Imipramine Increases Norepinephrine and Serotonin in the Salivary Glands of Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. In Vivo Microdialysis in Anesthetized Rats
2.4. Quantification of Monoamines in Dialysates
2.5. Statistical Analyses
3. Results
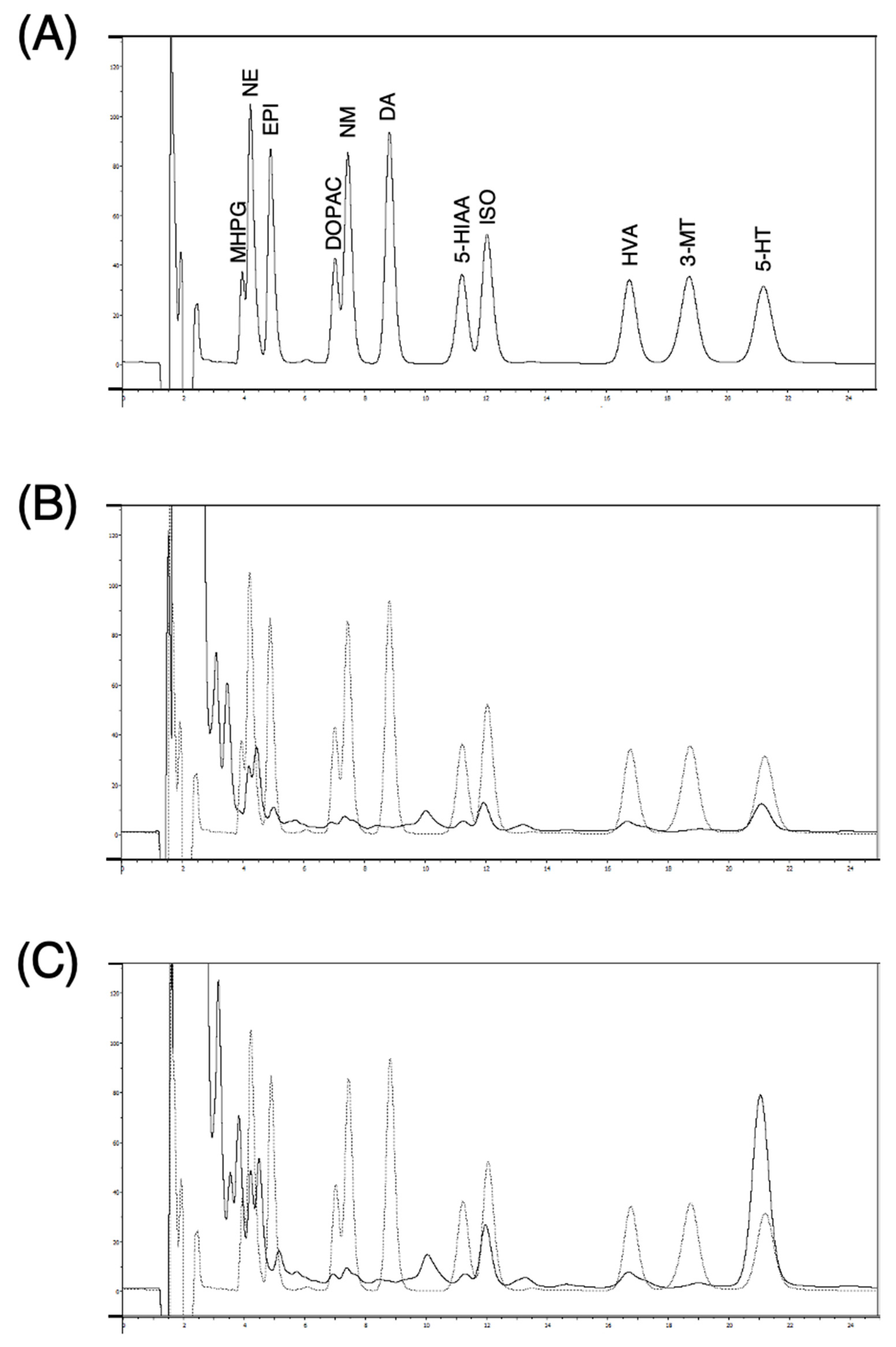
3.1. Conditions for Optimal Dialysis Perfusion Rate In Vitro
3.2. Monoamines in Dialysates from Submandibular Glands of Rats
3.3. Time Course of Norepinephrine and Serotonin Levels in Dialysate after Probe Implantation
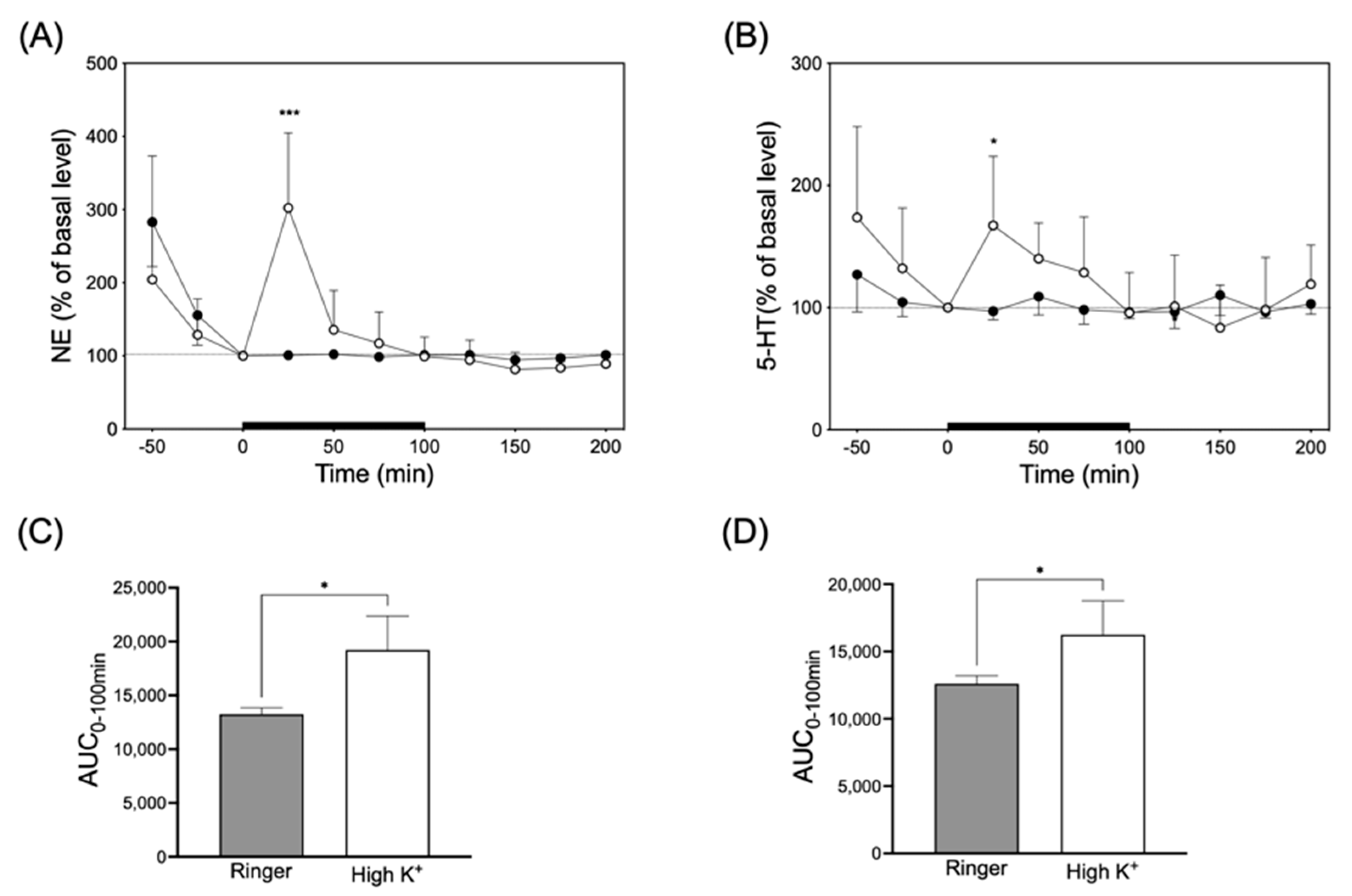
3.4. Infusion of High-K+ Solution
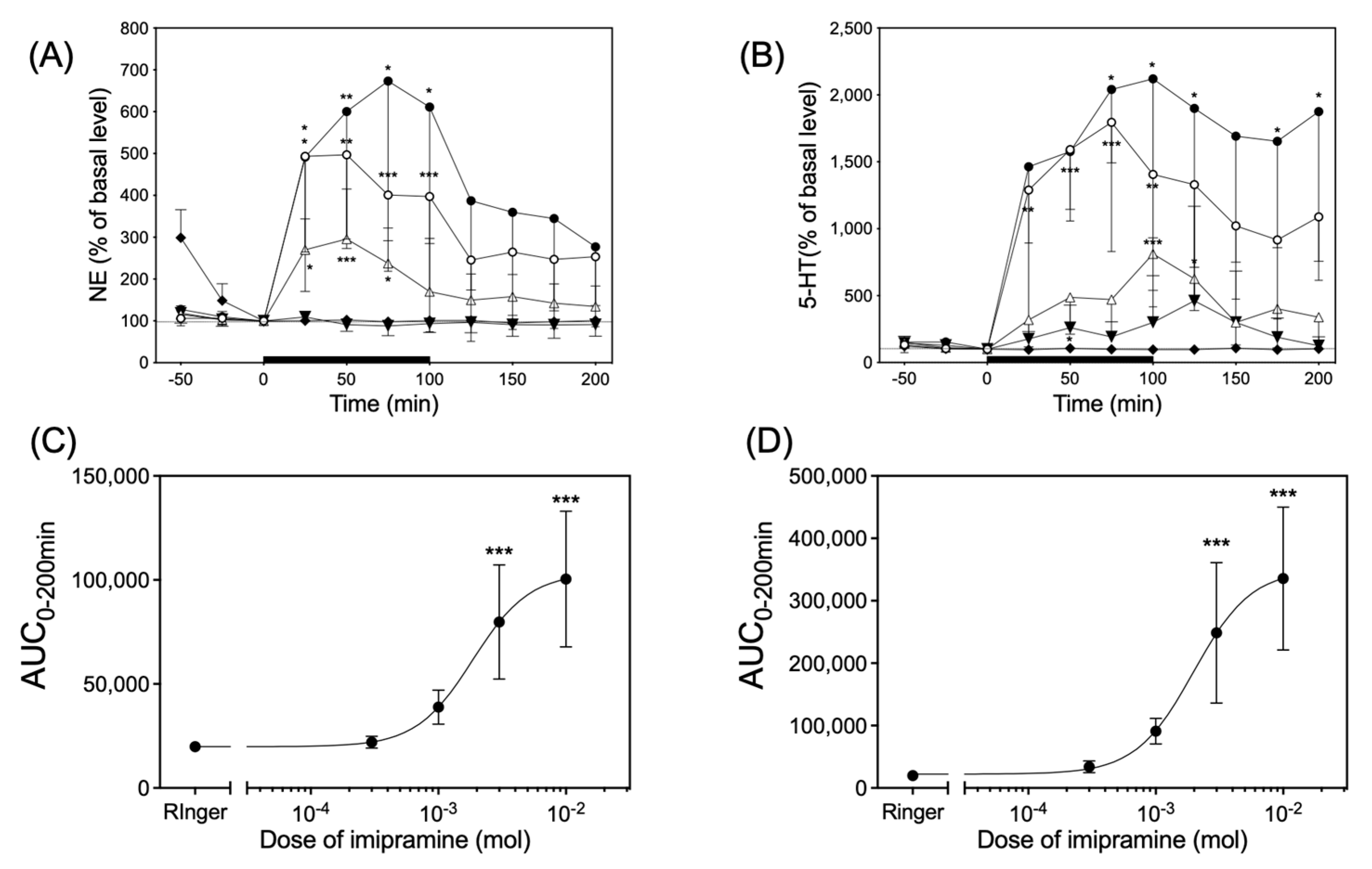
3.5. Infusion of Imipramine
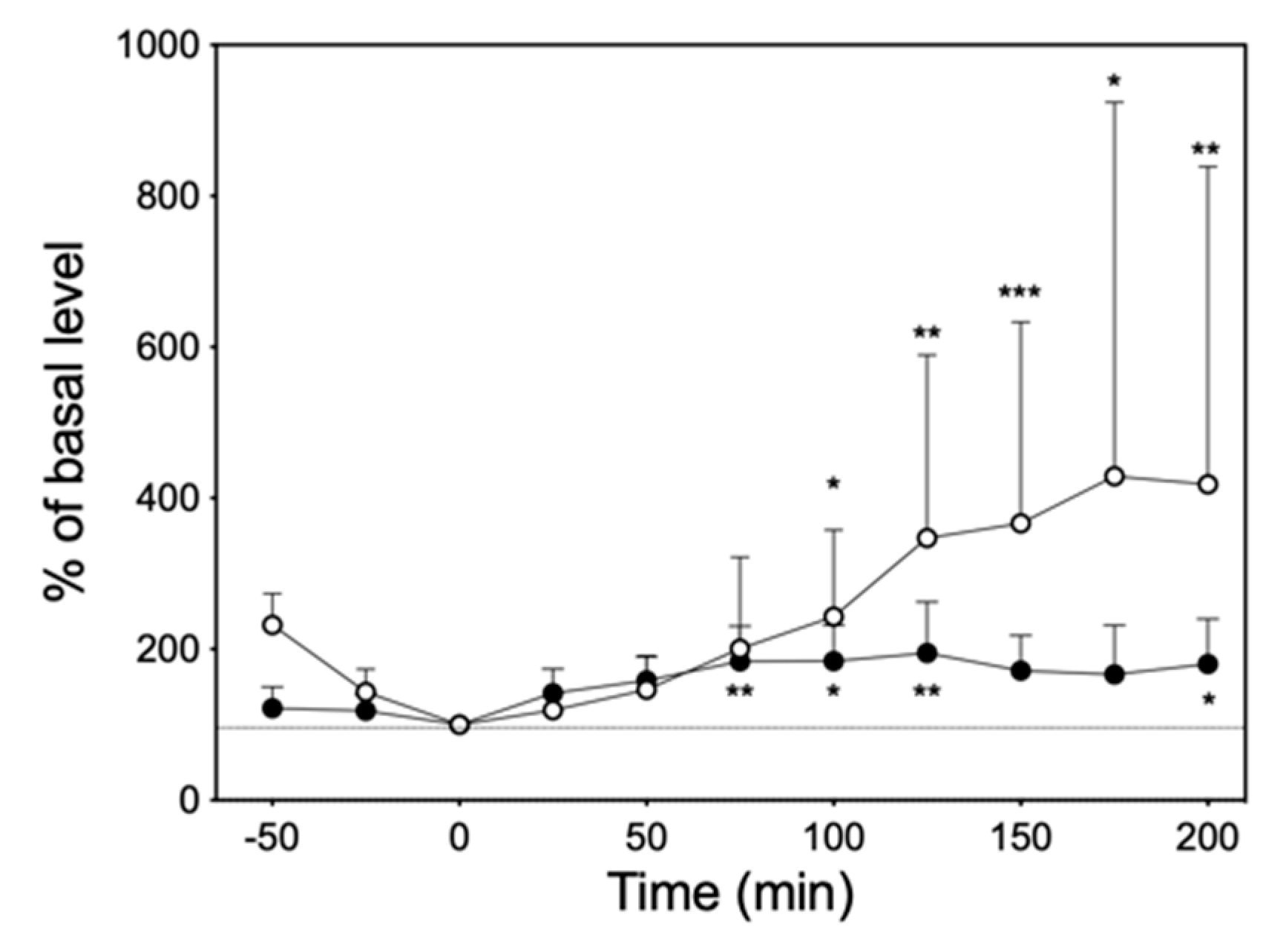
3.6. Intraperitoneal Administration of Imipramine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proctor, G.; Carpenter, G. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Tobin, G.; Giglio, D.; Lundgren, O. Muscarinic receptor subtypes in the alimentary tract. J. Physiol. Pharmacol. 2009, 60, 3–21. [Google Scholar]
- Scully, C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003, 9, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ramos Casals, M.; Tzioufas, A.; Stone, J.; Sisó, A.; Bosch, X. Treatment of primary Sjögren syndrome: A systematic review. JAMA J. Am. Med. Assoc. 2010, 304, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Dirix, P.; Nuyts, S.; Van den Bogaert, W. Radiation-induced xerostomia in patients with head and neck cancer: A literature review. Cancer 2006, 107, 2525–2534. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef]
- Khawam, E.A.; Laurencic, G.; Malone, D.A., Jr. Side effects of antidepressants: An overview. Clevel. Clin. J. Med. 2006, 73, 351–361. [Google Scholar] [CrossRef]
- Arany, S.; Kopycka-Kedzierawski, D.T.; Caprio, T.V.; Watson, G.E. Anticholinergic medication: Related dry mouth and effects on the salivary glands. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 662–670. [Google Scholar] [CrossRef]
- Huot, P.; Fox, S.H.; Brotchie, J.M. Monoamine reuptake inhibitors in parkinson’s disease. Park. Dis. 2015, 2015, 609428. [Google Scholar] [CrossRef]
- Licinio, J.; Wong, M.L. Pharmacogenomics of antidepressant treatment effects. Dial. Clin. Neurosci. 2011, 13, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Obata, H. Analgesic mechanisms of antidepressants for neuropathic pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef]
- Bourdon, D.M.; Camden, J.M.; Landon, L.A.; Levy, F.O.; Turner, J.T. Identification of the adenylyl cyclase-activating 5-hydroxytryptamine receptor subtypes expressed in the rat submandibular gland. Br. J. Pharmacol. 2000, 130, 104–108. [Google Scholar] [CrossRef]
- Turner, J.T.; Sullivan, D.M.; Rovira, I.; Camden, J.M. A regulatory role in mammalian salivary glands for 5-hydroxytryptamine receptors coupled to increased cyclic AMP production. J. Dent. Res. 1996, 75, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Kawaguchi, M. In Vivo Monitoring of Acetylcholine Release from Nerve Endings in Salivary Gland. Biology 2021, 10, 351. [Google Scholar] [CrossRef]
- Kitamura, Y.; Doi, M.; Kuwatsuka, K.; Onoue, Y.; Miyazaki, I.; Shinomiya, K.; Koyama, T.; Sendo, T.; Kawasaki, H.; Asanuma, M.; et al. Chronic treatment with imipramine and lithium increases cell proliferation in the hippocampus in adrenocorticotropic hormone-treated rats. Biol. Pharm. Bull. 2011, 34, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Fenton, E.Y.; Fournier, N.M.; Lussier, A.L.; Romay-Tallon, R.; Caruncho, H.J.; Kalynchuk, L.E. Imipramine protects against the deleterious effects of chronic corticosterone on depression-like behavior, hippocampal reelin expression, and neuronal maturation. Prog Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 52–59. [Google Scholar]
- Habib, M.; Shaker, S.; El-Gayar, N.; Aboul-Fotouh, S. The effects of antidepressants “fluoxetine and imipramine” on vascular abnormalities and Toll like receptor-4 expression in diabetic and non-diabetic rats exposed to chronic stress. PLoS ONE 2015, 10, e0120559. [Google Scholar] [CrossRef]
- Maldonado, J.O.; Riveros, P.P.; Chiorini, J.A. Myoepithelial Cell Function in Salivary Gland Physiology and Disease. In Sjögren’s Syndrome and Oral Health: Disease Characteristics and Management of Oral Manifestations; Cha, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Galligan, J.J.; Parkman, H. Recent advances in understanding the role of serotonin in gastrointestinal motility and functional bowel disorders. Neurogastroenterol. Motil. 2007, 19 (Suppl. S2), 1–4. [Google Scholar]
- Glatzle, J.; Sternini, C.; Robin, C.; Zittel, T.T.; Wong, H.; Reeve, J.R., Jr.; Raybould, H.E. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 2002, 123, 217–226. [Google Scholar] [CrossRef]
- Poole, D.P.; Xu, B.; Koh, S.L.; Hunne, B.; Coupar, I.M.; Irving, H.R.; Shinjo, K.; Furness, J.B. Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res. 2006, 325, 413–422. [Google Scholar] [CrossRef]
- Raybould, H.E.; Glatzle, J.; Robin, C.; Meyer, J.H.; Phan, T.; Wong, H.; Sternini, C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G367–G372. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol. Motil. 2007, 19 (Suppl. S2), 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Sanders, K.M.; Hirst, G.D. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol. Motil. 2004, 16 (Suppl. S1), 112–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Galligan, J.J. Synaptic activation and properties of 5-hydroxytryptamine(3) receptors in myenteric neurons of guinea pig intestine. J. Pharmacol. Exp. Ther. 1999, 290, 803–810. [Google Scholar]
- Fiorica-Howells, E.; Hen, R.; Gingrich, J.; Li, Z.; Gershon, M.D. 5-HT(2A) receptors: Location and functional analysis in intestines of wild-type and 5-HT(2A) knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G877–G893. [Google Scholar] [CrossRef] [PubMed]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Johnson, D.S.; Heinemann, S.F. Embryonic expression of the 5-HT3 receptor subunit, 5-HT3R-A, in the rat: An in situ hybridization study. Mol. Cell Neurosci. 1995, 6, 122–138. [Google Scholar] [CrossRef]
- Sadek, J. Antidepressants. In Clinician’s Guide to Psychopharmacology; Sadek, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Zolkowska, D.; Baumann, M.H.; Rothman, R.B. Chronic fenfluramine administration increases plasma serotonin (5-hydroxytryptamine) to nontoxic levels. J. Pharmacol. Exp. Ther. 2008, 324, 791–797. [Google Scholar] [CrossRef]
- Murai, S.; Saito, H.; Masuda, Y.; Itoh, T. Sex-dependent differences in the concentrations of the principal neurotransmitters, noradrenaline and acetylcholine, in the three major salivary glands of mice. Arch. Oral Biol. 1998, 43, 9–14. [Google Scholar] [CrossRef]
- Murai, S.; Saito, H.; Masuda, Y.; Itsukaichi, O.; Itoh, T. Basal levels of noradrenaline, dopamine, 5-hydroxytryptamine, and acetylcholine in the submandibular, parotid, and sublingual glands of mice and rats. Arch. Oral Biol. 1995, 40, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, Y.K. Molecular Neurobiology and Promising New Treatment in Depression. Int. J. Mol. Sci. 2016, 17, 381. [Google Scholar] [CrossRef]
- Koller, M.M.; Maeda, N.; Scarpace, P.J.; Humphreys-Beher, M.G. Desipramine changes salivary gland function, oral microbiota, and oral health in rats. Eur. J. Pharmacol. 2000, 408, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.R.; Fiais, G.A.; Oliveira, H.A.; Ribas, T.B.; Souza, R.O.; Tsosura, T.V.S.; Matsushita, D.H.; Ervolino, E.; Dornelles, R.C.M.; Nakamune, A.; et al. Assessment of redox state and biochemical parameters of salivary glands in rats treated with anti-obesity drug sibutramine hydrochloride. Clin. Oral Investig. 2022, 26, 5833–5846. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, M.; Winder, M.; Zawia, H.; Lödöen, I.; Tobin, G.; Götrick, B. In vivo studies of effects of antidepressants on parotid salivary secretion in the rat. Arch. Oral Biol. 2016, 67, 54–60. [Google Scholar] [CrossRef]
- Scarpace, P.J.; Koller, M.M.; Rajakumar, G. Desipramine desensitizes β-adrenergic signal transduction in rat salivary glands. Neuropharmacology 1992, 31, 1305–1309. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirose, K.; Yoshikawa, M.; Kan, T.; Miura, M.; Watanabe, M.; Matsuda, M.; Kobayashi, H.; Kawaguchi, M.; Ito, K.; Suzuki, T. Imipramine Increases Norepinephrine and Serotonin in the Salivary Glands of Rats. Biology 2024, 13, 679. https://doi.org/10.3390/biology13090679
Shirose K, Yoshikawa M, Kan T, Miura M, Watanabe M, Matsuda M, Kobayashi H, Kawaguchi M, Ito K, Suzuki T. Imipramine Increases Norepinephrine and Serotonin in the Salivary Glands of Rats. Biology. 2024; 13(9):679. https://doi.org/10.3390/biology13090679
Chicago/Turabian StyleShirose, Kosuke, Masanobu Yoshikawa, Takugi Kan, Masaaki Miura, Mariko Watanabe, Mitsumasa Matsuda, Hiroyuki Kobayashi, Mitsuru Kawaguchi, Kenji Ito, and Takeshi Suzuki. 2024. "Imipramine Increases Norepinephrine and Serotonin in the Salivary Glands of Rats" Biology 13, no. 9: 679. https://doi.org/10.3390/biology13090679



