Nonindigenous Plant Advantage in Native and Exotic Australian Grasses under Experimental Drought, Warming, and Atmospheric CO2 Enrichment
Abstract
:1. Introduction
2. Methods
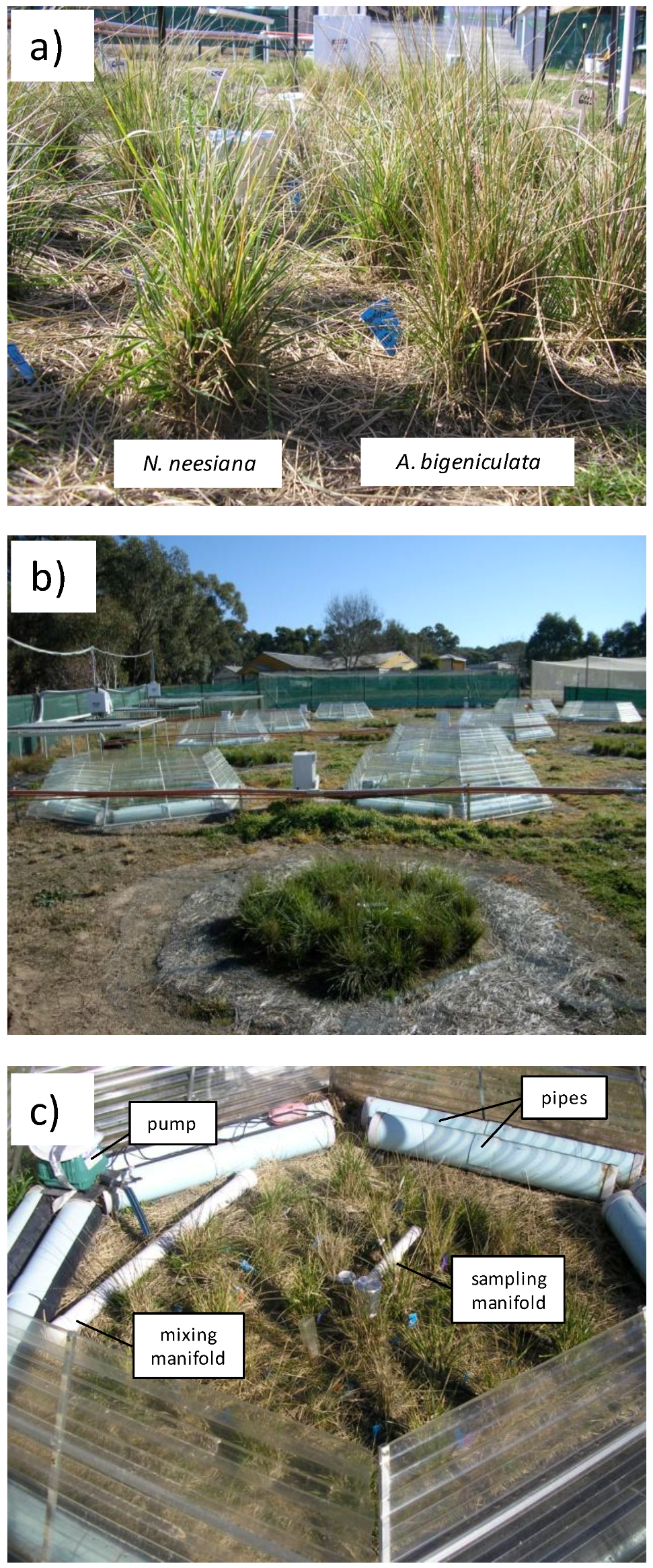
2.1. Study Species
2.2. Site Selection and Description

2.3. Specimen Collection and Propagation
| Collection site | |||||
|---|---|---|---|---|---|
| Braidwood | Bungendore | Nanima | Woodstock | ||
| Average Tmax (°C) | |||||
| Summer | 24.9 | 26.9 | 27.4 | 30.0 | |
| Autumn | 19.1 | 19.7 | 20.1 | 22.3 | |
| Winter | 12.4 | 11.9 | 12.0 | 13.7 | |
| Spring | 18.8 | 19.4 | 19.7 | 22.0 | |
| Annual | 18.8 | 19.5 | 19.8 | 22.0 | |
| Average Tmin (°C) | |||||
| Summer | 11.9 | 12.6 | 12.8 | 14.4 | |
| Autumn | 7.0 | 7.1 | 7.3 | 8.8 | |
| Winter | 1.3 | 1.1 | 1.4 | 2.9 | |
| Spring | 6.3 | 6.4 | 6.5 | 7.7 | |
| Annual | 6.6 | 6.8 | 7.0 | 8.5 | |
| Total precipitation (mm) | |||||
| Summer | 208.7 | 166.8 | 152.6 | 177.2 | |
| Autumn | 192.4 | 151.5 | 147.2 | 140.5 | |
| Winter | 159.0 | 156.9 | 175.0 | 177.0 | |
| Spring | 186.8 | 184.0 | 183.8 | 182.8 | |
| Annual | 746.9 | 659.2 | 658.6 | 677.5 | |
| Total PET (mm) | |||||
| Summer | 409.0 | 462.0 | 467.9 | 518.4 | |
| Autumn | 214.0 | 238.4 | 238.5 | 269.7 | |
| Winter | 120.2 | 124.4 | 121.9 | 132.0 | |
| Spring | 298.5 | 317.9 | 315.9 | 346.4 | |
| Annual | 1,041.7 | 1,142.7 | 1,144.2 | 1,266.5 | |
2.4. Field Trial Design and Establishment

2.5. Field Trial Treatments and Climate Scenarios
2.6. Data Collection
2.7. Data Analyses
| Dependent variable | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf elongation rate (LER) | Height to width ratio (HWR) | Tussock width (Wid) | Tussock volume (Vol) | Height to width ratio (HWR) | Biomass accumulation rate (BAR) | |||||||||||
| Experimental phase measured | I | I | I | I | I | I | ||||||||||
| Month | February | February | February | April | April | Feb-April | ||||||||||
| Units | mm day−1 | none | mm | cm3 | none | mg day−1 | ||||||||||
| Transformation | sqrt(×) | ln(×) | sqrt(×) | sqrt(×) | ln(×) | sqrt(×) | ||||||||||
| Model fixed effects (F, p) | ||||||||||||||||
| Species | 52.2 | *** | 831.2 | *** | 540.3 | *** | 399.9 | *** | 719.1 | *** | 18.5 | *** | ||||
| Site | 129.8 | *** | 82.1 | *** | 49.7 | *** | 35.7 | *** | 31.8 | *** | 57.0 | *** | ||||
| Species × Site | 115.2 | *** | 30.3 | *** | 6.3 | *** | 3.9 | * | 4.4 | ** | 28.0 | *** | ||||
| Planting | 68.5 | *** | 2.8 | ns | 58.0 | *** | 75.1 | *** | - | 103.2 | *** | |||||
| Quadrant (Quad) | 5.4 | ** | 2.8 | * | 2.3 | ns | 0.1 | ns | 1.4 | ns | 5.6 | *** | ||||
| Quadrant Position (Qpos) | 1.4 | ns | 25.1 | *** | 18.1 | *** | 50.4 | *** | 78.5 | *** | 1.1 | ns | ||||
| Quad × Qpos | 1.3 | ns | 3.1 | * | 1.5 | ns | 1.6 | ns | 3.5 | * | 0.8 | ns | ||||
| Quad × Species | 1.1 | ns | 1.4 | ns | 2.0 | ns | 2.8 | * | 0.9 | ns | 3.6 | * | ||||
| Species × Qpos | 2.0 | ns | 0.5 | ns | 0.0 | ns | 2.7 | ns | 2.1 | ns | 0.1 | ns | ||||
| Quad × Species × Qpos | 0.3 | ns | 0.7 | ns | 0.8 | ns | 0.5 | ns | - | 0.1 | ns | |||||
| Random effect (Z, p) | ||||||||||||||||
| Clone line | 2.8 | ** | 3.7 | *** | 2.1 | * | 3.0 | ** | 0.1 | ns | 4.7 | *** | ||||
| Block | 1.4 | ns | 0.4 | ns | 1.2 | ns | - | 0.8 | ns | 1.2 | ns | |||||
| Plot (Block) | 1.2 | ns | 1.5 | ns | 1.6 | ns | 2.1 | * | 0.2 | ns | 2.4 | ** | ||||
| Estimated population means | ||||||||||||||||
| A. bigeniculata | Braidwood | 2.94 | cd | 0.00 | cd | 17.3 | bc | 17.6 | c | −0.12 | c | 13.8 | bc | |||
| Bungendore | 3.08 | bc | 0.26 | a | 15.8 | d | 13.9 | d | 0.07 | a | 12.5 | c | ||||
| Nanima | 3.14 | b | 0.07 | bc | 17.2 | c | 18.2 | c | −0.12 | bc | 16.4 | b | ||||
| Woodstock | 3.13 | bc | 0.15 | ab | 16.8 | c | 16.0 | cd | −0.03 | b | 16.1 | b | ||||
| N. neesiana | Braidwood | 2.76 | d | −0.50 | f | 20.4 | a | 27.8 | a | −0.48 | e | 15.9 | b | |||
| Bungendore | 2.40 | e | −0.32 | e | 18.0 | b | 21.9 | b | −0.39 | d | 12.0 | c | ||||
| Nanima | 2.38 | e | −0.61 | f | 19.7 | a | 28.4 | a | −0.53 | e | 13.8 | bc | ||||
| Woodstock | 3.86 | a | −0.07 | d | 20.4 | a | 22.5 | b | −0.35 | d | 24.6 | a | ||||
| Dependent Variable | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf elongation rate (LER) | Biomass accumulation rate (BAR) | Leaf elongation rate (LER) | Basal diameter (Basal D) | |||||||||||||||||||||||
| Experimental phase measured | II | II | III | III | ||||||||||||||||||||||
| Month | June | June | August | November | ||||||||||||||||||||||
| Units | mm day−1 | mg day−1 | mm day−1 | mm | ||||||||||||||||||||||
| Transformation | none | ln(×) | none | none | ||||||||||||||||||||||
| Model fixed effects (F,p) | ||||||||||||||||||||||||||
| Species | 60.7 | *** | 65.1 | *** | 166.1 | *** | 155.5 | *** | ||||||||||||||||||
| Site | 44.2 | *** | 23.1 | *** | 15.4 | *** | 10.8 | *** | ||||||||||||||||||
| Species × Site | 31.2 | *** | 10.5 | *** | 36.8 | *** | 0.7 | ns | ||||||||||||||||||
| Climate treatment (Treat) | 3.2 | ns | 2.4 | ns | 10.6 | *** | 1.3 | ns | ||||||||||||||||||
| Treat × Species | 6.5 | *** | 5.2 | ** | 2.9 | * | 1.3 | ns | ||||||||||||||||||
| Treat × Site | 1.8 | ns | 0.6 | ns | 1.9 | ns | 0.9 | ns | ||||||||||||||||||
| Treat × Species x Site | 0.9 | ns | 0.6 | ns | 0.9 | ns | 0.5 | ns | ||||||||||||||||||
| Planting | 31.3 | *** | 99.4 | *** | 37.7 | *** | 2.2 | ns | ||||||||||||||||||
| Quadrant (Quad) | 1.4 | ns | 0.9 | ns | 1.8 | ns | 1.0 | ns | ||||||||||||||||||
| Quadrant position (Qpos) | 0.3 | ns | 1.0 | ns | 15.9 | *** | 24.9 | *** | ||||||||||||||||||
| Treat × Quad | 2.0 | * | 0.8 | ns | 0.3 | ns | - | |||||||||||||||||||
| Treat × Qpos | 4.0 | ** | 3.5 | * | 4.0 | * | 0.5 | ns | ||||||||||||||||||
| Random effects (Z, p) | ||||||||||||||||||||||||||
| Clone line | 1.6 | ns | 2.7 | ** | 3.8 | *** | - | |||||||||||||||||||
| Block | 1.4 | ns | 1.3 | ns | 1.3 | ns | 0.1 | ns | ||||||||||||||||||
| Plot(Block) | 2.3 | * | 2.2 | * | 2.1 | * | 1.0 | ns | ||||||||||||||||||
| Estimated species x treatment means | ||||||||||||||||||||||||||
| Phase II | Phase III | Species | ||||||||||||||||||||||||
| C1 | C | Aubi | 2.48 | bc | 2.73 | b | 5.87 | b | 57.1 | b | ||||||||||||||||
| C2 | +D | Aubi | 2.56 | b | 2.74 | b | 6.15 | b | 47.2 | b | ||||||||||||||||
| +W | +DW | Aubi | 3.05 | a | 3.20 | ab | 7.33 | a | 59.4 | b | ||||||||||||||||
| +WCO2 | +DWCO2 | Aubi | 3.05 | a | 3.10 | ab | 7.25 | a | 55.5 | b | ||||||||||||||||
| C1 | C | Nane | 2.37 | bc | 3.31 | a | 4.67 | c | 84.1 | a | ||||||||||||||||
| C2 | +D | Nane | 2.27 | c | 3.27 | a | 4.84 | c | 84.3 | a | ||||||||||||||||
| +W | +DW | Nane | 2.51 | bc | 3.42 | a | 5.49 | b | 86.5 | a | ||||||||||||||||
| +WCO2 | +DWCO2 | Nane | 2.48 | bc | 3.37 | a | 5.62 | b | 92.3 | a | ||||||||||||||||
| Dependent Variable | ||||||||||||||||||||||||||
| Basal expansion (BasalE) | Maximum stem length (MaxSL) | Above ground biomass (AGB) | Stem Prod Rate (StemPR) | Floret production rate (FlorPR) | ||||||||||||||||||||||
| Experimental phase measured | III | III | III | III | III | |||||||||||||||||||||
| Month | November | November | November | November | November | |||||||||||||||||||||
| Units | mm | cm | g | stems dm−2 | florets infl−1 | |||||||||||||||||||||
| Transformation | none | none | sqrt(×) | sqrt(×) | none | |||||||||||||||||||||
| Model fixed effects (F,P) | ||||||||||||||||||||||||||
| Species | 11.8 | *** | 0.2 | ns | 35.2 | *** | 39.0 | *** | 53.6 | *** | ||||||||||||||||
| Site | 2.5 | ns | 2.1 | ns | 7.3 | *** | 10.3 | *** | 0.9 | ns | ||||||||||||||||
| Species × Site | 1.4 | ns | 1.8 | ns | 2.8 | * | 3.1 | * | 2.0 | ns | ||||||||||||||||
| Climate treatment (Treat) | 1.1 | ns | 1.9 | ns | 0.6 | ns | 1.0 | ns | 2.9 | ns | ||||||||||||||||
| Treat × Species | 0.6 | ns | 3.9 | * | 1.3 | ns | 4.8 | ** | 3.0 | * | ||||||||||||||||
| Treat × Site | 0.9 | ns | 0.1 | ns | 0.6 | ns | 1.6 | ns | 0.8 | ns | ||||||||||||||||
| Treat × Species x Site | 0.5 | ns | 0.3 | ns | 0.5 | ns | 0.8 | ns | 0.8 | ns | ||||||||||||||||
| Planting | 1.6 | ns | 0.3 | ns | 2.4 | ns | 0.0 | ns | 1.0 | ns | ||||||||||||||||
| Quadrant (Quad) | 0.1 | ns | 0.8 | ns | 0.3 | ns | 0.5 | ns | 0.3 | ns | ||||||||||||||||
| Quadrant position (Qpos) | 2.0 | ns | 6.5 | * | 27.2 | *** | 0.4 | ns | 12.3 | *** | ||||||||||||||||
| Treat × Quad | - | - | - | - | - | |||||||||||||||||||||
| Treat × Qpos | 1.3 | ns | 1.3 | ns | 0.0 | ns | 0.8 | ns | 0.8 | ns | ||||||||||||||||
| Random effects (Z, P) | ||||||||||||||||||||||||||
| Clone line | - | - | - | - | - | |||||||||||||||||||||
| Block | 0.6 | ns | 0.2 | ns | 0.3 | ns | 0.5 | ns | - | |||||||||||||||||
| Plot(Block) | 0.6 | ns | 1.8 | * | 1.3 | ns | 0.8 | ns | 0.8 | ns | ||||||||||||||||
| Estimated species x treatment means | ||||||||||||||||||||||||||
| Phase II | Phase III | Species | ||||||||||||||||||||||||
| C1 | C | Aubi | 4.1 | ab | 137.4 | a | 7.6 | bc | 1.66 | b | 64.4 | a | ||||||||||||||
| C2 | +D | Aubi | 1.9 | ab | 125.2 | a | 7.4 | bc | 1.85 | a | 61.6 | a | ||||||||||||||
| +W | +DW | Aubi | 1.8 | b | 105.6 | a | 7.4 | bc | 1.50 | bcd | 43.3 | bc | ||||||||||||||
| +WCO2 | +DWCO2 | Aubi | 6.3 | ab | 110.1 | a | 7.0 | c | 1.61 | bc | 53.0 | ab | ||||||||||||||
| C1 | C | Nane | 7.6 | ab | 128.1 | a | 9.9 | a | 1.45 | bcd | 38.8 | bc | ||||||||||||||
| C2 | +D | Nane | 11.2 | ab | 112.5 | a | 8.3 | abc | 1.23 | d | 35.8 | c | ||||||||||||||
| +W | +DW | Nane | 12.4 | a | 116.3 | a | 9.7 | ab | 1.37 | bcd | 36.3 | bc | ||||||||||||||
| +WCO2 | +DWCO2 | Nane | 18.7 | a | 129.0 | a | 9.8 | ab | 1.32 | cd | 36.0 | c | ||||||||||||||
3. Results
3.1. Temperature and [CO2]
| Climate Treatment | Day | Night | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | Tav | Tmax | Temax | Tav | Tmin | Temin | |||
| May | Control | 9.9 | 15.5 | 20.7 | 3.0 | 0.3 | -3.1 | ||
| +W | +0.6 | +1.6 | +2.0 | +0.9 | +1.1 | +1.1 | |||
| +WCO2 | +0.5 | +1.0 | +1.1 | +1.1 | +1.3 | +1.3 | |||
| July | Control | 8.3 | 13.6 | 19.0 | 3.9 | 0.9 | −2.5 | ||
| +D | 0.0 | 0.0 | -0.4 | +0.1 | +0.1 | −0.2 | |||
| +DW | +0.5 | +1.0 | -0.1 | +0.4 | +0.5 | +0.2 | |||
| +DWCO2 | +0.4 | +0.6 | -0.2 | +0.4 | +0.6 | +0.8 | |||
| August | Control | 11.9 | 18.5 | 23.9 | 6.2 | 2.9 | −0.9 | ||
| +D | +0.5 | +0.9 | +0.7 | 0.0 | +0.1 | +0.2 | |||
| +DW | +1.0 | +2.0 | +2.4 | +0.6 | +1.0 | +1.6 | |||
| +DWCO2 | +1.1 | +1.5 | +2.2 | +0.8 | +1.3 | +2.0 | |||
| September | Control | 15.3 | 21.9 | 29.0 | 7.9 | 4.1 | −1.1 | ||
| +D | +0.9 | +1.4 | +1.6 | +0.3 | +0.4 | +0.6 | |||
| +DW | +1.9 | +3.1 | +3.1 | +0.8 | +1.0 | +1.1 | |||
| +DWCO2 | +1.5 | +1.7 | +1.0 | +1.2 | +1.6 | +2.2 | |||
| October | Control | 20.7 | 29.2 | 37.1 | 11.7 | 8.6 | 1.8 | ||
| +D | +0.8 | +0.8 | +0.7 | +0.3 | 0.0 | −0.3 | |||
| +DW | +1.1 | +1.9 | +2.3 | +0.7 | +0.4 | +0.4 | |||
| +DWCO2 | +1.1 | +0.6 | 0.0 | +0.8 | +0.5 | +0.8 | |||
3.2. Soil Water Content

3.3. Plant Growth and Reproduction




4. Discussion
4.1. Drought, Climate and Adaptation in A. bigeniculata and N. neesiana
4.2. Warming, Elevated Atmospheric CO2, and Drought Severity
4.3. Genetic Diversity and Evolutionary Adaptive Potential in N. neesiana and A. bigeniculata
5. Conclusions
Acknowledgements
References
- Dukes, J.S.; Mooney, H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999, 11, 135–139. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar]
- Hegel, G.C.; Zwiers, F.W.; Braconnot, P.; Gillett, N.P.; Luo, Y.; Marengo Orsini, L.A.; Nicholls, N.; Penner, J.E.; Stott, P.A. Understanding and attributing climate change. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, New York, NY, USA, 2007. [Google Scholar]
- Williamson, M. Invasions. Ecography 1999, 22, 5–12. [Google Scholar] [CrossRef]
- Marini, L.; Battisti, A.; Bona, E.; Federici, G.; Martini, F.; Pautasso, M.; Hulme, P.E. Alien and native plant life-forms respond differently to human and climate pressures. Glob. Ecol. Biogeogr. 2012, 21, 534–544. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Sexton, J.P.; McKay, J.K.; Sala, A. Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol. Appl. 2002, 12, 1652–1660. [Google Scholar] [CrossRef]
- Hulme, P.E. Phenotypic plasticity and plant invasions: Is it all Jack? Funct. Ecol. 2008, 22, 3–7. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Grewell, B.J.; D’Antonio, C.M.; Funk, J.L.; James, J.J.; Mollinari, N.; Parker, I.M.; Richards, C.L. A functional trait perspective on plant invasion. Annu. Bot. 2012, 110, 141–153. [Google Scholar] [CrossRef]
- Bradford, M.A.; Schumacher, H.B.; Catovsky, S.; Eggers, T.; Newingtion, J.E.; Tordoff, G.M. Impacts of invasive plant species on riparian plant assemblages: Interactions with elevated atmospheric carbon dioxide and nitrogen deposition. Oecologia 2007, 152, 791–803. [Google Scholar] [CrossRef]
- Song, L.; Wu, J.; Li, C.; Li, F.; Peng, S.; Chen, B. Different responses of invasive and native species to elevated CO2 concentration. Acta Oecol. 2009, 35, 128–135. [Google Scholar] [CrossRef]
- Manea, A.; Leishman, M.R. Competitive interactions between native and invasive exotic plant species are altered under elevated carbon dioxide. Oecologia 2011, 165, 735–744. [Google Scholar] [CrossRef]
- Maron, J.L.; Vilà, M.; Bommarco, R.; Elmendorf, S.; Beardsley, P. Rapid evolution of an invasive plant. Ecol. Monogr. 2004, 74, 261–280. [Google Scholar] [CrossRef]
- Whitney, K.D.; Gabler, C.A. Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasion potential. Divers. Distrib. 2008, 14, 569–580. [Google Scholar] [CrossRef]
- Dlugosch, K.M.; Parker, I.M. Founding events in species invasions: Genetic variation, adaptive evolution, and the role of multiple introductions. Mol. Ecol. 2008, 17, 431–449. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Turner, N.C.; Nicolle, D.; Schumacher, J. Leaf and wood carbon isotope ratios, specific leaf areas and wood growth of Eucalyptus species across a rainfall gradient in Australia. Tree Physiol. 2006, 26, 479–492. [Google Scholar] [CrossRef]
- Maron, J.L.; Elmendorf, S.C.; Vilà, M. Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum. Evolution 2007, 61, 1912–1924. [Google Scholar] [CrossRef]
- Etterson, J.R.; Delf, D.E.; Craig, T.P.; Ando, Y.; Ohgushi, T. Parallel patterns of clinal variation in Solidago altissima in its native range in central USA and its invasive range in Japan. Botany 2008, 86, 91–97. [Google Scholar] [CrossRef]
- Monty, A.; Mahy, G. Clinal differentiation during invasion: Senecio inaequidens (Asteraceae) along altitudinal gradients in Europe. Oecologia 2009, 159, 305–315. [Google Scholar] [CrossRef]
- Hodgins, K.A.; Rieseberg, L. Genetic differentiation in life-history traits of introduced and native common ragweed (Ambrosia artemisiifolia) populations. J. Evol. Biol. 2011, 24, 2731–2749. [Google Scholar] [CrossRef]
- Baker, H.G. The evolution of weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar]
- Vilá, M.; Weiner, J. Are invasive species better competitors than native plant species?—Evidence from pair-wise experiments. Oikos 2004, 105, 229–238. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Rejmanek, M.; Richardson, D.M. What attributes make some plant species more invasive? Ecology 1996, 77, 1655–1661. [Google Scholar] [CrossRef]
- Byers, J.E. Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 2002, 97, 449–458. [Google Scholar] [CrossRef]
- Etterson, J.R.; Shaw, R.G. Constraint on adaptive evolution in response to global warming. Science 2001, 294, 151–154. [Google Scholar] [CrossRef]
- Blows, M.W.; Chenowith, S.; Hinde, E. Orientation of the genetic variance-covariance matrix and the fitness surface for multiple male sexually-selected traits. Am. Nat. 2004, 163, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Ellstrand, N.C.; Schierenbeck, K.A. Hybridization as a stimulus for the evolution of invasiveness in plants. Proc. Nat. Acad. Sci. USA 2000, 97, 7043–7050. [Google Scholar] [CrossRef]
- Bridle, J.R.; Vines, T.H. Limits to evolution at range margins: When and why does evolution fail? Trends Ecol. Evol. 2007, 22, 140–147. [Google Scholar] [CrossRef]
- Phillips, P.C. Maintenance of polygenic variation via a migration-selection balance under uniform selection. Evolution 1996, 50, 1334–1339. [Google Scholar] [CrossRef]
- Lenormand, T. Gene flow and the limits of natural selection. Trends. Ecol. Evol. 2002, 17, 183–189. [Google Scholar] [CrossRef]
- Colautti, R.I.; Eckert, C.G.; Barret, S.C.H. Evolutionary constraints on adaptive evolution during range expansion in an invasive plant. Proc. Roy. Soc. B 2010, 2077, 1799–1806. [Google Scholar] [CrossRef]
- White, T.A.; Campbell, BD.; Kemp, P.D.; Hunt, C.L. Impacts of extreme climatic events on competition during grassland invasions. Glob. Chang. Biol. 2001, 7, 1–13. [Google Scholar] [CrossRef]
- Gutschick, V.P.; BassiriRad, H. Extreme events as shaping physiology, ecology, and evolution of plants: Toward a unified definition and evaluation of their consequences. New Phytol. 2003, 160, 21–42. [Google Scholar] [CrossRef]
- Yurkonis, K.A.; Meiners, S.J. Drought impacts and recovery are driven by local variation in species turnover. Plant Ecol. 2006, 184, 325–336. [Google Scholar] [CrossRef]
- Godfree, R.; Lepschi, B.; Reside, A.; Bolger, T.; Robertson, B.; Marshall, D.; Carnegie, M. Multiscale topoedpahic heterogeneity increase resilience and resistance of a dominant grassland species to extreme drought and climate change. Glob. Chang Biol. 2011, 17, 943–958. [Google Scholar] [CrossRef]
- Smith, M.D. An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. J. Ecol. 2011, 99, 656–663. [Google Scholar] [CrossRef]
- Godfree, R.C. Extreme climatic events as drivers of ecosystem change. In Diversity of Ecosystems; Ali, M., Ed.; Intech Publishers: Rijeka, Croatia, 2012; pp. 339–366. [Google Scholar]
- Stampfli, A.; Zieter, M. Plant regeneration directs changes in grassland composition after extreme drought: A 13-year study in southern Switzerland. J. Ecol. 2004, 92, 5668–5676. [Google Scholar]
- Sheppard, C.S.; Alexander, J.M.; Billeter, R. The invasion of plant communities following extreme weather events under ambient and elevated temperature. Plant Ecol. 2012, 213, 1289–1301. [Google Scholar] [CrossRef]
- Bell, J.L.; Sloan, L.C.; Snyder, M.A. Regional changes in extreme climatic events: A future climate scenario. J. Clim. 2004, 17, 81–87. [Google Scholar] [CrossRef]
- Beniston, M.; Stephenson, D.B. Extreme climatic events and their evolution under changing climatic conditions. Glob. Planet. Change 2004, 44, 1–9. [Google Scholar] [CrossRef]
- Planton, S.; Déqué, M.; Chauvin, F.; Terray, L. Expected impacts of climate change on extreme climate events. C. R. Geosci. 2008, 340, 564–574. [Google Scholar] [CrossRef]
- Climate change in Australia. Available online: http://www.climatechangeinaustralia.gov.au/ (accessed on 22 August 2012).
- Barkworth, M.E. Nassella (Gramineae, Stipeae): Revised interpretation and nomenclatural changes. Taxon 1990, 39, 597–614. [Google Scholar] [CrossRef]
- Barkworth, M.E.; Arriaga, M.O.; Smith, J.F.; Jacobs, S.W.L.; Valdés-Reyna, J.; Bushman, B.S. Molecules and morphology in South American Stipeae (Poaceae). Syst. Bot. 2008, 33, 719–731. [Google Scholar] [CrossRef]
- Everett, J.; Jacobs, S.W.L.; Nairn, L. Poaceae 2. In Flora of Australia 44a; Wilson, A., Ed.; ABRS, Canberra/CSIRO Publishing: Melbourne, Australia, 2009; pp. 64–67. [Google Scholar]
- Barkworth, M.E.; Torres, M.A. Distribution and diagnostic characters of Nassella (Poaceae: Stipeae). Taxon 2001, 50, 439–468. [Google Scholar] [CrossRef]
- Jacobs, S.W.L.; Everett, J.; Torres, M.A. Nassella tenuissima (Gramineae) recorded from Australia, a potential new weed related to Serrated Tussock. Telopea 1998, 8, 41–44. [Google Scholar]
- McLaren, D.A.; Stajsic, V.; Iacomis, L. The distribution, impacts and identification of exotic stipoid grasses in Australia. Plant Prot. Q. 2004, 19, 59–66. [Google Scholar]
- Moore, R.M. South-eastern temperate woodlands and grasslands. In Australian Grasslands; Moore, R.M., Ed.; Australian National University Press: Canberra, Australia, 1973; pp. 169–190. [Google Scholar]
- Benson, J.S. The native grasslands of the Monaro region: Southern Tablelands of NSW. Cunninghamia 1994, 3, 609–650. [Google Scholar]
- Kirkpatrick, J.; McDougall, K.; Hyde, M. Australia's Most Threatened Ecosystem: The Southeastern Lowland Native Grasslands; Surry Beatty & Sons: Chipping Norton, NSW, Australia, 1995. [Google Scholar]
- Lunt, I.D.; Morgan, J.W. Can competition from Themeda triandra inhibit invasion by the perennial exotic grass Nassella neesiana in native grasslands? Plant Prot. Q. 2000, 15, 92–94. [Google Scholar]
- Gardener, M.R.; Whalley, R.B.D.; Sindel, B.M. Ecology of Nassella neesiana, Chilean needle grass, in pastures on the Northern Tablelands of New South Wale. I. Seed Production and dispersal. Aust. J. Agric. Res. 2003, 54, 613–619. [Google Scholar] [CrossRef]
- Dyksterhuis, E.J. Axillary cleistogenes in Stipa leucotricha and their role in nature. Ecology 1945, 26, 195–199. [Google Scholar] [CrossRef]
- Brown, W.V. The relation of soil moisture to cleistogamy in Stipa leucotricha. Bot. Gaz. 1952, 113, 438–444. [Google Scholar]
- Council of Heads of Australasian Herbaria (CHAH). Available online: http://www.chah.gov.au/ (accessed 22 August 2012).
- SILO enhanced climate data bank hosted by the Queensland Climate Change Centre of Excellence. Available online: http://www.longpaddock.qld.gov.au/silo/ (accessed 22 August 2012).
- Falconer, D.S. Introduction to Quantitative Genetics, 2nd ed; Longman Scientific and Technical, copublished with John Wiley and Sons: New York, NY, USA, 1981; p. 340. [Google Scholar]
- Baltunis, B.S.; Wu, H.X.; Dungey, H.S.; Mullin, T.J.; Brawner, J.T. Comparisons of genetic parameters and clonal value predictions from clonal trials and seedling base population trials of radiata pine. Tree Genet. Genomes 2009, 5, 269–278. [Google Scholar] [CrossRef]
- Godfree, R.; Robertson, B.; Bolger, T.; Carnegie, M.; Young, A. An improved hexagon open-top chamber system for stable diurnal and nocturnal warming and atmospheric carbon dioxide enrichment. Glob. Chang Biol. 2011, 17, 439–451. [Google Scholar] [CrossRef]
- Ivkovich, M. Genetic variation of wood properties in Balsam Poplar (Populus balsamifera L.). Silvae Genet. 1996, 45, 2–3. [Google Scholar]
- Aronson, J.; Kigel, J.; Shmida, A. Reproductive allocation strategies in desert and Mediterranean populations of annual plants grown with and without stress. Oecologia 1993, 93, 336–342. [Google Scholar] [CrossRef]
- Bolger, T.P.; Rivelli, A.R.; Garden, D.L. Drought resistance of native and introduced perennial grasses in south-eastern Australia. Aust. J. Agric. Res. 2005, 56, 1261–1267. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Weiner, J.; Thomas, S.C. Size variability and competition in plant monocultures. Oikos 1986, 47, 211–222. [Google Scholar] [CrossRef]
- Wilson, J.B. The effect of initial advantage on the course of plant competition. Oikos 1988, 51, 19–25. [Google Scholar] [CrossRef]
- Wedin, D.; Tilman, D. Competition among grasses along a nitrogen gradient: Initial conditions and mechanisms of competition. Ecol. Monogr. 993, 63, 199–229. [Google Scholar] [CrossRef]
- Bentley, A.R.; Petrovic, T.; Griffiths, S.P.; Burgess, L.W.; Summerell, B.A. Crop pathogens and other Fusarium species associated with Austrostipa aristiglumis. Aust. Plant Pathol. 2007, 36, 434–438. [Google Scholar] [CrossRef]
- Liu, H.; Stiling, P. Testing the enemy release hypothesis: A review and meta-analysis. Biol. Invasions 2006, 8, 1535–1545. [Google Scholar] [CrossRef]
- Nicholls, N. The changing nature of Australian droughts. Clim. Change 2004, 63, 323–336. [Google Scholar] [CrossRef]
- Cai, W.; Cowan, T. Evidence from impacts of rising temperature on inflows to the Murray-Darling Basin. Geophys. Res. Lett. 2008, 35, L07701. [Google Scholar]
- Eamus, D. The interaction of rising CO2 and temperatures with water use efficiency. Plant Cell Environ. 1991, 14, 843–852. [Google Scholar] [CrossRef]
- Betts, R.A.; Boucher, O.; Collins, M.; Cox, P.M.; Falloon, P.D.; Gedney, N.; Hemming, D.L.; Huntingford, C.; Jones, C.D.; Sexton, D.M.H.; et al. Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature 2007, 448, 1037–1042. [Google Scholar] [CrossRef]
- Leipprand, A.; Gerten, D. Global effects of doubled atmosphere CO2 content on evapotranspiration, soil moisture and runoff under potential natural vegetation. Hydrol. Sci. J. 2006, 51, 171–185. [Google Scholar] [CrossRef]
- Kergoat, L.; Lafont, S.; Douvilee, H.; Berthelot, B.; Dedieu, G.; Planton, S.; Royer, J.-F. Impact of doubled CO2 on global-scale leaf area index and evapotranspiration: Conflicting stomatal conductance and LAI responses. J. Geophys. Res. 2002, 107, 4808. [Google Scholar]
- Gedney, N.; Cox, P.M.; Betts, R.A.; Boucher, O.; Huntingford, C.; Stott, P.A. Detection of a direct carbon dioxide effect in continental river runoff records. Nature 2006, 439, 835–838. [Google Scholar] [CrossRef]
- Alkama, R.; Kageyama, M.; Ramstein, G. Relative contributions of climate change, stomatal closure, and leaf area index changes to 20th and 21st century runoff change: A modeling approach using the Organizing Carbon and Hydrology in Dynamic Ecosystems (ORCHIDEE) land surface model. J. Geophys. Res. 2010, 115, D17112. [Google Scholar] [CrossRef]
- Leuzinger, S.; Körner, C. Rainfall distribution is the main driver of runoff under future CO2-concentration in a temperate deciduous forest. Glob. Chang Biol. 2010, 16, 246–254. [Google Scholar] [CrossRef]
- Murray, S.J.; Foster, P.N.; Prentice, I.C. Future global water resources with respect to climate change and water withdrawals as estimated by a dynamic global vegetation model. J. Hydrol. 2012, 448–449, 14–29. [Google Scholar]
- Hovenden, M.J.; Wills, K.E.; Vander Schoor, J.K.; Williams, A.L.; Newton, P.C.D. Flowering phenology in a species-rich temperate grassland is sensitive to warming but not elevated CO2. New Phytol. 2008, 178, 815–822. [Google Scholar] [CrossRef]
- Dieleman, W.I.J.; Vicca, S.; Dijkstra, F.A.; Hagedorn, F.; Hovenden, M.J.; Larsen, K.S.; Morgan, J.A.; Volder, A.; Beier, C.; Dukes, J.S.; et al. Simple additive effects are rare: A quantified review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob. Chang. Biol. 2012, 18, 2681–2693. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gordon, C.; Llorens, L.; Nielsen, T.; Tietema, A.; Beier, C.; Bruna, P.; Emmett, B.; Estiarte, M.; Gorissen, A. Nonintrusive field experiments show different plant responses to warming and drought among sites, seasons, and species in a north-south European Gradient. Ecosystems 2004, 7, 598–612. [Google Scholar]
- Murphy, B.F.; Timbal, B. A review of recent climate variability and climate change in southeastern Australia. Int. J. Climatol. 2008, 28, 859–879. [Google Scholar] [CrossRef]
- Hoffman, A.A.; Blows, M.W. Species borders—Ecological and evolutionary perspectives. Trends Ecol. Evol. 1994, 9, 223–227. [Google Scholar] [CrossRef]
- Arnaud-Haond, S.; Teixeira, S.; Massa, S.I.; Billot, C.; Saenger, P.; Coupland, G.; Duarte, C.M.; Serråo, E.A. Genetic structure at range edge: Low diversity and high inbreeding in Southeast Asian mangrove (Avicennia marina) populations. Mol. Ecol. 2000, 15, 3515–3525. [Google Scholar]
- Mandák, B.; Zákravský, P.; Kořínková, D.; Dostál, P.; Plačová, I. Low population differentiation and high genetic diversity in the invasive species Carduus acanthoides L. (Asteraceae) within its native range in the Czech Republic. Biol. J. Linn. Soc. Lond. 2009, 98, 596–607. [Google Scholar] [CrossRef]
- Kirk, H.; Paul, J.; Straka, J.; Freeland, J.R. Long distance dispersal and high genetic diversity are implicated in the invasive spread of the common reed, Phragmites australis, in northeastern North America. Am. J. Bot. 2011, 98, 1180–1190. [Google Scholar]
- Schoen, D.J.; Brown, A.H.D. Intraspecific variation in population gene diversity and effective population size correlates with the mating system of plants. Proc. Nat. Acad. Sci. USA 1991, 88, 4494–4497. [Google Scholar] [CrossRef]
- Wright, S.I.; Ness, R.W.; Foxe, J.P.; Barrett, S.C.H. Genomic consequences of outcrossing and selfing in plants. Int. J. Plant Sci. 2008, 169, 105–118. [Google Scholar] [CrossRef]
- Bossdorf, O.; Auge, H.; Lafuma, L.; Rogers, W.E.; Siemann, E.; Prati, D. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 2005, 144, 1–11. [Google Scholar] [CrossRef]
- Chapin, F.S.; Autumn, K.; Pugnaire, F. Evolution of suites of traits in response to environmental stress. Am. Nat. 1993, 142, 78–92. [Google Scholar]
- Vitasse, Y.; Delzon, S.; Bresson, C.C.; Michalet, R.; Kremer, A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res. 2009, 39, 1259–1269. [Google Scholar] [CrossRef]
- Liancourt, P.; Tielbörger, K. Competition and a short growing season lead to ecotypic differentiation at the two extremes of the ecological range. Funct. Ecol. 2009, 23, 397–404. [Google Scholar] [CrossRef]
- He, W.M.; Thelen, G.C.; Ridenour, W.M.; Callaway, R.M. Is there a risk to living large? Large size correlates with reduced growth when stressed for knapweed populations. Biol. Invasions 2010, 12, 3591–3598. [Google Scholar] [CrossRef]
- Ebeling, S.K.; Stöcklin, J.; Hensen, I.; Auge, H. Multiple garden experiments suggest lack of local adaptation in an invasive ornamental plant. J. Plant Ecol. 2011, 4, 209–220. [Google Scholar] [CrossRef]
- Kawakami, T.; Morgan, T.J.; Nippert, J.B.; Ocheltree, T.W.; Keith, R.; Dhakal, P.; Ungerer, M.C. Natural selection drives clinal life history patterns in the perennial sunflower species, Helianthus maximiliani. Mol. Ecol. 2011, 20, 2318–2328. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Godfree, R.C.; Robertson, B.C.; Gapare, W.J.; Ivković, M.; Marshall, D.J.; Lepschi, B.J.; Zwart, A.B. Nonindigenous Plant Advantage in Native and Exotic Australian Grasses under Experimental Drought, Warming, and Atmospheric CO2 Enrichment. Biology 2013, 2, 481-513. https://doi.org/10.3390/biology2020481
Godfree RC, Robertson BC, Gapare WJ, Ivković M, Marshall DJ, Lepschi BJ, Zwart AB. Nonindigenous Plant Advantage in Native and Exotic Australian Grasses under Experimental Drought, Warming, and Atmospheric CO2 Enrichment. Biology. 2013; 2(2):481-513. https://doi.org/10.3390/biology2020481
Chicago/Turabian StyleGodfree, Robert C., Bruce C. Robertson, Washington J. Gapare, Miloš Ivković, David J. Marshall, Brendan J. Lepschi, and Alexander B. Zwart. 2013. "Nonindigenous Plant Advantage in Native and Exotic Australian Grasses under Experimental Drought, Warming, and Atmospheric CO2 Enrichment" Biology 2, no. 2: 481-513. https://doi.org/10.3390/biology2020481




