The Role of Milk Oligosaccharides in Enhancing Intestinal Microbiota, Intestinal Integrity, and Immune Function in Pigs: A Comparative Review
Abstract
:Simple Summary
Abstract
1. Introduction
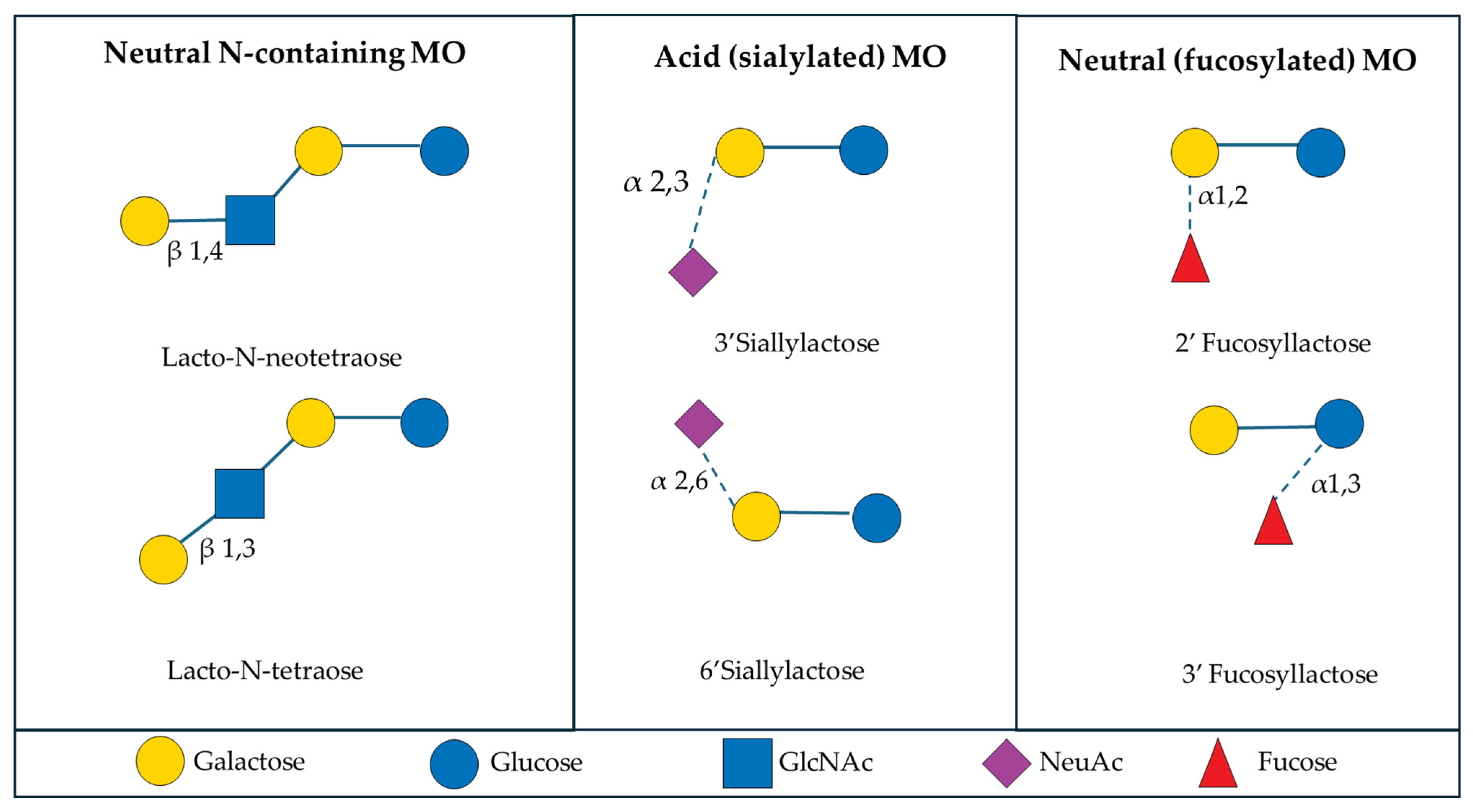
2. Characterization of Milk Oligosaccharides
Characterization of Milk Oligosaccharides from Human, Bovine, and Porcine Sources
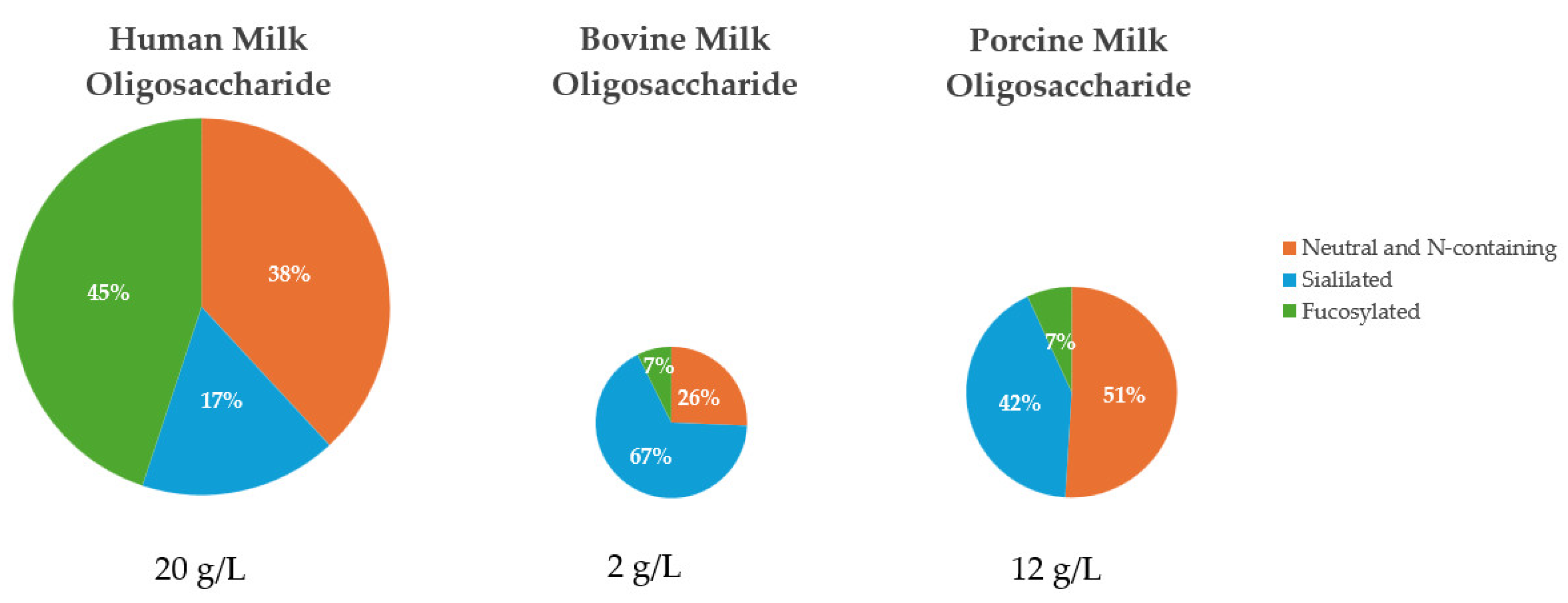
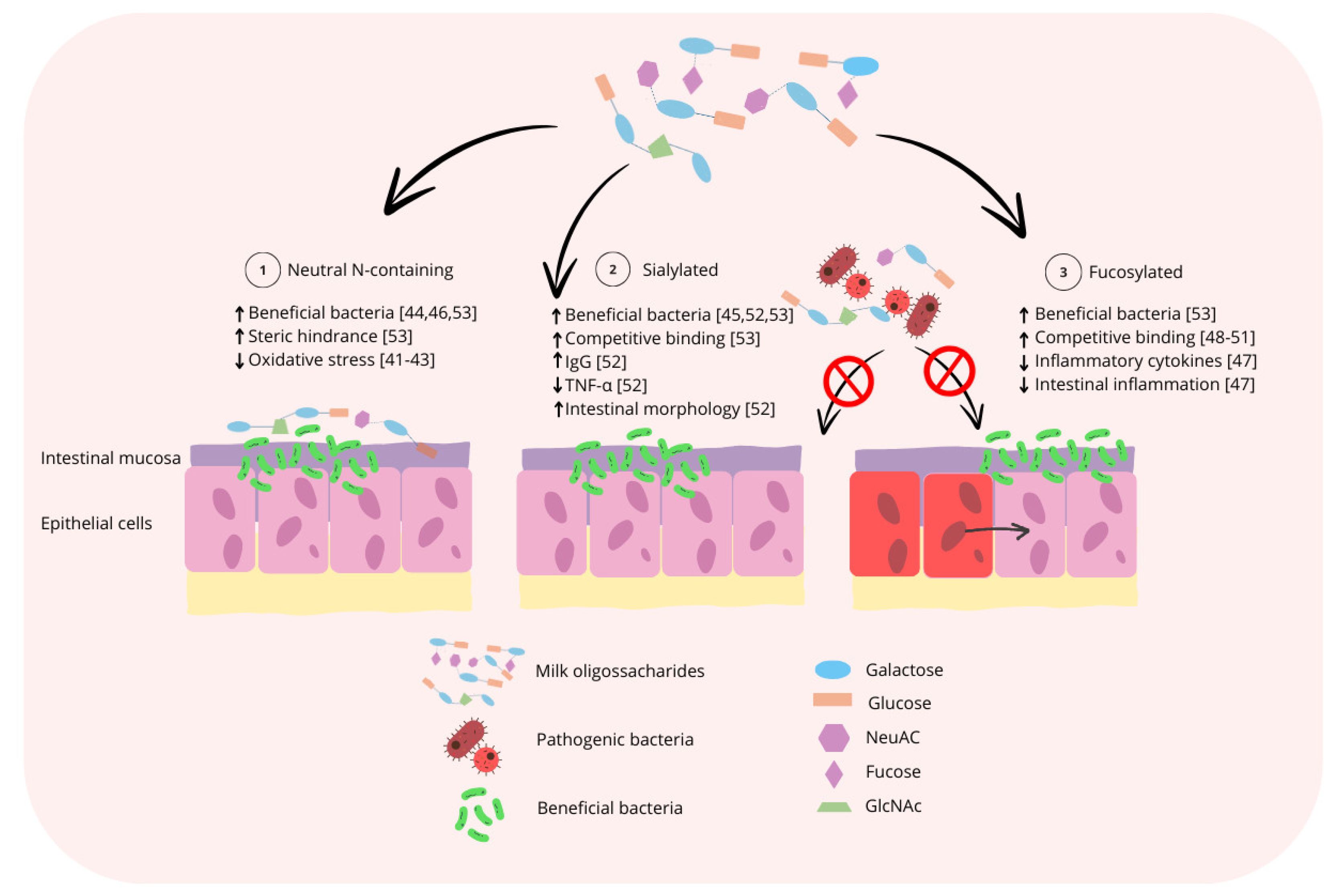
3. Functional Role of Milk Oligosaccharides
3.1. Influence on the Intestinal Microbiota
3.2. Immunomodulatory Properties
3.3. Impact on Intestinal Development, Nutrient Absorption, and Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Tissier, H. Recherches Sur La Flore Intestinale Des Nourrissons: (État Normal et Pathologique). Ph.D. Thesis, BIU Santé, Paris, France, 1900. [Google Scholar]
- Schönfeld, H. Über Die Beziehungen Der Einzelnen Bestandteile Der Frauenmilch Zur Bifidusflora. Jahrb. Der Kinderh 1926, 113, 19–60. [Google Scholar]
- Gauhe, A.; György, P.; Hoover, J.R.E.; Kuhn, R.; Rose, C.S.; Ruelius, H.W.; Zilliken, F. Bifidus Factor. IV. Preparations Obtained from Human Milk. Arch. Biochem. Biophys. 1954, 48, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Kobata, A.; Yamashita, K.; Tachibana, Y. [21] Oligosaccharides from Human Milk. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; pp. 216–220. [Google Scholar]
- Kunz, C.; Rudloff, S. Biological Functions of Oligosaccharides in Human Milk. Acta Paediatr. 1993, 82, 903–912. [Google Scholar] [CrossRef]
- Albrecht, S.; Lane, J.A.; Mariño, K.; Al Busadah, K.A.; Carrington, S.D.; Hickey, R.M.; Rudd, P.M. A Comparative Study of Free Oligosaccharides in the Milk of Domestic Animals. Br. J. Nutr. 2014, 111, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, J.; Frese, S.A.; Mills, D.A.; Barile, D. Characterization of Porcine Milk Oligosaccharides during Early Lactation and Their Relation to the Fecal Microbiome. J. Dairy Sci. 2016, 99, 7733–7743. [Google Scholar] [CrossRef]
- Messer, M.; Urashima, T. Evolution of Milk Oligosacharides and Lactose. Trends Glycosci. Glycotechnol. 2002, 14, 153–176. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Durham, S.D.; Wei, Z.; Lemay, D.G.; Lange, M.C.; Barile, D. Creation of a Milk Oligosaccharide Database, MilkOligoDB, Reveals Common Structural Motifs and Extensive Diversity across Mammals. Sci. Rep. 2023, 13, 10345. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; van Stigt, A.H.; Mank, M.; Willemsen, L.E.M.; Stahl, B.; Garssen, J.; van’t Land, B. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front. Pediatr. 2018, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Jantscher-Krenn, E. Structure-Function Relationships of Human Milk Oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef]
- Morozov, V.; Hansman, G.; Hanisch, F.; Schroten, H.; Kunz, C. Human Milk Oligosaccharides as Promising Antivirals. Mol. Nutr. Food Res. 2018, 62, 1700679. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, T.; Yoshida, A. Biochemical Evidence That Secretor Gene, Se, Is a Structural Gene Encoding a Specific Fucosyltransferase. Proc. Natl. Acad. Sci. USA 1984, 81, 4193–4197. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, G.; Shevlyakova, M.; Charpagne, A.; Marquis, J.; Vogel, M.; Kirsten, T.; Kiess, W.; Austin, S.; Sprenger, N.; Binia, A. Time of Lactation and Maternal Fucosyltransferase Genetic Polymorphisms Determine the Variability in Human Milk Oligosaccharides. Front. Nutr. 2020, 7, 574459. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Derraik, J.G.B.; Binia, A.; Sprenger, N.; Vickers, M.H.; Cutfield, W.S. Maternal and Infant Factors Influencing Human Milk Oligosaccharide Composition: Beyond Maternal Genetics. J. Nutr. 2021, 151, 1383–1393. [Google Scholar] [CrossRef]
- Bode, L. The Functional Biology of Human Milk Oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, Z.A.; Wang, B.; Jahan, M.; Wang, Z.; Wynn, P.C.; Du, Y. Characterization of Porcine Milk Oligosaccharides over Lactation between Primiparous and Multiparous Female Pigs. Sci. Rep. 2018, 8, 4688. [Google Scholar] [CrossRef]
- Cheng, L.; Xu, Q.; Yang, K.; He, J.; Chen, D.; Du, Y.; Yin, H. Annotation of Porcine Milk Oligosaccharides throughout Lactation by Hydrophilic Interaction Chromatography Coupled with Quadruple Time of Flight Tandem Mass Spectrometry. Electrophoresis 2016, 37, 1525–1531. [Google Scholar] [CrossRef]
- Urashima, T.; Kitaoka, M.; Asakuma, S.; Messer, M. Milk Oligosaccharides. In Advanced Dairy Chemistry; Springer: New York, NY, USA, 2009; pp. 295–349. [Google Scholar]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The Predominance of Type I Oligosaccharides Is a Feature Specific to Human Breast Milk. Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Lebrilla, C.B. Analysis and Role of Oligosaccharides in Milk. BMB Rep. 2012, 45, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Niñonuevo, M.R.; Perkins, P.D.; Francis, J.; Lamotte, L.M.; LoCascio, R.G.; Freeman, S.L.; Mills, D.A.; German, J.B.; Grimm, R.; Lebrilla, C.B. Daily Variations in Oligosaccharides of Human Milk Determined by Microfluidic Chips and Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Totten, S.M.; Zivkovic, A.M.; Wu, S.; Ngyuen, U.; Freeman, S.L.; Ruhaak, L.R.; Darboe, M.K.; German, J.B.; Prentice, A.M.; Lebrilla, C.B. Comprehensive Profiles of Human Milk Oligosaccharides Yield Highly Sensitive and Specific Markers for Determining Secretor Status in Lactating Mothers. J. Proteome Res. 2012, 11, 6124–6133. [Google Scholar] [CrossRef]
- Robinson, R.C. Structures and Metabolic Properties of Bovine Milk Oligosaccharides and Their Potential in the Development of Novel Therapeutics. Front. Nutr. 2019, 6, 50. [Google Scholar] [CrossRef]
- Fong, B.; Ma, K.; McJarrow, P. Quantification of Bovine Milk Oligosaccharides Using Liquid Chromatography–Selected Reaction Monitoring–Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 9788–9795. [Google Scholar] [CrossRef]
- ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H. Functional Role and Mechanisms of Sialyllactose and Other Sialylated Milk Oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Kirmiz, N.; Robinson, R.C.; Shah, I.M.; Barile, D.; Mills, D.A. Milk Glycans and Their Interaction with the Infant-Gut Microbiota. Annu. Rev. Food Sci. Technol. 2018, 9, 429–450. [Google Scholar] [CrossRef]
- Hobbs, M.; Jahan, M.; Ghorashi, S.A.; Wang, B. Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns. Foods 2021, 10, 473. [Google Scholar] [CrossRef]
- Mudd, A.T.; Salcedo, J.; Alexander, L.S.; Johnson, S.K.; Getty, C.M.; Chichlowski, M.; Berg, B.M.; Barile, D.; Dilger, R.N. Porcine Milk Oligosaccharides and Sialic Acid Concentrations Vary Throughout Lactation. Front. Nutr. 2016, 3, 39. [Google Scholar] [CrossRef]
- Baker, D.H. Animal Models in Nutrition Research. J. Nutr. 2008, 138, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Difilippo, E.; Pan, F.; Logtenberg, M.; Willems, R.H.A.M.; Braber, S.; Fink-Gremmels, J.; Schols, H.A.; Gruppen, H. Milk Oligosaccharide Variation in Sow Milk and Milk Oligosaccharide Fermentation in Piglet Intestine. J. Agric. Food Chem. 2016, 64, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning Stress and Gastrointestinal Barrier Development: Implications for Lifelong Gut Health in Pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef]
- Macdonald, A.A.; Bosma, A.A. Notes on Placentation in the Suina. Placenta 1985, 6, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Kim, S.W. Intestinal Microbiota and Its Interaction to Intestinal Health in Nursery Pigs. Anim. Nutr. 2022, 8, 169–184. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Z.; Zhang, S.; Page, G.; Jaworski, N.W. The Role of Lactose in Weanling Pig Nutrition: A Literature and Meta-Analysis Review. J. Anim. Sci. Biotechnol. 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.B.; Purvis, J.M.; Kim, S.W. Dose–Response and Functional Role of Whey Permeate as a Source of Lactose and Milk Oligosaccharides on Intestinal Health and Growth of Nursery Pigs. J. Anim. Sci. 2021, 99, skab008. [Google Scholar] [CrossRef]
- Thomson, P.; Medina, D.A.; Garrido, D. Human Milk Oligosaccharides and Infant Gut Bifidobacteria: Molecular Strategies for Their Utilization. Food Microbiol. 2018, 75, 37–46. [Google Scholar] [CrossRef]
- Herfel, T.M.; Jacobi, S.K.; Lin, X.; Jouni, Z.E.; Chichlowski, M.; Stahl, C.H.; Odle, J. Dietary Supplementation of Bifidobacterium Longum Strain AH1206 Increases Its Cecal Abundance and Elevates Intestinal Interleukin-10 Expression in the Neonatal Piglet. Food Chem. Toxicol. 2013, 60, 116–122. [Google Scholar] [CrossRef]
- Pang, J.; Liu, Y.; Kang, L.; Ye, H.; Zang, J.; Wang, J.; Han, D. Bifidobacterium Animalis Promotes the Growth of Weaning Piglets by Improving Intestinal Development, Enhancing Antioxidant Capacity, and Modulating Gut Microbiota. Appl. Environ. Microbiol. 2022, 88, e01296-22. [Google Scholar] [CrossRef]
- James, K.; Motherway, M.O.; Bottacini, F.; van Sinderen, D. Bifidobacterium Breve UCC2003 Metabolises the Human Milk Oligosaccharides Lacto-N-Tetraose and Lacto-N-Neo-Tetraose through Overlapping, yet Distinct Pathways. Sci. Rep. 2016, 6, 38560. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.; O’Connell Motherway, M.; Ventura, M.; van Sinderen, D. Metabolism of Sialic Acid by Bifidobacterium Breve UCC2003. Appl. Environ. Microbiol. 2014, 80, 4414–4426. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The Human Milk Oligosaccharide 2′-Fucosyllactose Modulates CD14 Expression in Human Enterocytes, Thereby Attenuating LPS-Induced Inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Marriage, B.J.; Buck, R.H.; Goehring, K.C.; Oliver, J.S.; Williams, J.A. Infants Fed a Lower Calorie Formula With 2′FL Show Growth and 2 ′ FL Uptake Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-T. Effects of Fucosylated Milk of Goat and Mouse on Helicobacter Pylori Binding to Lewis b Antigen. World J. Gastroenterol. 2004, 10, 2063. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Pickering, L.K.; McCluer, R.H.; Cleary, T.G. Fucosylated Oligosaccharides of Human Milk Protect Suckling Mice from Heat-Stabile Enterotoxin of Escherichia Coli. J. Infect. Dis. 1990, 162, 1075–1080. [Google Scholar] [CrossRef]
- Crane, J.K.; Azar, S.S.; Stam, A.; Newburg, D.S. Oligosaccharides from Human Milk Block Binding and Activity of the Escherichia Coli Heat-Stable Enterotoxin (STa) in T84 Intestinal Cells. J. Nutr. 1994, 124, 2358–2364. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter Jejuni Binds Intestinal H(O) Antigen (Fucα1, 2Galβ1, 4GlcNAc), and Fucosyloligosaccharides of Human Milk Inhibit Its Binding and Infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef]
- Li, D.; Chen, D.; Yu, B.; Luo, Y.; He, J. Effect of Sialyllactose Administration on Growth Performance and Intestinal Epithelium Development in Suckling Piglets. Anim. Feed Sci. Technol. 2022, 284, 115205. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human Milk Oligosaccharides Inhibit the Adhesion to Caco-2 Cells of Diarrheal Pathogens: Escherichia Coli, Vibrio Cholerae, and Salmonella Fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Jiang, L.; Wang, W.; Li, T.; Zuo, B.; Li, Z.; Wang, J. Differences in the Gut Microbiota Establishment and Metabolome Characteristics Between Low- and Normal-Birth-Weight Piglets During Early-Life. Front. Microbiol. 2018, 9, 1798. [Google Scholar] [CrossRef] [PubMed]
- Kabat, A.M.; Srinivasan, N.; Maloy, K.J. Modulation of Immune Development and Function by Intestinal Microbiota. Trends Immunol. 2014, 35, 507–517. [Google Scholar] [CrossRef]
- Jin, J.; Jia, J.; Zhang, L.; Chen, Q.; Zhang, X.; Sun, W.; Ma, C.; Xu, F.; Zhan, S.; Ma, L.; et al. Jejunal Inflammatory Cytokines, Barrier Proteins and Microbiome-Metabolome Responses to Early Supplementary Feeding of Bamei Suckling Piglets. BMC Microbiol. 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W.; et al. The Dynamic Distribution of Porcine Microbiota across Different Ages and Gastrointestinal Tract Segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef] [PubMed]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet Shapes the Gut Microbiome of Pigs during Nursing and Weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of Human Milk Oligosaccharides by Gut-Related Microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Berri, M.; Estellé, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.-J.; Chevaleyre, C.; Billon, Y.; Doré, J.; et al. Early-Life Establishment of the Swine Gut Microbiome and Impact on Host Phenotypes. Environ. Microbiol. Rep. 2015, 7, 554–569. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]
- Tao, N.; Ochonicky, K.L.; German, J.B.; Donovan, S.M.; Lebrilla, C.B. Structural Determination and Daily Variations of Porcine Milk Oligosaccharides. J. Agric. Food Chem. 2010, 58, 4653–4659. [Google Scholar] [CrossRef]
- Huang, A.; Cai, R.; Wang, Q.; Shi, L.; Li, C.; Yan, H. Dynamic Change of Gut Microbiota During Porcine Epidemic Diarrhea Virus Infection in Suckling Piglets. Front. Microbiol. 2019, 10, 322. [Google Scholar] [CrossRef]
- He, K.; Yan, W.; Sun, C.; Liu, J.; Bai, R.; Wang, T.; Qian, W. Alterations in the Diversity and Composition of Gut Microbiota in Weaned Piglets Infected with Balantioides Coli. Vet. Parasitol. 2020, 288, 109298. [Google Scholar] [CrossRef]
- Fouhse, J.M.; Zijlstra, R.T.; Willing, B.P. The Role of Gut Microbiota in the Health and Disease of Pigs. Anim. Front. 2016, 6, 30–36. [Google Scholar] [CrossRef]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernández-Barranco, A.; Margolles, A.; los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and Development of Intestinal Microbiota in Preterm Neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef]
- Prasoodanan, P.K.V.; Sharma, A.K.; Mahajan, S.; Dhakan, D.B.; Maji, A.; Scaria, J.; Sharma, V.K. Western and Non-Western Gut Microbiomes Reveal New Roles of Prevotella in Carbohydrate Metabolism and Mouth–Gut Axis. Npj Biofilms Microbiomes 2021, 7, 77. [Google Scholar] [CrossRef]
- Ramayo-Caldas, Y.; Mach, N.; Lepage, P.; Levenez, F.; Denis, C.; Lemonnier, G.; Leplat, J.-J.; Billon, Y.; Berri, M.; Doré, J.; et al. Phylogenetic Network Analysis Applied to Pig Gut Microbiota Identifies an Ecosystem Structure Linked with Growth Traits. ISME J. 2016, 10, 2973–2977. [Google Scholar] [CrossRef]
- Ivarsson, E.; Roos, S.; Liu, H.Y.; Lindberg, J.E. Fermentable Non-Starch Polysaccharides Increases the Abundance of Bacteroides–Prevotella–Porphyromonas in Ileal Microbial Community of Growing Pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef]
- Lallès, J.P.; Sève, B.; Pié, S.; Blazy, F.; Laffitte, J.; Oswald, I.P. Weaning Is Associated with an Upregulation of Expression of Inflammatory Cytokines in the Intestine of Piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef]
- Venema, K. Intestinal Fermentation of Lactose and Prebiotic Lactose Derivatives, Including Human Milk Oligosaccharides. Int. Dairy J. 2012, 22, 123–140. [Google Scholar] [CrossRef]
- Solomons, N. Fermentation, Fermented Foods and Lactose Intolerance. Eur. J. Clin. Nutr. 2002, 56, S50–S55. [Google Scholar] [CrossRef]
- Pierce, K.M.; Sweeney, T.; Brophy, P.O.; Callan, J.J.; McCarthy, P.; O’Doherty, J.V. Dietary Manipulation Post Weaning to Improve Piglet Performance and Gastro-Intestinal Health. Anim. Sci. 2005, 81, 347–356. [Google Scholar] [CrossRef]
- Hoeflinger, J.L.; Davis, S.R.; Chow, J.; Miller, M.J. In Vitro Impact of Human Milk Oligosaccharides on Enterobacteriaceae Growth. J. Agric. Food Chem. 2015, 63, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 12, 91–119. [Google Scholar]
- Grulee, C.G.; Sanford, H.N. The Influence of Breast and Artificial Feeding Oninfantile Eczema. J. Pediatr. 1936, 9, 223–225. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Takkinen, H.-M.; Niemelä, O.; Kaila, M.; Erkkola, M.; Ahonen, S.; Haapala, A.-M.; Kenward, M.G.; Pekkanen, J.; Lahesmaa, R.; et al. Timing of Infant Feeding in Relation to Childhood Asthma and Allergic Diseases. J. Allergy Clin. Immunol. 2013, 131, 78–86. [Google Scholar] [CrossRef]
- Lowe, A.J.; Thien, F.C.K.; Stoney, R.M.; Bennett, C.M.; Hosking, C.S.; Hill, D.J.; Carlin, J.B.; Abramson, M.J.; Dharmage, S.C. Associations between Fatty Acids in Colostrum and Breast Milk and Risk of Allergic Disease. Clin. Exp. Allergy 2008, 38, 1745–1751. [Google Scholar] [CrossRef]
- Wijga, A.H.; van Houwelingen, A.C.; Kerkhof, M.; Tabak, C.; de Jongste, J.C.; Gerritsen, J.; Boshuizen, H.; Brunekreef, B.; Smit, H.A. Breast Milk Fatty Acids and Allergic Disease in Preschool Children: The Prevention and Incidence of Asthma and Mite Allergy Birth Cohort Study. J. Allergy Clin. Immunol. 2006, 117, 440–447. [Google Scholar] [CrossRef]
- Lee, M.-T.; Wu, C.-C.; Ou, C.-Y.; Chang, J.-C.; Liu, C.-A.; Wang, C.-L.; Chuang, H.; Kuo, H.-C.; Hsu, T.-Y.; Chen, C.-P.; et al. A Prospective Birth Cohort Study of Different Risk Factors for Development of Allergic Diseases in Offspring of Non-Atopic Parents. Oncotarget 2017, 8, 10858–10870. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2016, 69, 41–51. [Google Scholar] [CrossRef]
- Comstock, S.S.; Li, M.; Wang, M.; Monaco, M.H.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Dietary Human Milk Oligosaccharides but Not Prebiotic Oligosaccharides Increase Circulating Natural Killer Cell and Mesenteric Lymph Node Memory T Cell Populations in Noninfected and Rotavirus-Infected Neonatal Piglets. J. Nutr. 2017, 147, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Aldredge, D.L.; Geronimo, M.R.; Hua, S.; Nwosu, C.C.; Lebrilla, C.B.; Barile, D. Annotation and Structural Elucidation of Bovine Milk Oligosaccharides and Determination of Novel Fucosylated Structures. Glycobiology 2013, 23, 664–676. [Google Scholar] [CrossRef]
- Donovan, S.M. Human Milk Oligosaccharides—The Plot Thickens. Br. J. Nutr. 2009, 101, 1267. [Google Scholar] [CrossRef] [PubMed]
- Hester, S.N.; Chen, X.; Li, M.; Monaco, M.H.; Comstock, S.S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Human Milk Oligosaccharides Inhibit Rotavirus Infectivity in Vitro and in Acutely Infected Piglets. Br. J. Nutr. 2013, 110, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Firon, N.; Ofek, I.; Sharon, N. Carbohydrate Specificity of the Surface Lectins of Escherichia Coli, Klebsiella Pneumoniae, and Salmonella Typhimurium. Carbohydr. Res. 1983, 120, 235–249. [Google Scholar] [CrossRef]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Fahey, G.C., Jr.; Miller, M.J.; Kuhlenschmidt, M.S.; Donovan, S.M. Human Milk Oligosaccharides Shorten Rotavirus-Induced Diarrhea and Modulate Piglet Mucosal Immunity and Colonic Microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef]
- Daniels, V.C.; Monaco, M.H.; Wang, M.; Hirvonen, J.; Jensen, H.M.; Ouwehand, A.C.; Mukherjea, R.; Dilger, R.N.; Donovan, S.M. Evaluation of 2’-Fucosyllactose and Bifidobacterium Longum Subspecies Infantis on Growth, Organ Weights, and Intestinal Development of Piglets. Nutrients 2021, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated With Excessive Weight Gain During Exclusive Breastfeeding—An Explorative Study. Front. Pediatr. 2019, 7, 297. [Google Scholar] [CrossRef]
- Davis, J.C.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and Morbidity of Gambian Infants Are Influenced by Maternal Milk Oligosaccharides and Infant Gut Microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef]
- Loutet, M.G.; Narimani, A.; Qamar, H.; Yonemitsu, C.; Pell, L.G.; Mahmud, A.A.; Ahmed, T.; Bode, L.; Bassani, D.G.; Roth, D.E. Associations between Human Milk Oligosaccharides and Infant Growth in a Bangladeshi Mother–Infant Cohort. Pediatr. Res. 2023, 96, 356–364. [Google Scholar] [CrossRef]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between Human Milk Oligosaccharides and Infant Body Composition in the First 6 Mo of Life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.H.; Wang, M.; Pan, X.; Li, Q.; Richards, J.D.; Chichlowski, M.; Berg, B.M.; Dilger, R.N.; Donovan, S.M. Evaluation of Sialyllactose Supplementation of a Prebiotic-Containing Formula on Growth, Intestinal Development, and Bacterial Colonization in the Neonatal Piglet. Curr. Dev. Nutr. 2018, 2, nzy067. [Google Scholar] [CrossRef] [PubMed]
- Golden, R.K.; Sutkus, L.T.; Bauer, L.L.; Donovan, S.M.; Dilger, R.N. Determining the Safety and Efficacy of Dietary Supplementation with 3′-Sialyllactose or 6′-Sialyllactose on Growth, Tolerance, and Brain Sialic Acid Concentrations. Front. Nutr. 2023, 10, 1278804. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-Chain Fatty Acids Can Improve Lipid and Glucose Metabolism Independently of the Pig Gut Microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 61. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Jiao, A.; Diao, H.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Wang, Q.; Wang, H.; et al. Infusion of Short Chain Fatty Acids in the Ileum Improves the Carcass Traits, Meat Quality and Lipid Metabolism of Growing Pigs. Anim. Nutr. 2021, 7, 94–100. [Google Scholar] [CrossRef]
- Cho, S.; Zhu, Z.; Li, T.; Baluyot, K.; Howell, B.R.; Hazlett, H.C.; Elison, J.T.; Hauser, J.; Sprenger, N.; Wu, D.; et al. Human Milk 3’-Sialyllactose Is Positively Associated with Language Development during Infancy. Am. J. Clin. Nutr. 2021, 114, 588–597. [Google Scholar] [CrossRef]
- Jorgensen, J.M.; Young, R.; Ashorn, P.; Ashorn, U.; Chaima, D.; Davis, J.C.; Goonatilleke, E.; Kumwenda, C.; Lebrilla, C.B.; Maleta, K.; et al. Associations of Human Milk Oligosaccharides and Bioactive Proteins with Infant Growth and Development among Malawian Mother-Infant Dyads. Am. J. Clin. Nutr. 2021, 113, 209–220. [Google Scholar] [CrossRef]
- Cowardin, C.A.; Ahern, P.P.; Kung, V.L.; Hibberd, M.C.; Cheng, J.; Guruge, J.L.; Sundaresan, V.; Head, R.D.; Barile, D.; Mills, D.A.; et al. Mechanisms by Which Sialylated Milk Oligosaccharides Impact Bone Biology in a Gnotobiotic Mouse Model of Infant Undernutrition. Proc. Natl. Acad. Sci. USA 2019, 116, 11988–11996. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Delgado-Garcia, J.M.; Buck, R.; Rueda, R.; Martin, M.J. Oral Supplementation of 2′-Fucosyllactose during Lactation Improves Memory and Learning in Rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef]
- Lee, S.; Goodson, M.; Vang, W.; Kalanetra, K.; Barile, D.; Raybould, H. 2′-Fucosyllactose Supplementation Improves Gut-Brain Signaling and Diet-Induced Obese Phenotype and Changes the Gut Microbiota in High Fat-Fed Mice. Nutrients 2020, 12, 1003. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Goodson, M.L.; Vang, W.; Rutkowsky, J.; Kalanetra, K.; Bhattacharya, M.; Barile, D.; Raybould, H.E. Human Milk Oligosaccharide 2′-Fucosyllactose Supplementation Improves Gut Barrier Function and Signaling in the Vagal Afferent Pathway in Mice. Food Funct. 2021, 12, 8507–8521. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Dietary Oligofructose Alone or in Combination with 2′-Fucosyllactose Differentially Improves Recognition Memory and Hippocampal MRNA Expression. Nutrients 2020, 12, 2131. [Google Scholar] [CrossRef]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Human and Bovine Milk Oligosaccharides Elicit Improved Recognition Memory Concurrent With Alterations in Regional Brain Volumes and Hippocampal MRNA Expression. Front. Neurosci. 2020, 14, 770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gormley, A.; Garavito-Duarte, Y.; Kim, S.W. The Role of Milk Oligosaccharides in Enhancing Intestinal Microbiota, Intestinal Integrity, and Immune Function in Pigs: A Comparative Review. Biology 2024, 13, 663. https://doi.org/10.3390/biology13090663
Gormley A, Garavito-Duarte Y, Kim SW. The Role of Milk Oligosaccharides in Enhancing Intestinal Microbiota, Intestinal Integrity, and Immune Function in Pigs: A Comparative Review. Biology. 2024; 13(9):663. https://doi.org/10.3390/biology13090663
Chicago/Turabian StyleGormley, Alexa, Yesid Garavito-Duarte, and Sung Woo Kim. 2024. "The Role of Milk Oligosaccharides in Enhancing Intestinal Microbiota, Intestinal Integrity, and Immune Function in Pigs: A Comparative Review" Biology 13, no. 9: 663. https://doi.org/10.3390/biology13090663





