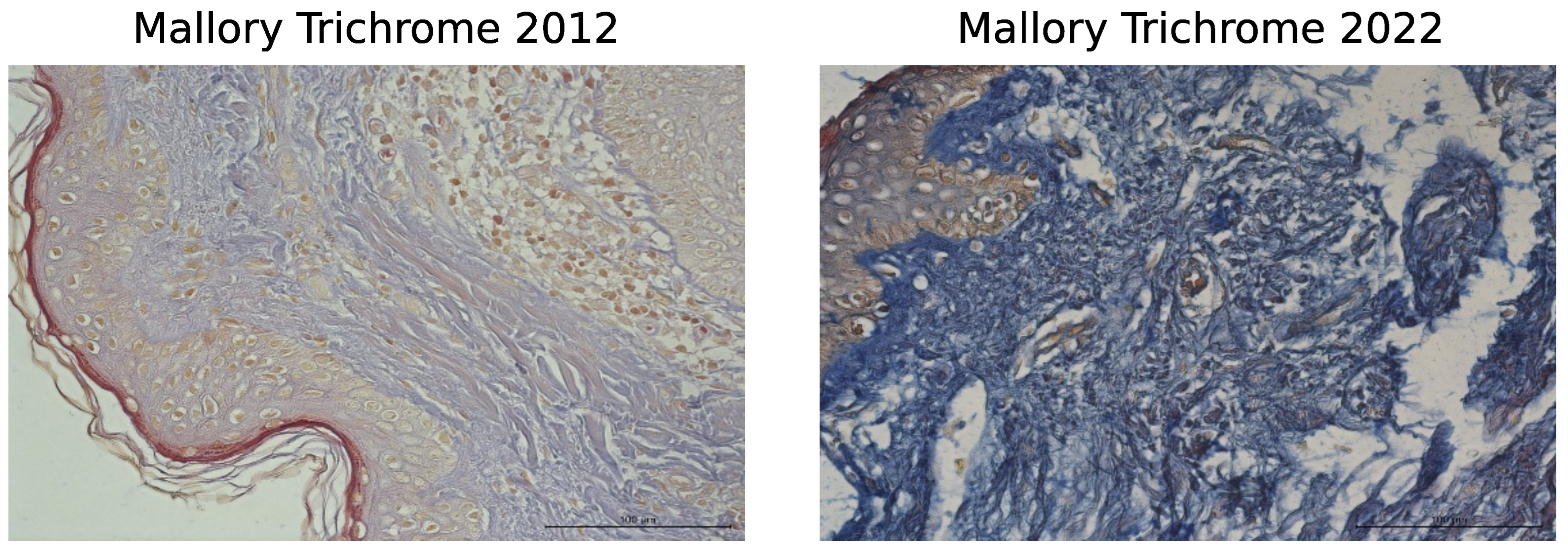
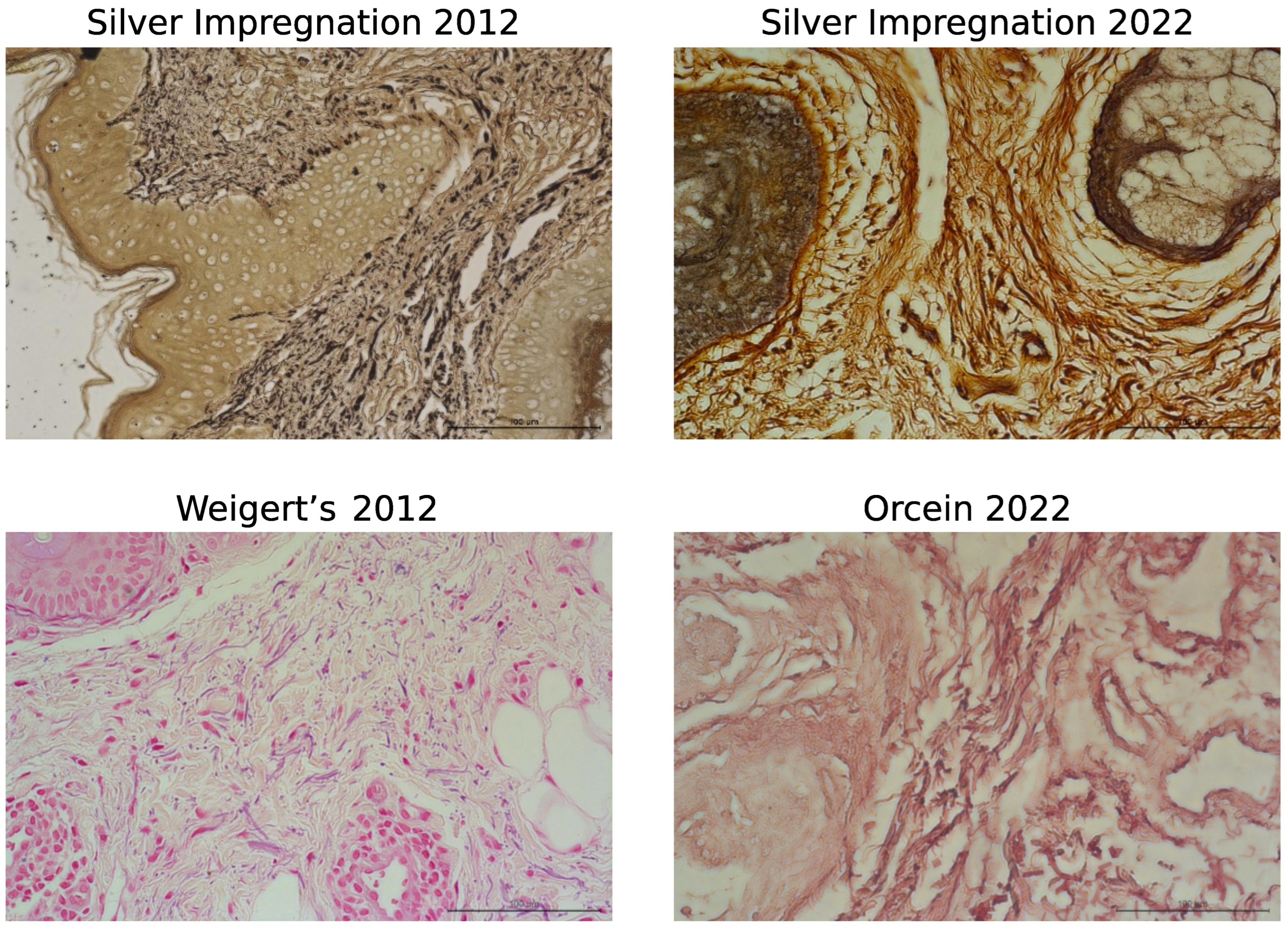
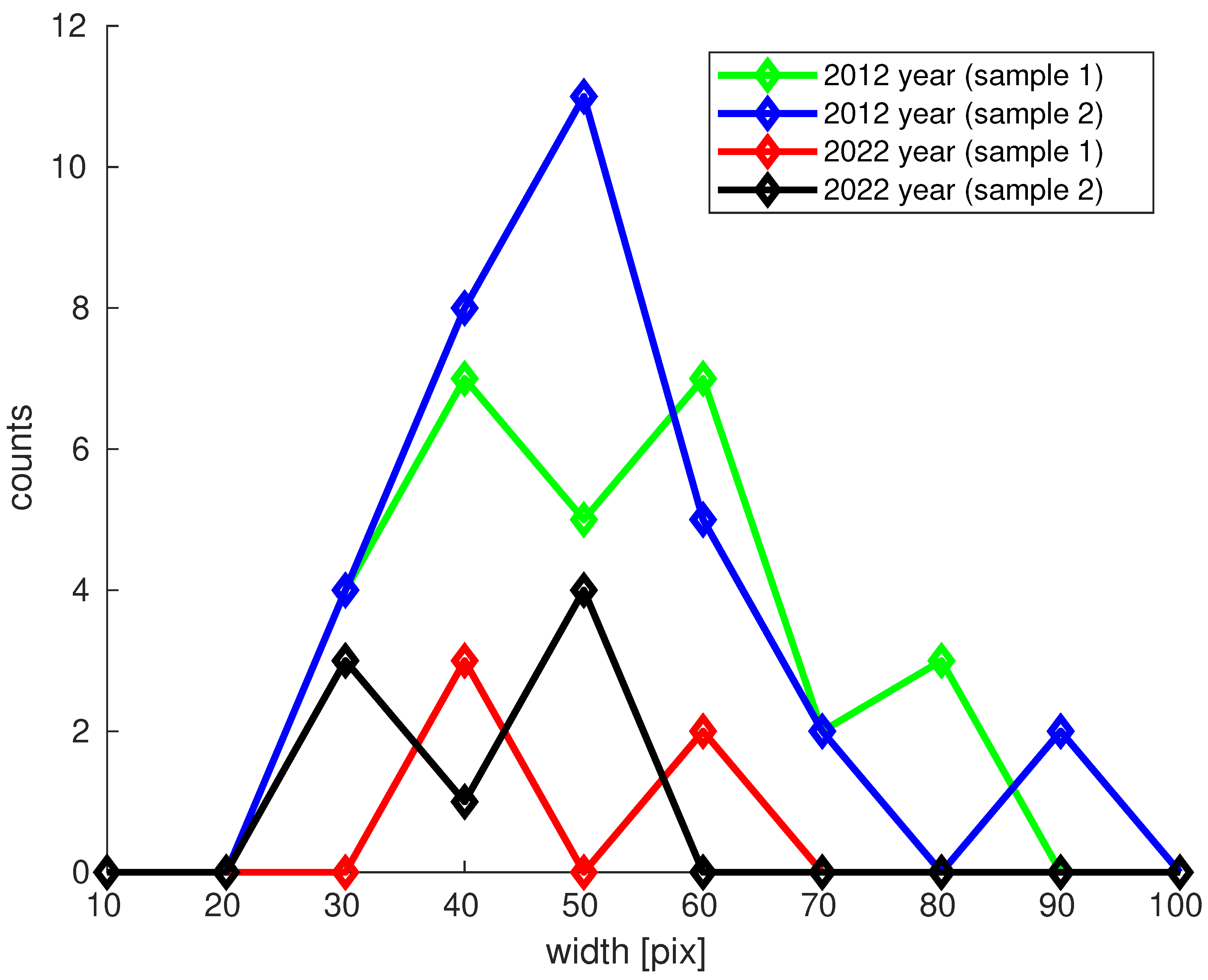
| Staining Procedure | Case | Mean [%] | Mean CI [%] | Statistical Difference |
|---|---|---|---|---|
| H&E | 2012 sample 1 | 57.3 | (56.4; 58.1) | Yes, but 2022 |
| 2012 sample 2 | 40.7 | (40.6; 40.7) | samples are | |
| 2022 sample 1 | 54.7 | (54.1; 55.2) | between 2012 | |
| 2022 sample 2 | 54.3 | (53.7; 54.9) | samples | |
| Mallory | 2012 | 60.8 | (59.7; 61.9) | yes |
| trichrome | 2022 | 79.2 | (77.3; 81.1) | |
| Silver | 2012 | 78.7 | (76.9; 80.3) | yes |
| impregnation | 2022 | 59.5 | (58.8; 60.6) | |
| Weigert’s | 2012 | 48.6 | (48.5; 48.8) | yes |
| Orcein | 2022 | 52.6 | (52.0; 53.1) |
| No. of Collagen Bundles | Mean [%] | Mean CI [%] | Max. Width of Bundle [pix] | |
|---|---|---|---|---|
| 2012 | 60 (sample 1 + sample 2) | 51.1 | (47.0; 55.1) | 91.1 |
| 2022 | 13 (sample 1 + sample 2) | 43.7 | (38.1; 49.3) | 61.1 |
| Staining Procedure | Case | k | k CI | CI | |
|---|---|---|---|---|---|
| H&E | 2012 sample 1 | 0.6923 | (0.6693; 0.7154) | 3.1394 | (3.0592; 3.2216) |
| 2012 sample 2 | 1.0058 | (0.9839; 1.0277) | 2.9690 | (2.9049; 3.0346) | |
| 2022 sample 1 | 0.5864 | (0.5713; 0.6015) | 3.2099 | (3.1535; 3.2674) | |
| 2022 sample 2 | 0.5947 | (0.5792; 0.6102) | 2.8896 | (2.8373; 2.9428) | |
| Mallory | 2012 | 0.7199 | (0.6912; 0.7487) | 2.4000 | (2.3238; 2.4787) |
| trichrome | 2022 | 0.6121 | (0.5790; 0.6452) | 3.0085 | (2.8956; 3.1258) |
| Silver | 2012 | 0.4971 | (0.4693; 0.5249) | 3.3251 | (3.2146; 3.4393) |
| impregnation | 2022 | 0.8385 | (0.8083; 0.8686) | 3.1774 | (3.0778; 3.2803) |
| Weigert’s | 2012 | 1.1141 | (1.0876; 1.1406) | 3.5058 | (3.4173; 3.5966) |
| Orcein | 2022 | 0.5522 | (0.5397; 0.5647) | 1.7294 | (1.7029; 1.7564) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machaliński, B.; Oszutowska-Mazurek, D.; Mazurek, P.; Parafiniuk, M.; Szumilas, P.; Zawiślak, A.; Zaremba, M.; Stecewicz, I.; Zawodny, P.; Wiszniewska, B. Assessment of Extracellular Matrix Fibrous Elements in Male Dermal Aging: A Ten-Year Follow-Up Preliminary Case Study. Biology 2024, 13, 636. https://doi.org/10.3390/biology13080636
Machaliński B, Oszutowska-Mazurek D, Mazurek P, Parafiniuk M, Szumilas P, Zawiślak A, Zaremba M, Stecewicz I, Zawodny P, Wiszniewska B. Assessment of Extracellular Matrix Fibrous Elements in Male Dermal Aging: A Ten-Year Follow-Up Preliminary Case Study. Biology. 2024; 13(8):636. https://doi.org/10.3390/biology13080636
Chicago/Turabian StyleMachaliński, Bogusław, Dorota Oszutowska-Mazurek, Przemyslaw Mazurek, Mirosław Parafiniuk, Paweł Szumilas, Alicja Zawiślak, Małgorzata Zaremba, Iwona Stecewicz, Piotr Zawodny, and Barbara Wiszniewska. 2024. "Assessment of Extracellular Matrix Fibrous Elements in Male Dermal Aging: A Ten-Year Follow-Up Preliminary Case Study" Biology 13, no. 8: 636. https://doi.org/10.3390/biology13080636






