Analysis of Metabolic Profiles and Antioxidant Activity of Chinese Cordyceps, Ophiocordyceps sinensis, and Paecilomyces hepiali Based on Untargeted Metabolomics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Untargeted Metabolomics Profiling
2.2.1. QC Sample Preparation and Metabolites Extraction
2.2.2. LC-MS/MS Condition
2.2.3. Mass Spectrum Condition
2.2.4. Data Processing
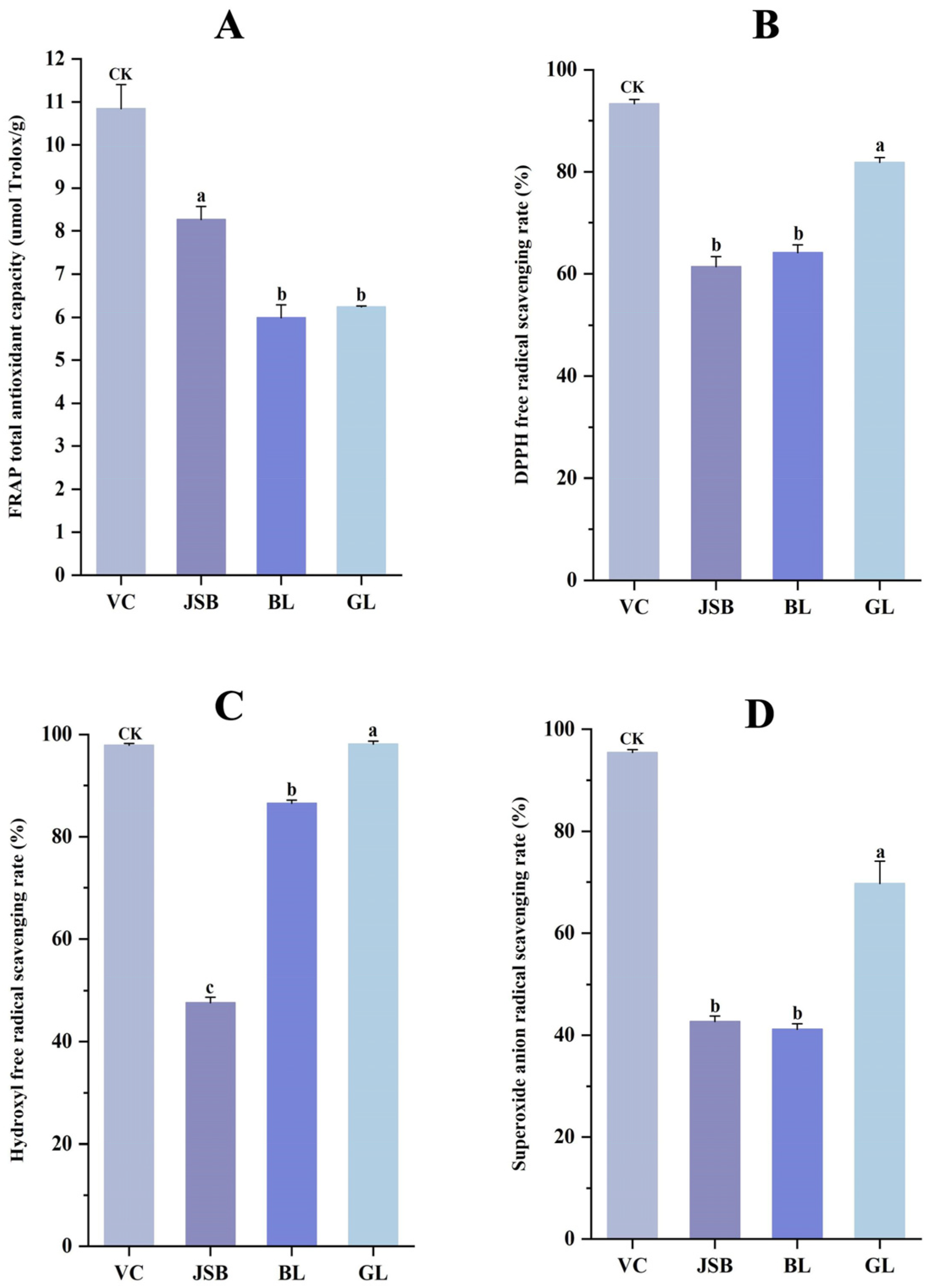
2.3. Determination of Antioxidant Activity
2.3.1. Determination of DPPH Free Radical Scavenging Ability
2.3.2. Determination of FRAP Total Antioxidant Capacity
2.3.3. Determination of Hydroxyl Free Radical Scavenging Ability
2.3.4. Determination of Superoxide Anion Radical Scavenging Capacity
2.4. Statistical Analyses
3. Results
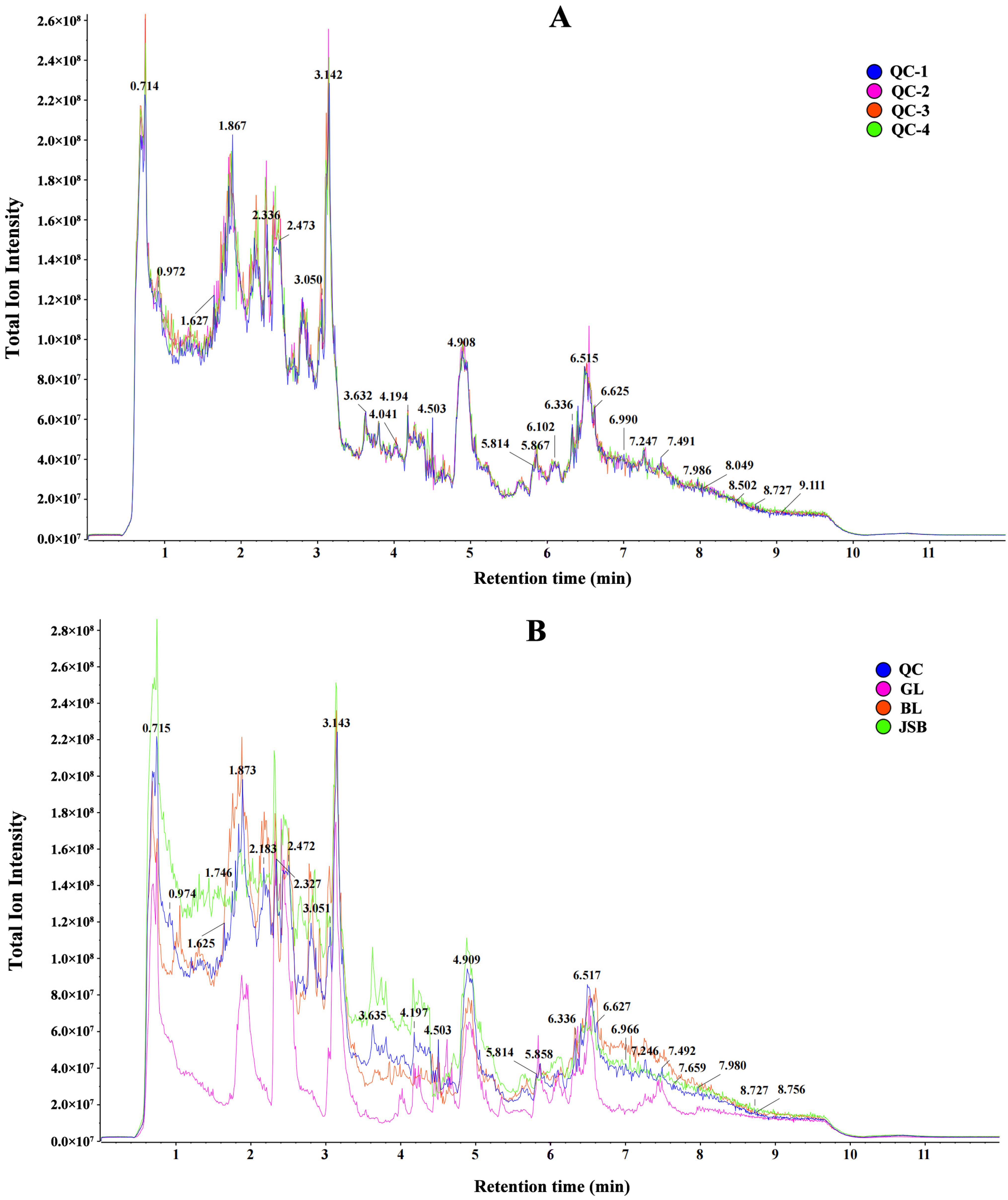
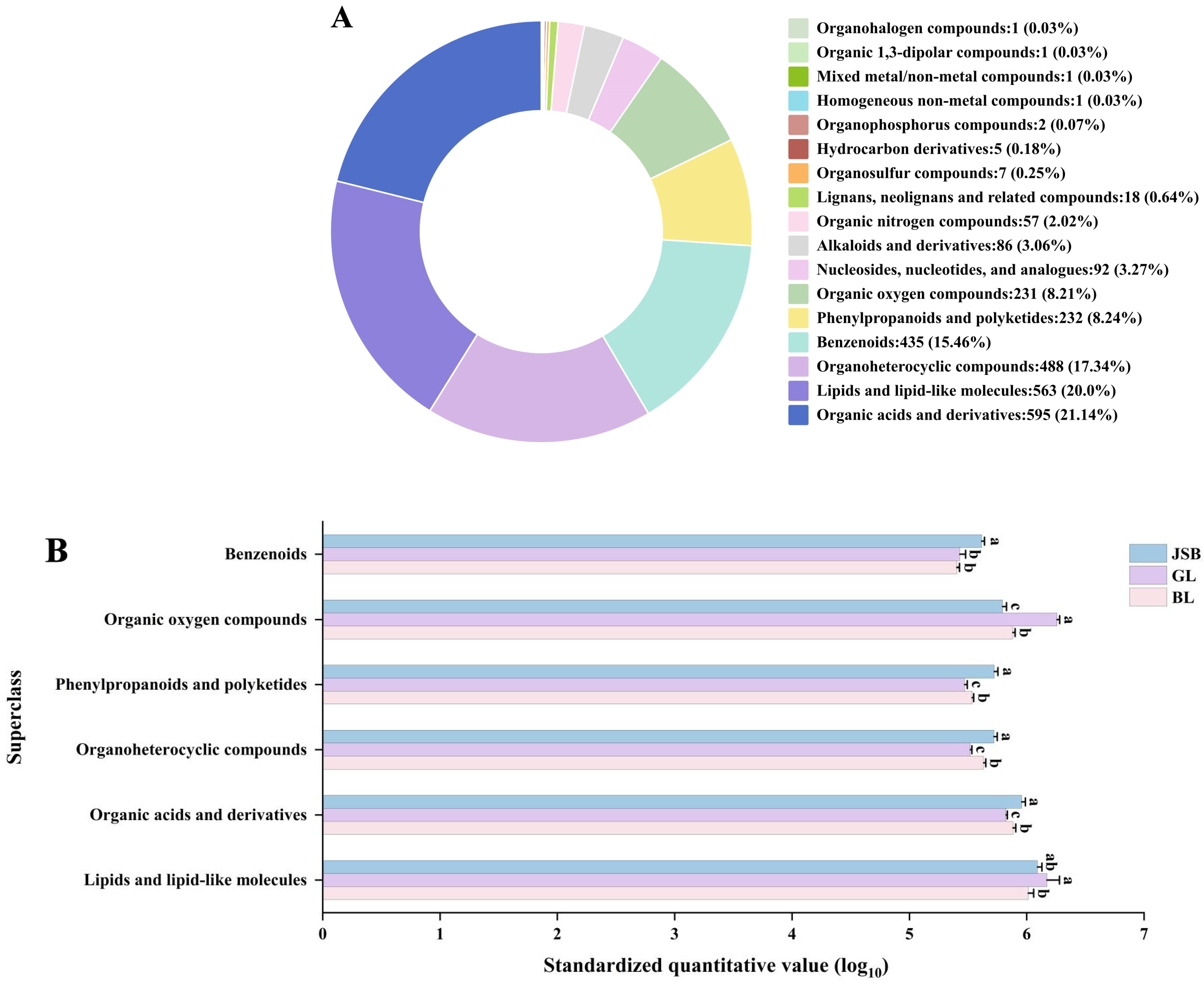
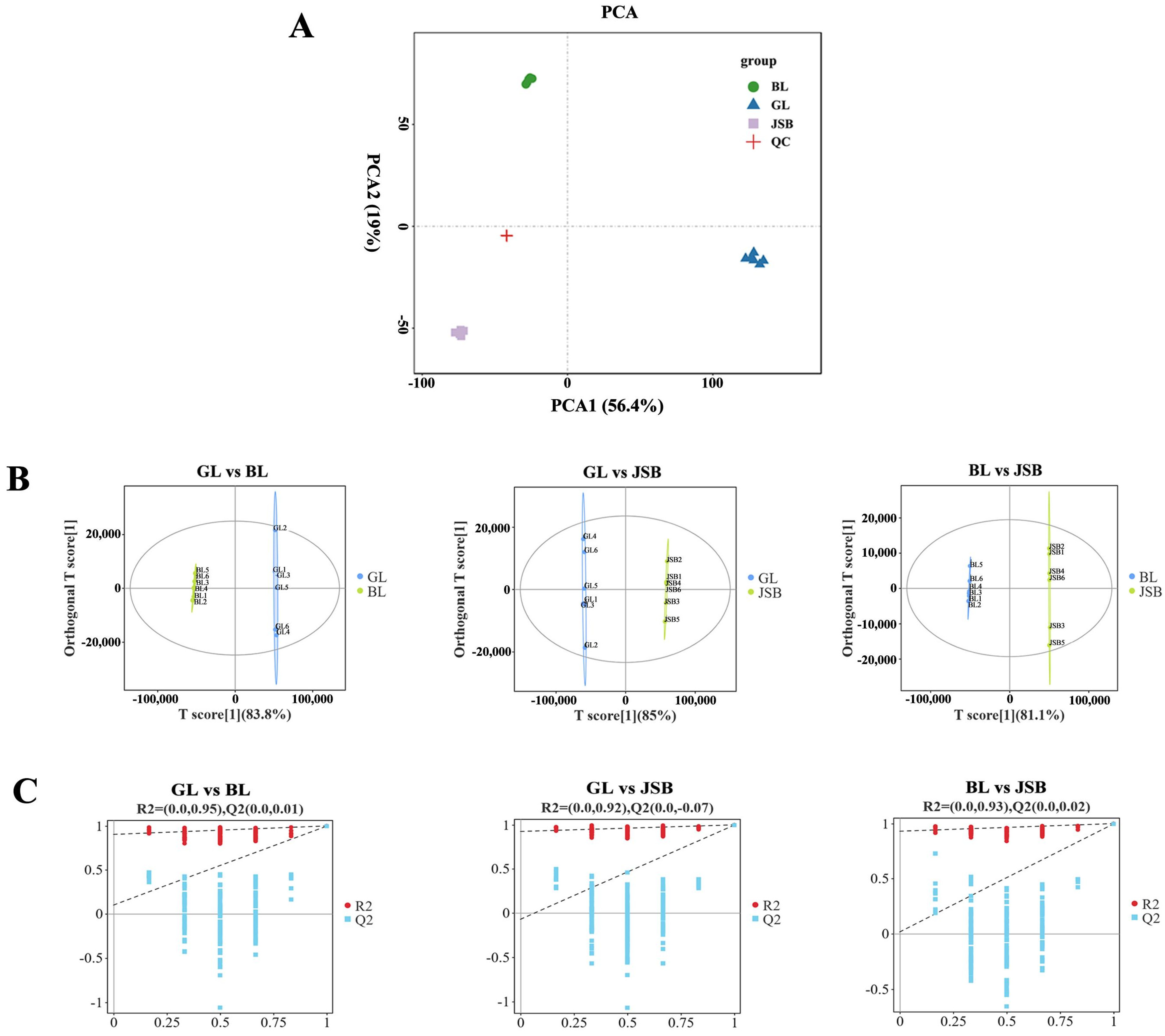
3.1. Metabolite Profiles Analysis
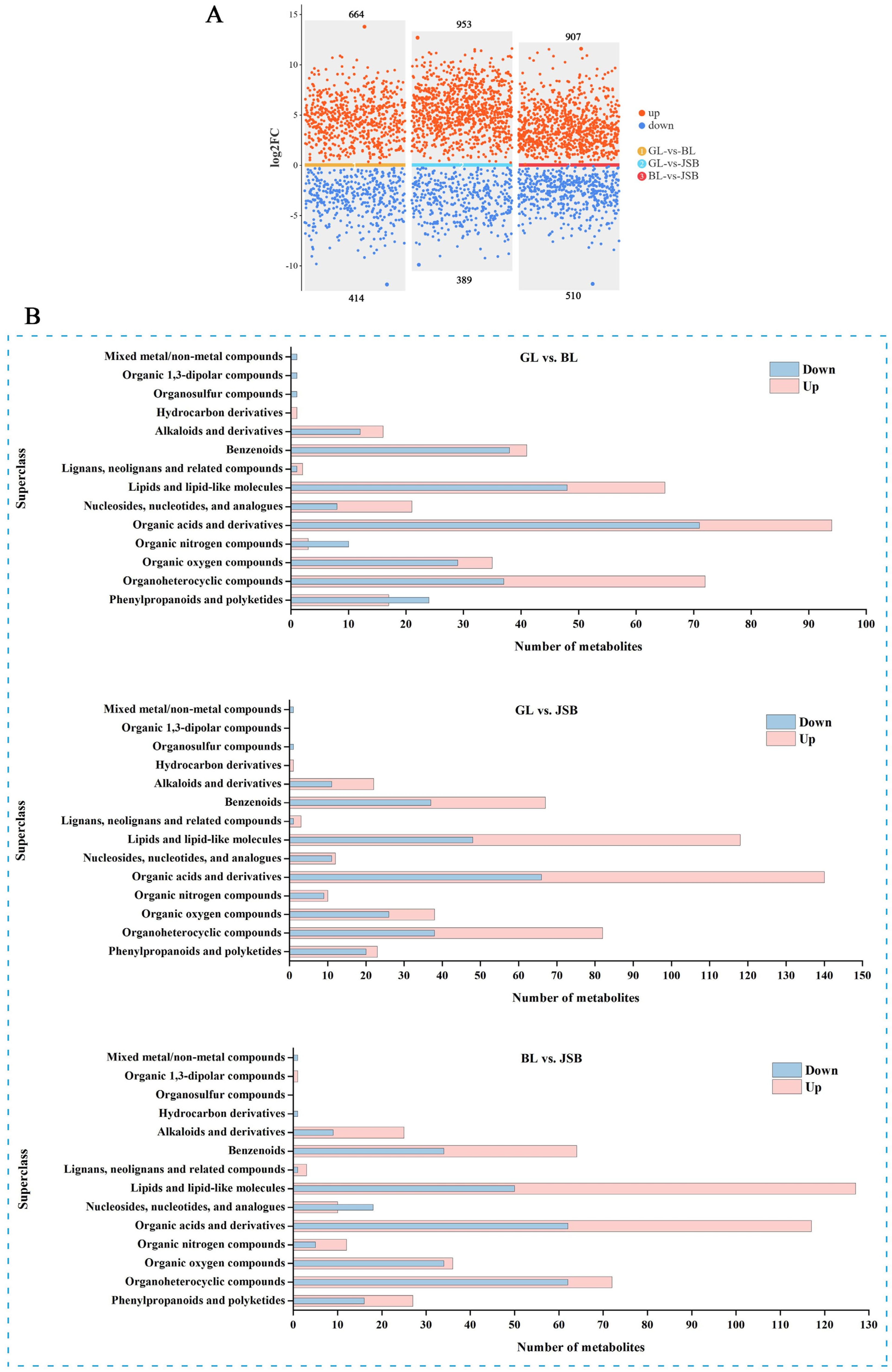
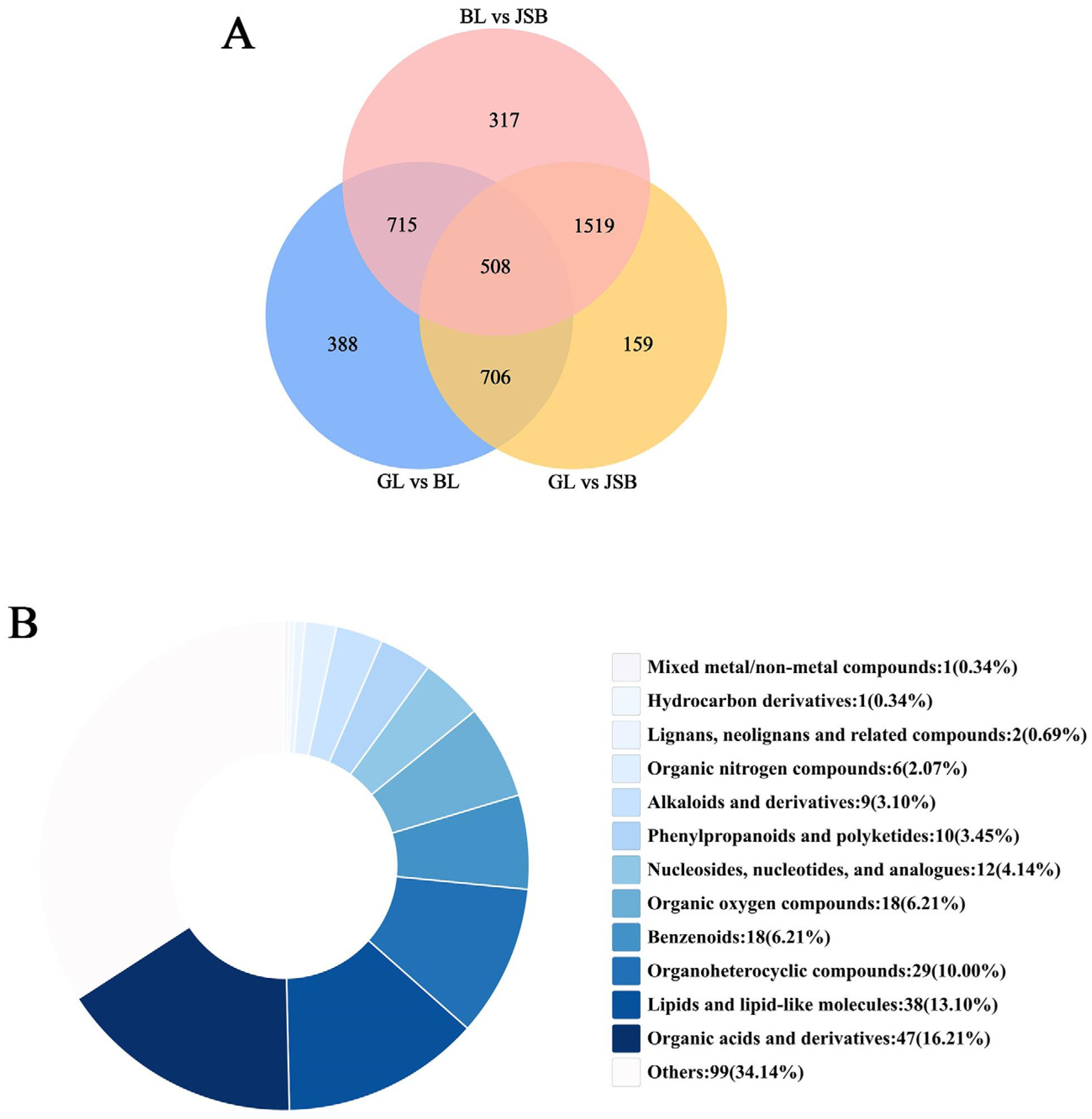
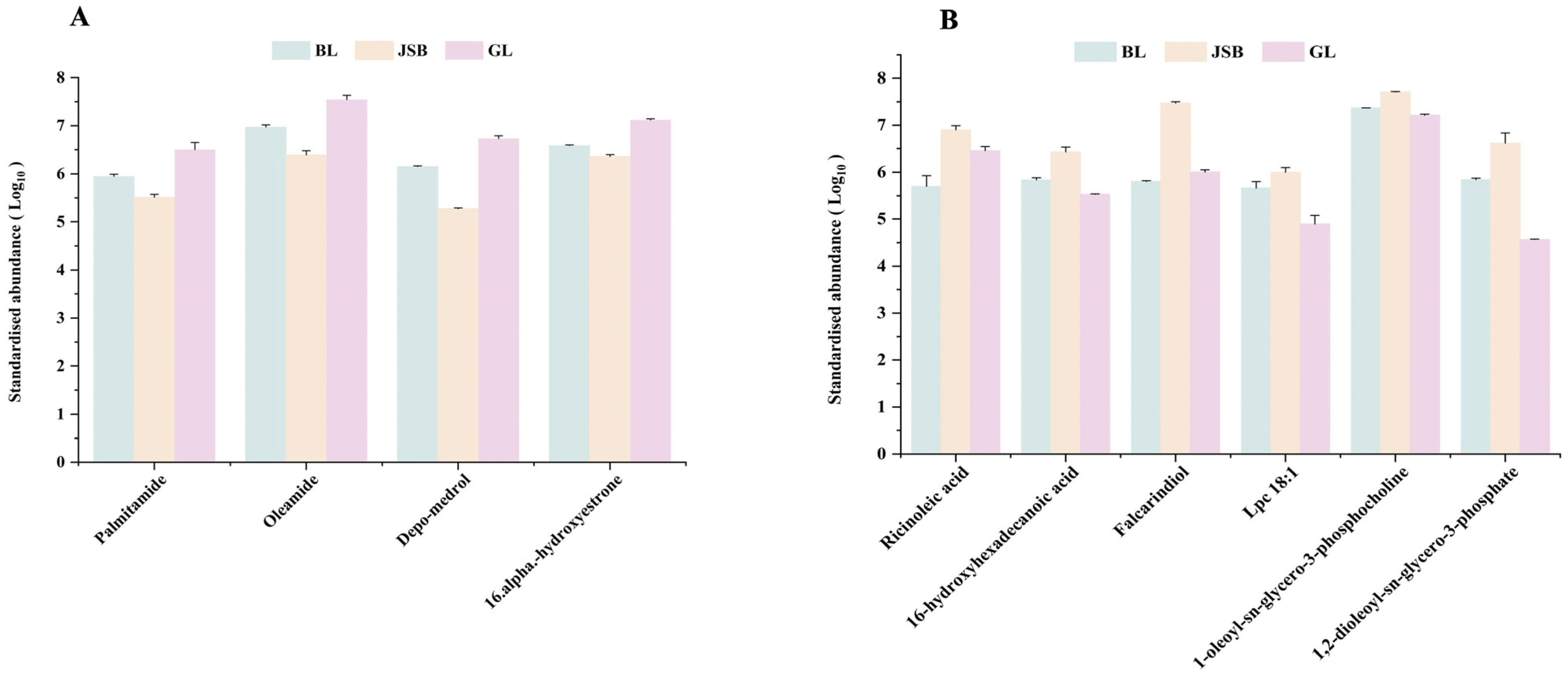
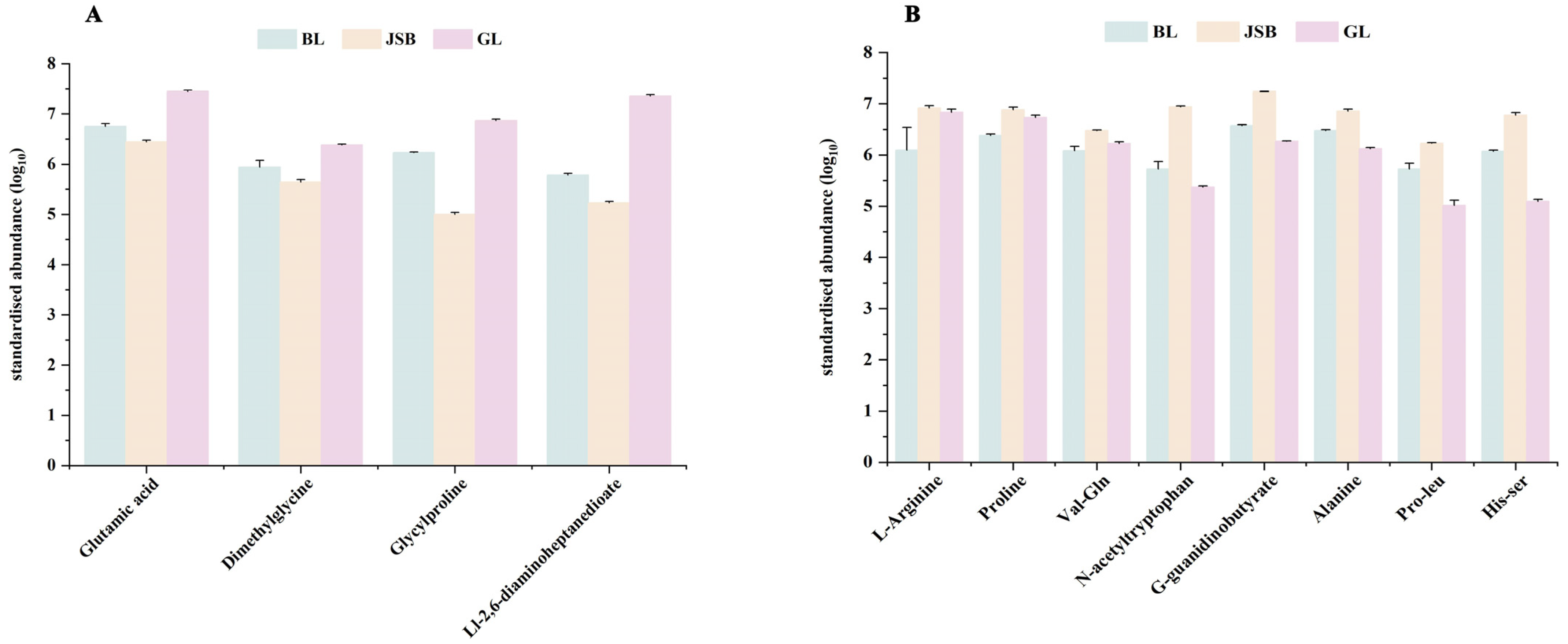
3.2. Screening of Differential Metabolites (DMs)
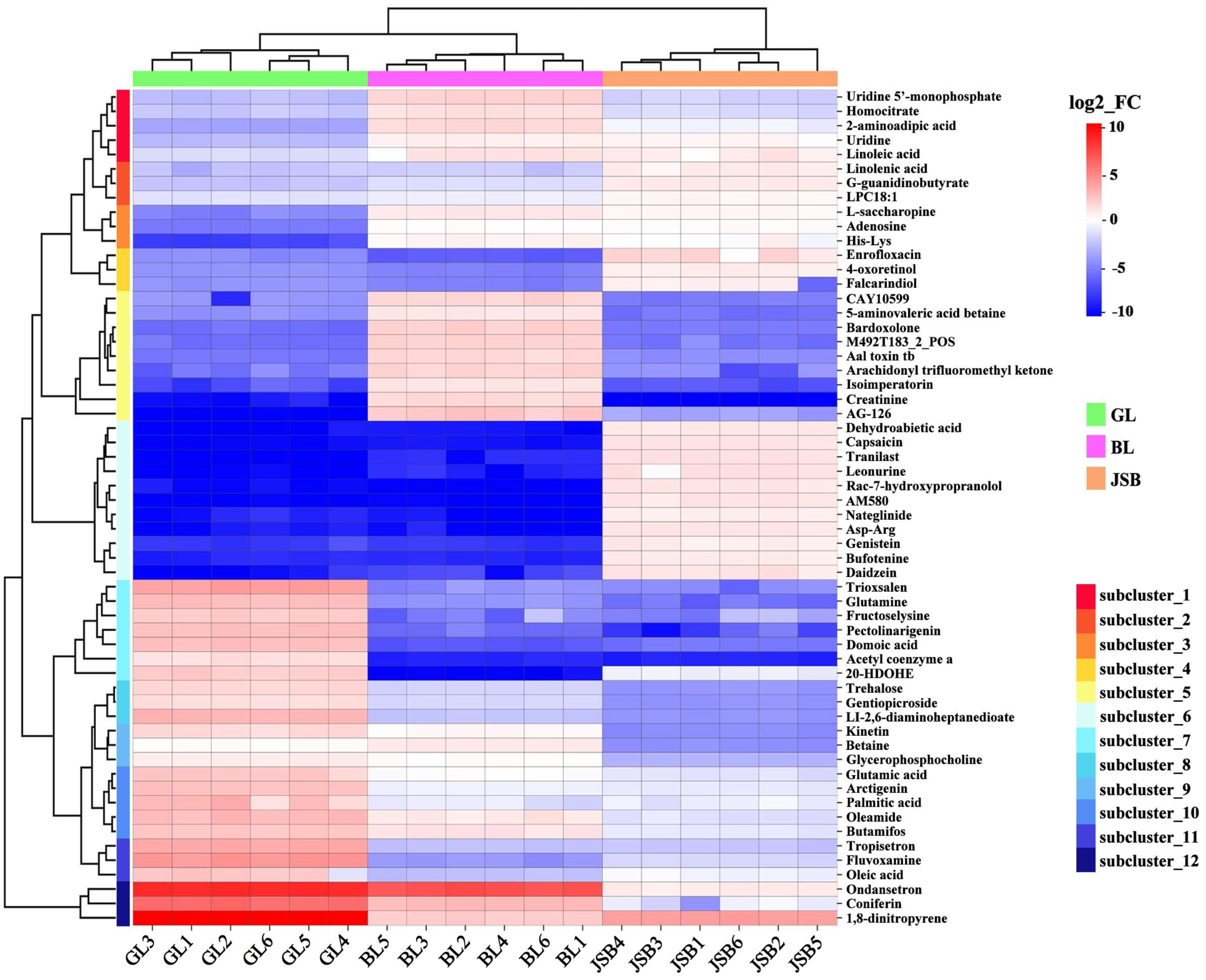
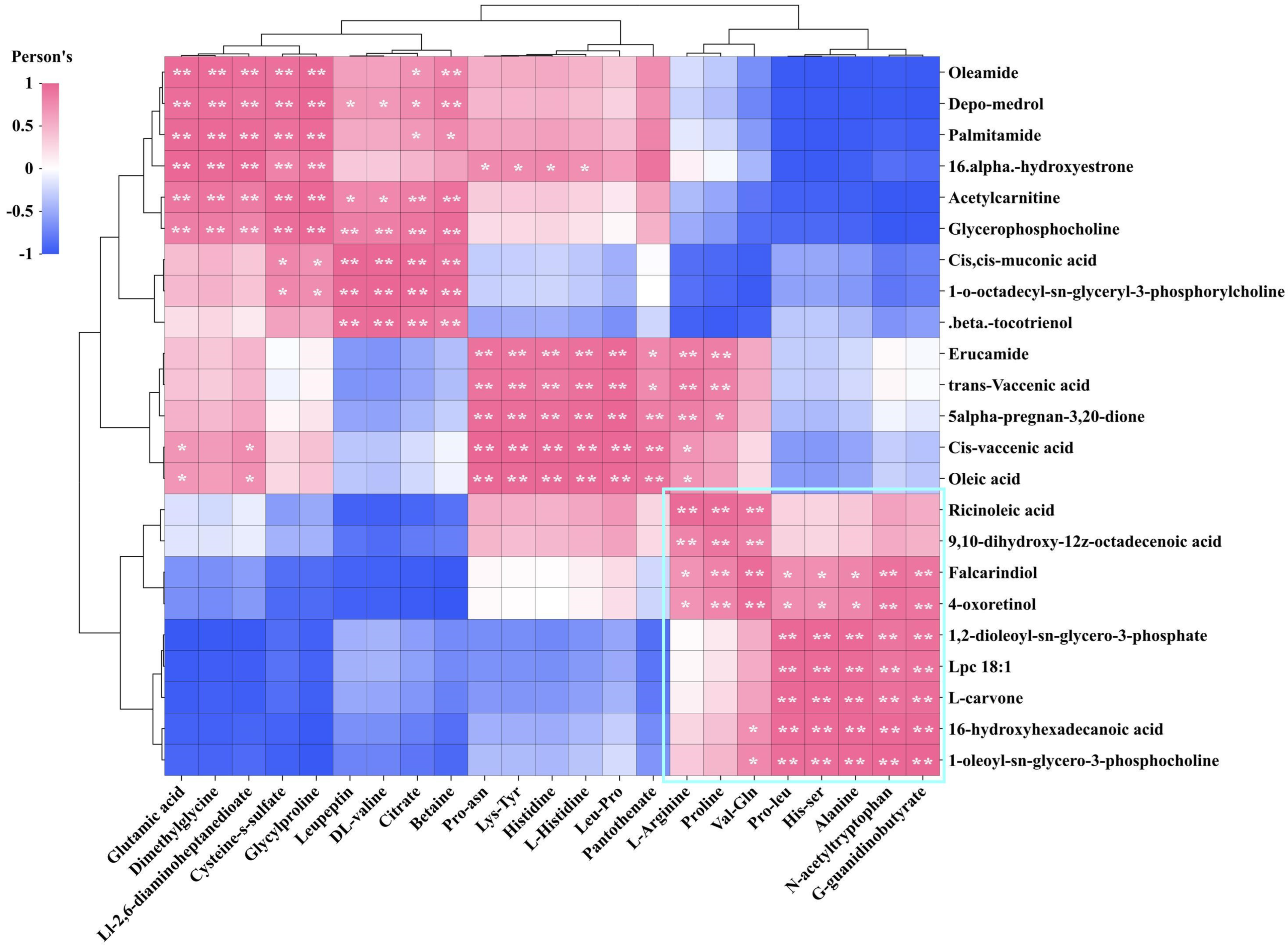
3.3. DMs Cluster Analysis
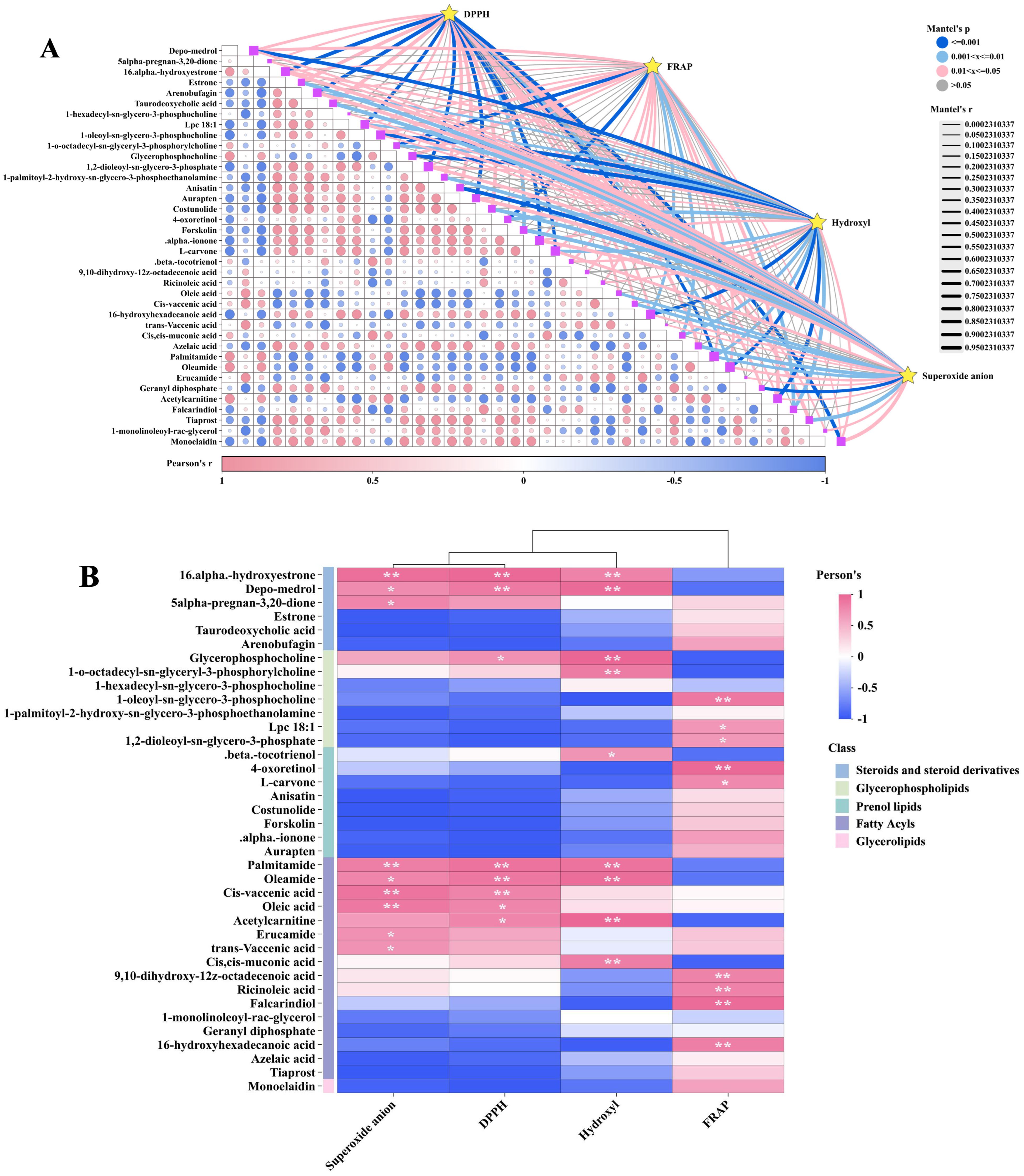
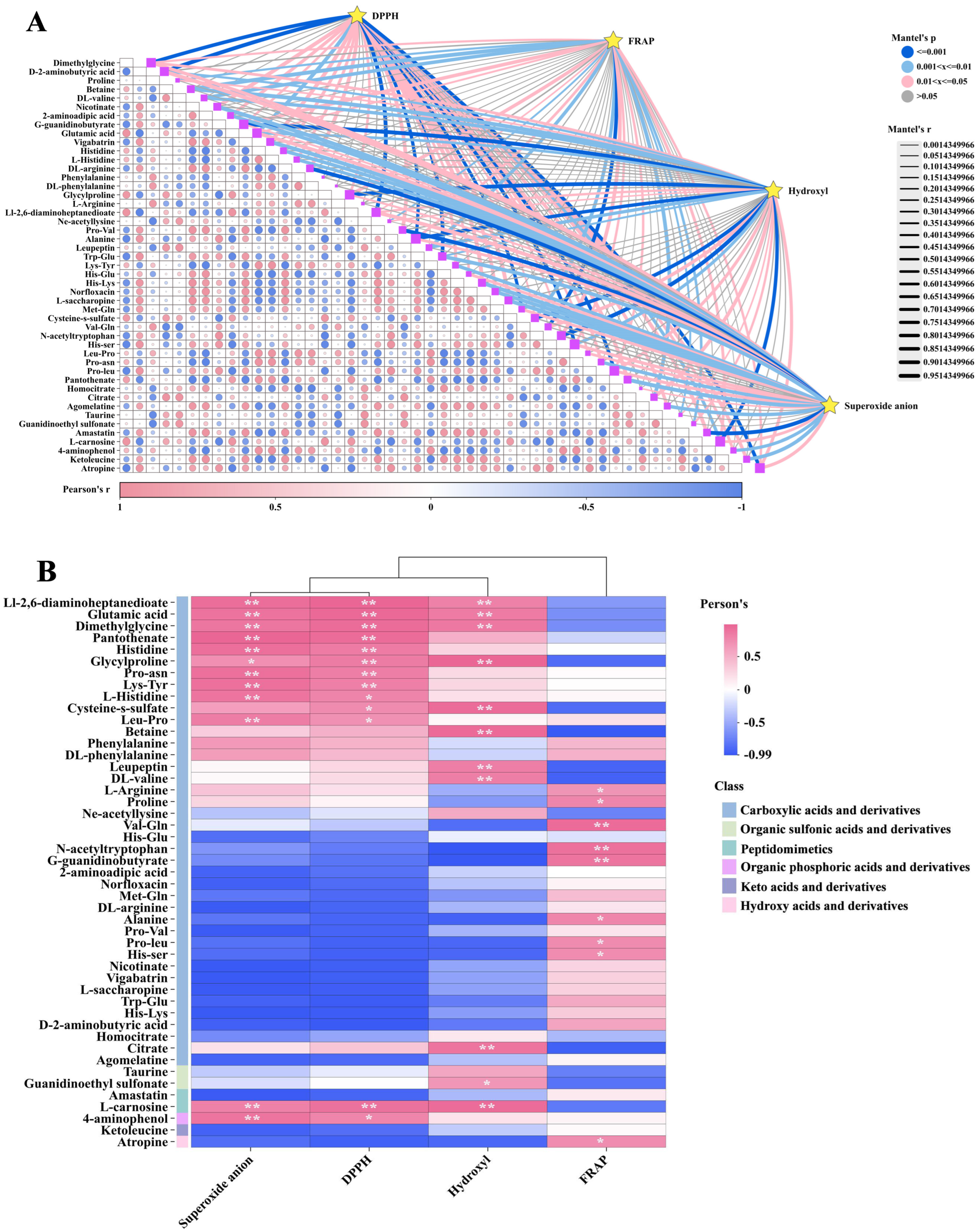
3.4. Correlation Analysis
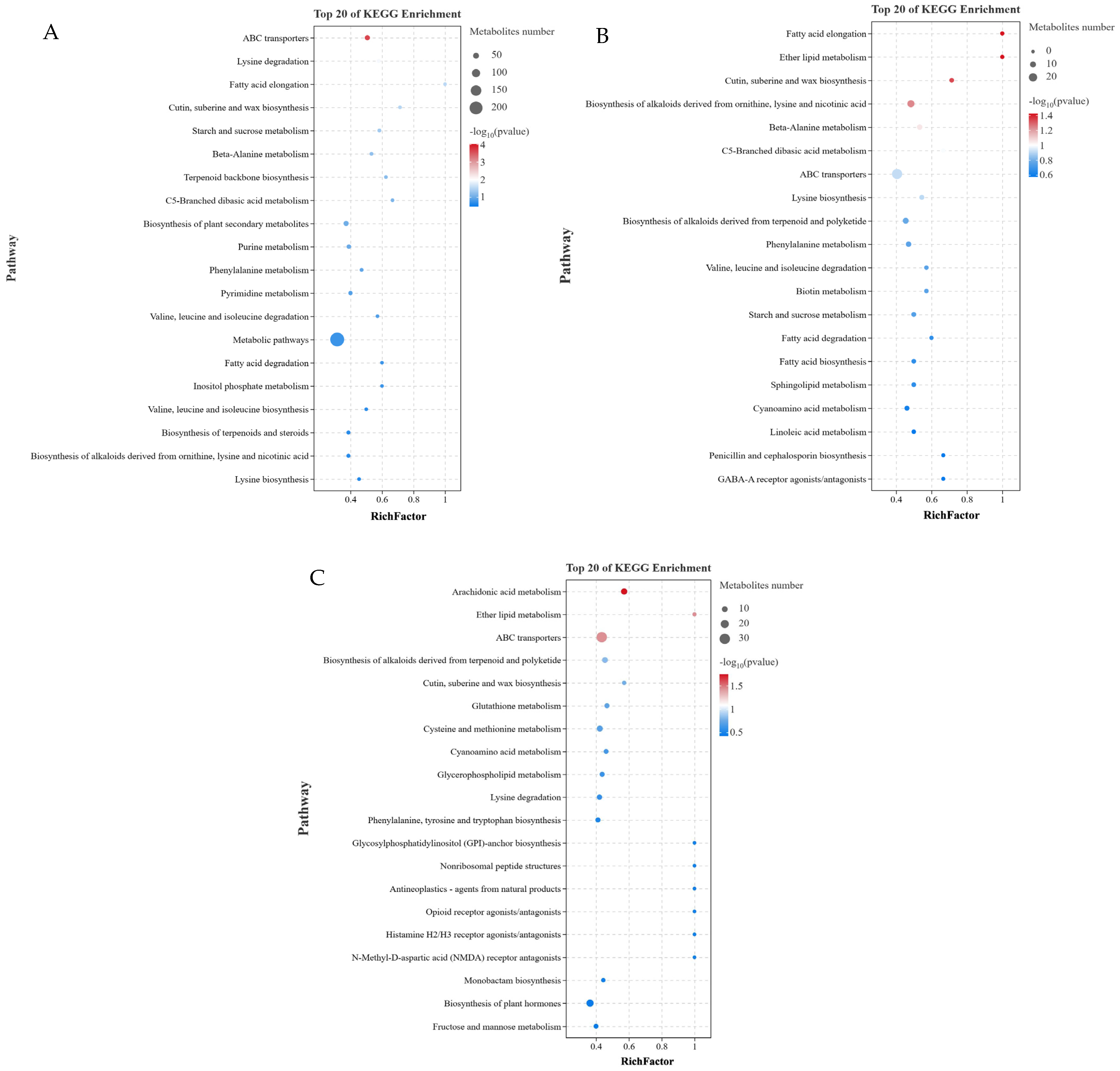
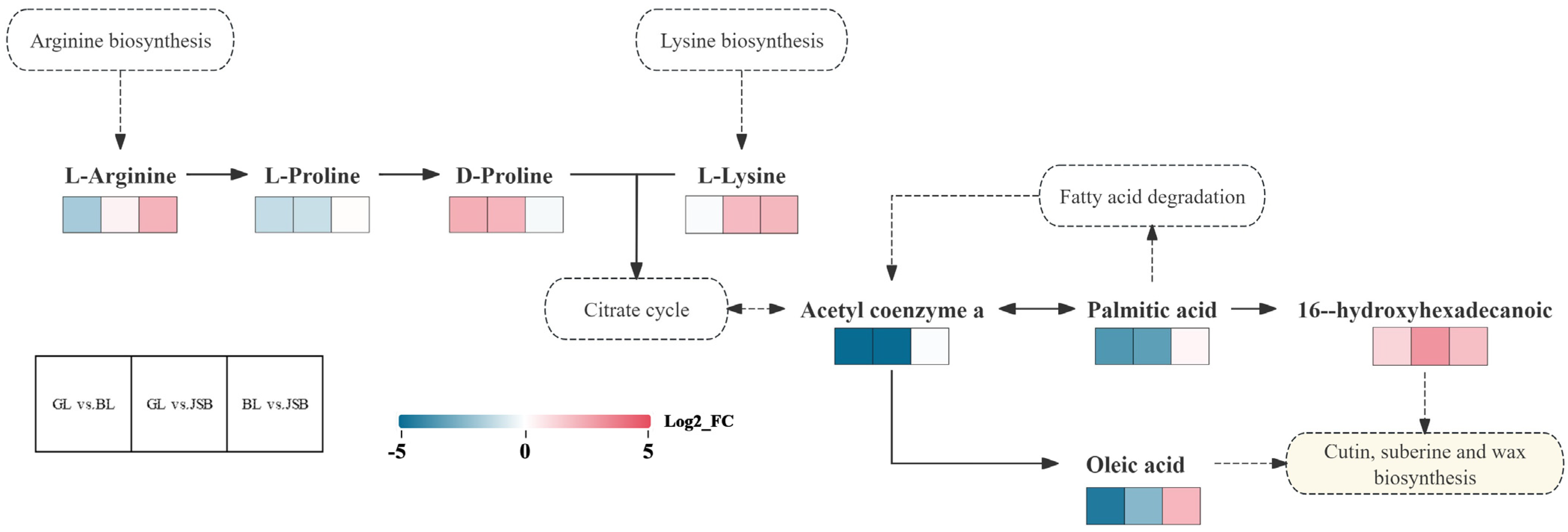
3.5. KEGG Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VC | Ascorbic acid |
| GL | Chinese cordyceps |
| CUR | Curtain gas |
| CAMERA | Collection of Algorithms of MEtabolite pRofile Annotation |
| DMs | Differential metabolites |
| DNA | Deoxyribonucleic acid |
| DP | Declustering potential |
| EDTA | Ethylenediaminetetraacetic acid |
| FRAP | FRAP total antioxidant capacity |
| HCA | Hierarchical Cluster Analysis |
| •OH | Hydroxyl free radical scavenging capacity |
| HMDB | Human Metabolome Database |
| LC-MS/MS | High-performance liquid chromatography-tandem mass spectrometry |
| ITS | Internal Transcribed Spacer |
| ISVF | IonSapary Voltage Floating |
| IDA | Information-dependent acquisition |
| KNN | K-Nearest Neighbor |
| KEGG | Kyoto Encyclopedia of Genes and Genomes database |
| R2Y | Model (for Y-variable dataset) Interpretability |
| Q2 | Model predictability |
| R2 | Model Interpretability |
| ESI− | Negative Electrospray Ionization |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| BL | Ophiocordyceps sinensis (O. sinensis) |
| JSB | Paecilomyces hepiali (P. hepiali) |
| PVP | Polyvinyl pyrrolidone |
| PCA | Principal Component Analysis |
| ESI+ | Positive Electrospray Ionization |
| PC1 | Principal Component 1 |
| PC2 | Principal Component 2 |
| QC | Quality control |
| RAPD | Random Amplified Polymorphic DNA |
| ROS | Reactive oxygen species |
| O2•− | Superoxide anion radical scavenging capacity |
| TIC | Total ion chromatograms |
| UHPLC | Ultra-high performance liquid chromatography |
| VIP | Variable importance |
| DPPH• | 2,2-diphenyl-1-picrylhydrazyl radical scavenging ability |
References
- Committee National Pharmacopoeia. Pharmacopoeia of People’s Republic of China [M]. Part 1; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Zhao, J.; Xie, J.; Wang, L.Y.; Li, S.P. Advanced development in chemical analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, A.; Yin, J.; Siu, K.; Wong, W.; Wu, J. Anti-inflammation activity of exopolysaccharides produced by a medicinal fungus Cordyceps sinensis Cs-HK1 in cell and animal models. Int. J. Biol. Macromol. 2020, 149, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237, 116113. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Jiang, C.; Huang, Q.; Sun, F. A comb-like branched β-d-glucan produced by a Cordyceps sinensis fungus and its protective effect against cyclophosphamide-induced immunosuppression in mice. Carbohydr. Polym. 2016, 142, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Y.; Chen, X.X.; Zheng, S.C.; Chen, P.; Mo, M.H. The Mechanisms of Pharmacological Activities of Ophiocordyceps sinensis Fungi. Phytother. Res. PTR 2016, 30, 1572–1583. [Google Scholar] [CrossRef]
- Zhou, X.; Gong, Z.; Su, Y.; Lin, J.; Tang, K. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef]
- Winkler, D. Yartsa Gunbu (Cordyceps sinensis) and the Fungal Commodification of Tibet’s Rural Economy. Econ. Bot. 2008, 62, 291–305. [Google Scholar] [CrossRef]
- Shrestha, U.; Bawa, K. Impact of climate change on potential distribution of Chinese caterpillar fungus (Ophiocordyceps sinensis) in Nepal Himalaya. PLoS ONE 2014, 9, e106405. [Google Scholar] [CrossRef]
- Zhou, X.; Li, L.; Tian, E. Advances in research of the artificial cultivation of Ophiocordyceps sinensis in China. Crit. Rev. Biotechnol. 2014, 34, 233–243. [Google Scholar] [CrossRef]
- Dong, C.; Guo, S.; Wang, W.; Liu, X. Cordyceps industry in China. Mycology 2015, 6, 121–129. [Google Scholar] [CrossRef]
- Ko, Y.; Liau, J.; Lee, C.; Chiu, C.; Martel, J.; Lin, C.; Tseng, S.; Ojcius, D.; Lu, C.; Lai, H.; et al. Isolation, Culture and Characterization of Hirsutella sinensis Mycelium from Caterpillar Fungus Fruiting Body. PLoS ONE 2017, 12, e0168734. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.; HywelJones, N.; Sung, J.; Luangsa-Ard, J.; Shrestha, B.; Spatafora, J. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Chioza, A.; Ohga, S. A Comparative Study on Chemical Composition and Pharmacological Effects of Paecilomyces hepiali and Wild Ophiocordyceps sinensis. Adv. Microbiol. 2014, 4, 839–848. [Google Scholar] [CrossRef]
- Bok, J.; Lermer, L.; Chilton, J.; Klingeman, H.; Towers, G. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Dan, A.; Hu, Y.; Chen, R.; Lin, X.; Wang, S. Advances in research on chemical constituents and pharmacological effects of Paecilomyces hepiali. Food Sci. Hum. Wellness 2021, 10, 401–407. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, N.; Qu, L. Determination of the anamorphof Cordyceps sinensis inferred from the analysis of the ribosomalDNA internal transcribed spacers and 5.8S rDNA. Biochem. Ecol. 2001, 29, 597–607. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, N.; Zhou, H. Differentiation of medicinal Cordyceps species by rDNA ITS sequence analysis. Planta Medica 2002, 68, 635–639. [Google Scholar]
- Liu, Z.; Yao, Y.; Liang, Z.; Liu, A.; Pegler, D.; Chase, M. Molecular evidence for the anamorph–teleomorph connection in Cordyceps sinensis. Mycol. Res. 2001, 105, 827–832. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, N.; Chen, Y.Q.; Li, T.; Qu, L. Molecular identification for the asexual stage of Cordyceps sinensis. Acta Sci. Nat. Univ. Sunyatseni 1999, 38, 121–123. (In Chinese) [Google Scholar]
- Li, Z.; Huang, B.; Fan, M. Molecular evidence for anamorph determination of Cordyceps sinensis (berk.) SACC.I.Relation Between Hirsutella sinensis and C. sinensis. Mycosystema 2000, 19, 60–64. (In Chinese) [Google Scholar]
- Tao, G.; Xiang, Q.; YU, Q.; Nie, S.; Xie, M. The immune modulation and antioxidative activities of artificial fermented Cordycepspowder in immune-suppressed mice. J. Nanchang Univ. (Nat. Sci.) 2016, 40, 156–160. (In Chinese) [Google Scholar]
- Rong, L.; Li, G.; Zhang, Y.; Xiao, Y.; Qiao, Y.; Yang, M.; Wei, L.; Bi, H.; Gao, T. Structure and immunomodulatory activity of a water-soluble α-glucan from Hirsutella sinensis mycelia. Int. J. Biol. Macromol. 2021, 189, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Chioza, A.; Ohga, S. Effects of hot-water extract of Paecilomyces hepiali on hypertension parameters in Spontaneously Hypertensive Rats. Adv. Microbiol. 2014, 4, 436–443. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, J.; Wang, X.; Hu, B.; Ye, H.; Fan, J.; Abid, M.; Zeng, X. Optimization for production of exopolysaccharides with antitumor activity in vitro from Paecilomyces hepiali. Carbohydr. Polym. 2014, 99, 226–234. [Google Scholar] [CrossRef]
- Jiang, L.; Bao, H.; Yang, M. Antitumor activity of a petroleum ether extractfrom Paecilomyces hepiali mycelium. Acta Edulis Fungi 2010, 17, 58–60. [Google Scholar]
- Zeng, Z.; Fu, Y.; Xiong, Y. Effects of Paecilomyces hepiali on Proliferation, Apoptosis and Expression of VEGF, MMP-2 of SMMC 7721 Cells. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 141–144. [Google Scholar]
- Thakur, A.; Ren, H.; Zhang, H. Pro-apoptotic effects of Paecilomyces hepiali, a Cordyceps sinensis extract on human lung adenocarcinomaA549 cells in vitro. J. Cancer Res. Ther. 2011, 7, 421–426. [Google Scholar] [PubMed]
- Wu, Y.; Chen, L.; Jin, C.; Guan, Y.; Yang, M.; Zhu, W. Chemical Constituents and Quality Control of Products of Fermented Cordyceps sinensis Powder. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 220–227. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R. The chemistry behind antioxidant capacity assays. Agric. Food Chem. 2005, 53, 1841. [Google Scholar] [CrossRef]
- Zahra, K.; Lefter, R.; Ali, A.; Abdellah, E.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Sergio, D.; Reed, T.; Paola, V.; Manuel, V. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar]
- Salehi, B.; Martorell, M.; Arbiser, J.; Sureda, A.; Martins, N.; Maurya, P.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or Negative Actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxidative Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Pandya, C.; Vekaria, H.; Joseph, B.; Slone, S.; Gensel, J.; Sullivan, P.; Miller, B. Hemoglobin induces oxidative stress and mitochondrial dysfunction in oligodendrocyte progenitor cells. Transl. Res. J. Lab. Clin. Med. 2021, 231, 13–23. [Google Scholar] [CrossRef]
- Borchani, C.; Besbes, S.; Masmoudi, M.; Blecker, C.; Paquot, M.; Attia, H. Effect of drying methods on physico-chemical and antioxidant properties of date fibre concentrates. Food Chem. 2011, 125, 1194–1201. [Google Scholar] [CrossRef]
- Ubando, R.; Navarro, O.; Valdivia, L. Mexican lime peel: Comparative study on contents of dietary fibre and associated antioxidant activity. Food Chem. 2005, 89, 57–61. [Google Scholar]
- Jabeen, K.; Malik, U.; Mansoor, S.; Shahzad, S.; Javed, A. Effect of oxidative stress and calcium deregulation on FAM26F (CALHM6) expression during hepatitis B virus infection. BMC Infect. Dis. 2021, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, J. Origin and concept of medicine food homology and its application in modern functional foods. J Food Funct. 2013, 4, 1727. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chen, S.; Yin, D.; Zhang, Y. Scavenging effect of Cordyceps growing in different environment on hydroxyl radical. Chin. Tradit. Herb. Drugs 2004, 35, 61–63. (In Chinese) [Google Scholar]
- Zhang, X.; Pu, Y.; Yin, L.; Zhong, W. Studies on the scavenging effect on superoxide anion free radical and hydroxyl free radical of Cordyceps sinensis. Chin. J. Gerontol. 2003, 23, 773–775. (In Chinese) [Google Scholar]
- Dong, C.; Yao, Y. In vitro evaluation of antioxidant activities of aqueous extracts from natural and cultured mycelia of Cordyceps sinensis. LWT—Food Sci. Technol. 2008, 41, 669–677. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Cheng, G.; Zhou, Y.; Guo, Q.; Wu, J.; Wong, Y.K.; Zhang, J.; Tang, H.; Wang, J. Cordyceps sinensis ameliorates idiopathic pulmonary fibrosis in mice via inhibiting mitochondrion-mediated oxidative stress. MedComm–Future Med. 2024, 3, e91. [Google Scholar] [CrossRef]
- Yan, X.F.; Zhang, Z.M.; Yao, H.Y.; Guan, Y.; Zhu, J.P.; Zhang, L.H.; Jia, Y.L.; Wang, R.W. Cardiovascular Protection and Antioxidant Activity of the Extracts from the Mycelia of Cordyceps Sinensis Act Partially Via Adenosine Receptors. Phytother. Res. 2013, 27, 1597–1604. [Google Scholar] [CrossRef]
- Yu, H.; Wang, B.; Huang, S.; Duh, P. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. Agric Food Chem 2006, 54, 3132–3138. [Google Scholar] [CrossRef]
- Guo, S.; Lin, M.; Xie, D.; Zhang, W.; Zhang, M.; Zhou, L.; Li, S.; Hu, H. Comparative metabolic profiling of wild Cordyceps species and their substituents by liquid chromatography-tandem mass spectrometry. Front Pharmacol 2022, 13, 1036589. [Google Scholar] [CrossRef]
- Azofeifa, G.; Quesada, S.; Boudard, F.; Morena, M.; Cristol, J.P.; Pérez, A.M.; Vaillant, F.; Michel, A. Antioxidant and anti-inflammatory in vitro activities of phenolic compounds from tropical highland blackberry (Rubus adenotrichos). J. Agric. Food Chem. 2013, 61, 5798–5804. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Ji, X.; Tang, J.; Zhang, J. Effects of Salt Stress on the Morphology, Growth and Physiological Parameters of Juglansmicrocarpa L. Seedlings. Plants 2022, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Yue, W.; Shen, Q.; Wang, W.; Meng, H.; Guo, Y.; Xu, W.; Guo, Y. Widely Targeted Metabolomics Analysis Reveals Great Changes in Nonvolatile Metabolites of Oolong Teas during Long-Term Storage. Molecules 2021, 26, 7278. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Jiang, Z.; Tao, M.; Wen, M.; Xiao, Z.; Zhang, L.; Zha, M.; Chen, J.; Liu, Z.; Zhang, L. Targeted and nontargeted metabolomics analysis for determining the effect of storage time on the metabolites and taste quality of keemun black tea. Food Chem. 2021, 359, 129950. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Q.; Shen, S.; Shan, X.; Hua, J.; Zhu, J.; Qiu, J.; Deng, Y.; Zhou, Q.; Jiang, Y. Non-targeted metabolomics and electronic tongue analysis reveal the effect of rolling time on the sensory quality and nonvolatile metabolites of congou black tea. Lwt 2022, 169, 113971. [Google Scholar] [CrossRef]
- Tang, R.; Qiu, X.; Cao, L.; Long, H.; Han, R. Stage- and Rearing-Dependent Metabolomics Profiling of Ophiocordyceps sinensis and Its Pipeline Products. Insects 2021, 12, 666. [Google Scholar] [CrossRef]
- Zhong, X.; Gu, L.; Xiong, W.; Wang, H.; Lian, D.; Zheng, Y.; Zhou, S.; Zhou, W.; Gu, J.L.; Shen, J.; et al. (1)H NMR spectroscopy-based metabolic profiling of Ophiocordyceps sinensis and Cordyceps militaris in water-boiled and 50% ethanol-soaked extracts. J. Pharm. Biomed. Anal. 2020, 180, 113038. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, X.; Song, Q.; Lu, W.; Wu, T.; Zhang, Q.; Li, C. Ophiocordyceps sinensis Determination and comparative analysis of 13 nucleosides and nucleobases in natural fruiting body of and its substitutes. Mycology 2017, 8, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Shi, L.; Zhang, F.; Chang, J.; Chu, X. High-throughput screening of the nucleosides and nucleotides using characteristic structural fragments fusion. J. Pharm. Biomed. Anal. 2019, 175, 112787. [Google Scholar] [CrossRef]
- Lin, M.; Guo, S.; Xie, D.; Li, S.; Hu, H. Lipidomic profiling of wild cordyceps and its substituents by liquid chromatography-electrospray ionization-tandem mass spectrometry. LWT—Food Sci. Technol. 2022, 16, 113497. [Google Scholar] [CrossRef]
- Wang, T.; Tang, C.; Xiao, M.; Cao, Z.; He, M.; Qi, J.; Li, Y.; Li, X. Effect of Air Drying on the Metabolic Profile of Fresh Wild and Artificial Cordyceps sinensis. Foods 2023, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Yuan, H.; He, N. From Tea Leaves to Factories: A Review of Research Progress in l-Theanine Biosynthesis and Production. J. Agric. Food Chem. 2021, 69, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kang, J.; Zhu, H.; Wang, K.; Han, Z.; Wang, L.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. L-Theanine and Immunity: A Review. Molecules 2023, 28, 3846. [Google Scholar] [CrossRef]
- Wang, L.; Brennan, M.; Li, S.; Zhao, H.; Wlange, K.; Brennan, C. How does the tea L-theanine buffer stress and anxiety. Food Sci. Hum. Wellness 2022, 11, 9. [Google Scholar] [CrossRef]
- Sharma, E.; Joshi, R.; Gulati, A. l-Theanine: An astounding sui generis integrant in tea. Food Chem 2018, 242, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, H.; Liu, Y.; Li, C.; Fang, Z.; Hu, B.; Li, X.; Zeng, Z.; Liu, Y. Changes in flavor and biological activities of Lentinula edodes hydrolysates after Maillard reaction. Food Chem. 2024, 431, 137138. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, F.; Tsim, K. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef]
- Shiao, L.; Hsieh, C.; Chang, D. A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom DongChongXiaCao, its counterfeit and mimic, and fermented mycelium of Cordyceps sinensis. Food Chem. 2002, 78, 463–469. [Google Scholar]
- Zhang, P.; Zhou, Y.; Yang, M.; Liu, W.; Wei, F.; Ma, S.; Liu, B. Determination of cordycepic acid in Cordyceps sinensis and fermented Cordycepspreparation by HPLC-RID. Chin. J. Pharm. Anal. 2016, 36, 1671–1678. (In Chinese) [Google Scholar]
- Tang, Y.; Li, M.; Yu, X. Comparing Research on Content Determination of Mannitol about the Cordyceps sinensis and Fermentation of Cordyceps militaris. J. Chengdu Univ. Tradit. Chin. Med. 2009, 32, 85–88. (In Chinese) [Google Scholar]
- Xiao, M.; Wang, T.; Tang, C.; He, M.; Li, Y.; Li, X. Effects of Drying Methods on Morphological Characteristics, Metabolite Content, and Antioxidant Capacity of Cordyceps sinensis. Foods 2024, 13, 1639. [Google Scholar] [CrossRef]
- Wang, J.; Kan, L.; Nie, S.; Chen, H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured Cordyceps sinensis. LWT—Food Sci. Technol. 2015, 63, 2–7. [Google Scholar] [CrossRef]
- Goodenowe, D.; Haroon, J.; Kling, M.; Zielinski, M.; Mahdavi, K.; Habelhah, B.; Shtilkind, L.; Jordan, S. Targeted Plasmalogen Supplementation: Effects on Blood Plasmalogens, Oxidative Stress Biomarkers, Cognition, and Mobility in Cognitively Impaired Persons. Front. Cell Dev. Biol. 2022, 10, 864842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, Y.; Li, W.; Wang, Z.; Chen, N.; Li, S. Effects of Dietary Phospholipid Level on Growth Performance, Antioxidant Capacity and Fat Deposition of Juvenile Snakehead (Channa arqus). J. Guangdong Ocean Univ. 2023, 43, 9–18. (In Chinese) [Google Scholar]
- Zhang, Y.; Liu, J.; Wang, Y.; Sun, C.; Li, W.; Qiu, J.; Qiao, Y.; Wu, F.; Huo, X.; An, Y.; et al. Nucleosides and amino acids, isolated from Cordyceps sinensis, protected against cyclophosphamide-induced myelosuppression in mice. Nat. Prod. Res. 2022, 36, 6056–6059. [Google Scholar] [CrossRef]
- Nakai, S.; Tateoka, Y.; Miyaguchi, Y.; Takahashi, M.; Ogita, H. Associations Between Habitual Dietary Behaviors and Glutamic Acid Levels in Human Milk. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2023, 39, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Petroff, O.A. GABA and glutamate in the human brain. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Steel, A.; Mikkelsen, M.; Edden, R.A.E.; Robertson, C.E. Regional balance between glutamate+glutamine and GABA+ in the resting human brain. NeuroImage 2020, 220, 117112. [Google Scholar] [CrossRef]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Guo, L.; Xu, X.; Wu, C.; Lin, L.; Zhou, S.; Luan, T.; Wang, J. Fatty acid composition of lipids in wild Cordyceps sinensis from major habitats in China. Biomed. Prev. Nutr. 2012, 2, 42–50. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Peng, F.; Lu, R.; Zhou, H.; Bao, G.; Wang, B.; Huang, B.; Li, Z.; Hu, F. Metabolomic variation in wild and cultured cordyceps and mycelia of Isaria cicadae. Biomed. Chromatogr. BMC 2019, 33, e4478. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, X.; Wang, T.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Fan, Y.; Li, Y. Characterization of Metabolite Landscape Distinguishes Medicinal Fungus Cordyceps sinensis and other Cordyceps by UHPLC-Q Exactive HF-X Untargeted Metabolomics. Molecules 2023, 28, 7745. [Google Scholar] [CrossRef] [PubMed]
- Segla Koffi Dossou, S.; Xu, F.; You, J.; Zhou, R.; Li, D.; Wang, L. Widely targeted metabolome profiling of different colored sesame (Sesamum indicum L.) seeds provides new insight into their antioxidant activities. Food Res. Int. 2022, 151, 110850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, L.; Liu, Y.; Zhan, S.; Wu, Z.; Luo, S.; Zhang, X. Dietary flavonoids: A novel strategy for the amelioration of cognitive impairment through intestinal microbiota. J. Sci. Food Agric. 2023, 103, 488–495. [Google Scholar] [CrossRef]
- Yao, X.; Jiao, S.; Qin, M.; Hu, W.; Yi, B.; Liu, D. Vanillic Acid Alleviates Acute Myocardial Hypoxia/Reoxygenation Injury by Inhibiting Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 8348035. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yan, Y.; Jiang, Y.; Meng, X. Ellagic Acid and Its Anti-Aging Effects on Central Nervous System. Int. J. Mol. Sci. 2022, 23, 10937. [Google Scholar] [CrossRef]

| Number | Species | Origin | Weight |
|---|---|---|---|
| GL | Chinese Cordyceps | Guoluo Tibetan Autonomous Prefecture, Qinghai Province | 2 g |
| BL | Ophiocordyceps sinensis | Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd. (Hangzhou, China) | 2 g |
| JSB | Paecilomyces hepiali | Jiangxi Jinshui Bao Pharmaceutical Co., Ltd. (Nanchang, China) | 2 g |
| Time (min) | Mobile Phase B |
|---|---|
| 0–0.5 | 95% |
| 0.5–7 | 65% |
| 7–8 | 40% |
| 8–9 | 40% |
| 9–9.1 | 95% |
| 9.1–12 | 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Tang, C.-Y.; Wang, T.; Xiao, M.-J.; Li, Y.-L.; Li, X.-Z. Analysis of Metabolic Profiles and Antioxidant Activity of Chinese Cordyceps, Ophiocordyceps sinensis, and Paecilomyces hepiali Based on Untargeted Metabolomics. Biology 2024, 13, 683. https://doi.org/10.3390/biology13090683
He M, Tang C-Y, Wang T, Xiao M-J, Li Y-L, Li X-Z. Analysis of Metabolic Profiles and Antioxidant Activity of Chinese Cordyceps, Ophiocordyceps sinensis, and Paecilomyces hepiali Based on Untargeted Metabolomics. Biology. 2024; 13(9):683. https://doi.org/10.3390/biology13090683
Chicago/Turabian StyleHe, Min, Chu-Yu Tang, Tao Wang, Meng-Jun Xiao, Yu-Ling Li, and Xiu-Zhang Li. 2024. "Analysis of Metabolic Profiles and Antioxidant Activity of Chinese Cordyceps, Ophiocordyceps sinensis, and Paecilomyces hepiali Based on Untargeted Metabolomics" Biology 13, no. 9: 683. https://doi.org/10.3390/biology13090683





