Disintegrated Waste-Activated Sludge (NO2/FNA Method) as a Source of Carbon for Denitrification in the Mainstream of a WWTP
Abstract
:1. Introduction
- Released SCOD is tested as a carbon source for denitrification in the classical activated sludge process. The potential of the obtained carbon sources is evaluated based on NUR and then compared to those obtained for acetate and mechanically treated wastewater. Moreover, the SCOD yield per g of the volatile solids (VS) of the WAS is assessed.
- The obtained experimental results are used in mass balance calculations and process simulations to show the potential of such an application at the full scale.
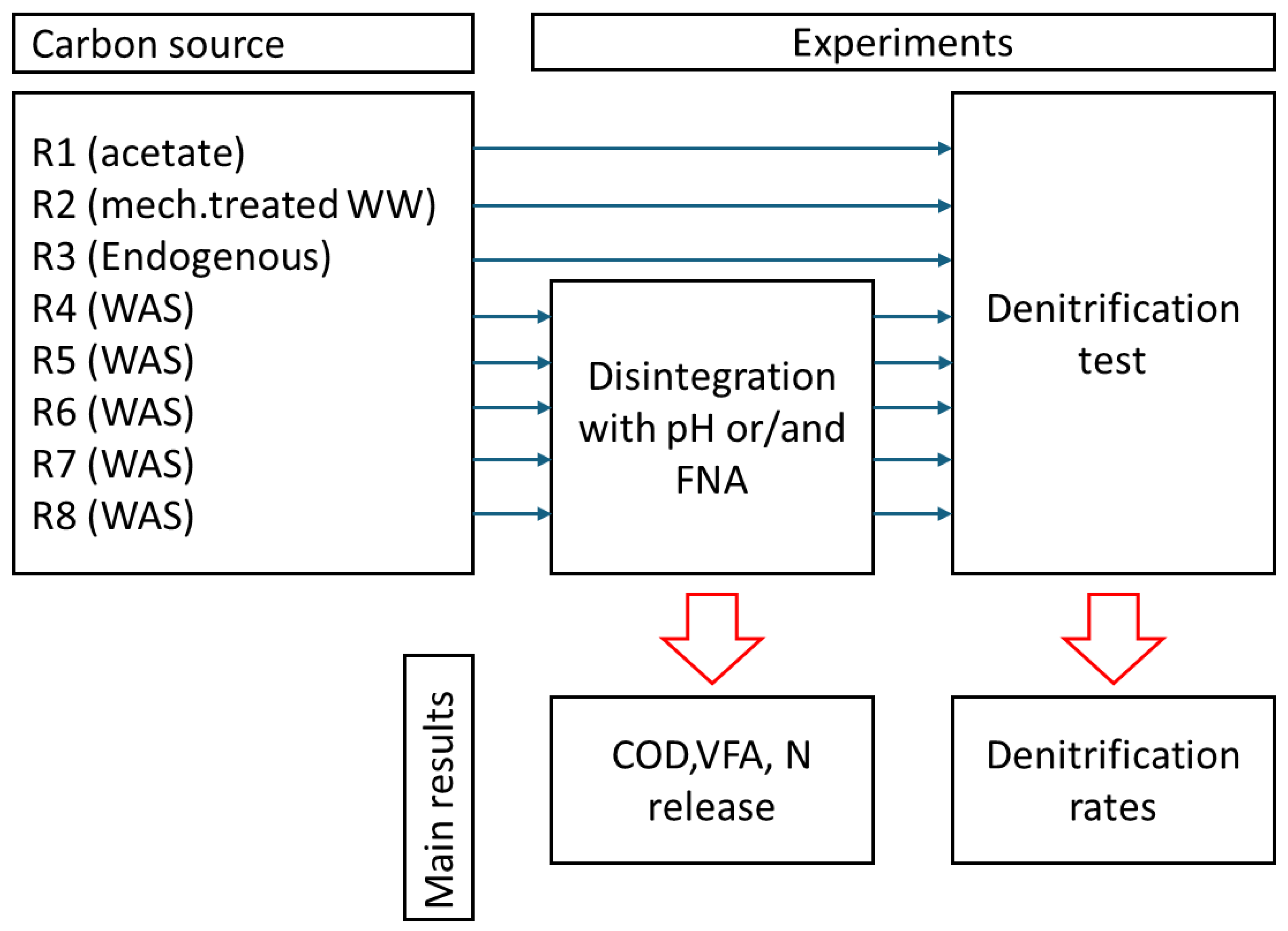
2. Materials and Methods
2.1. Wastewater and Sludge Sources
2.2. NO2/FNA Disintegrated WAS
2.3. Denitrification
2.4. Analytical Methods
2.5. Simulation of WWTP Operation
3. Results and Discussion
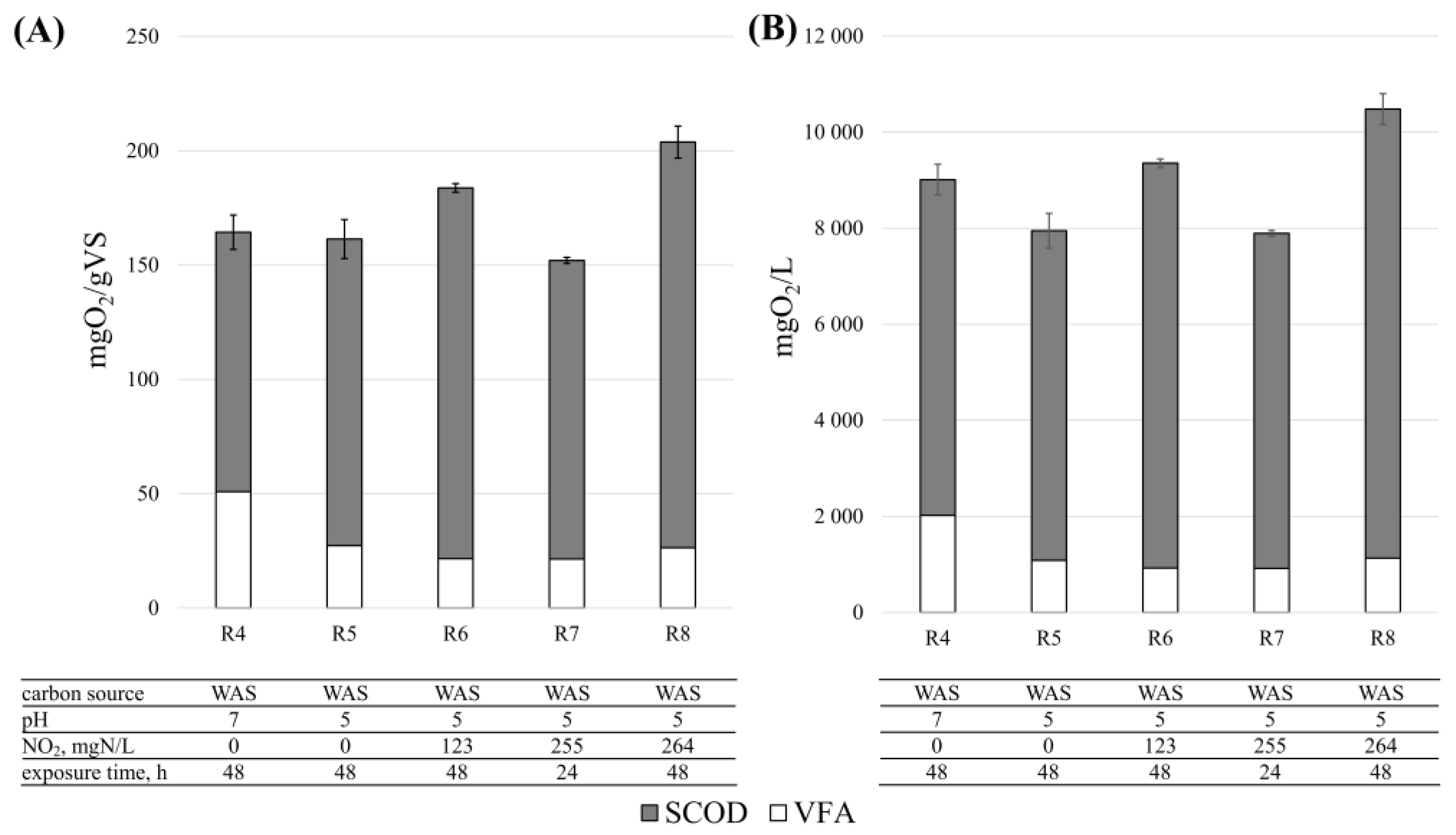
3.1. Disintegration Efficiency—SCOD
3.2. Disintegration Efficiency—Volatile Fatty Acids
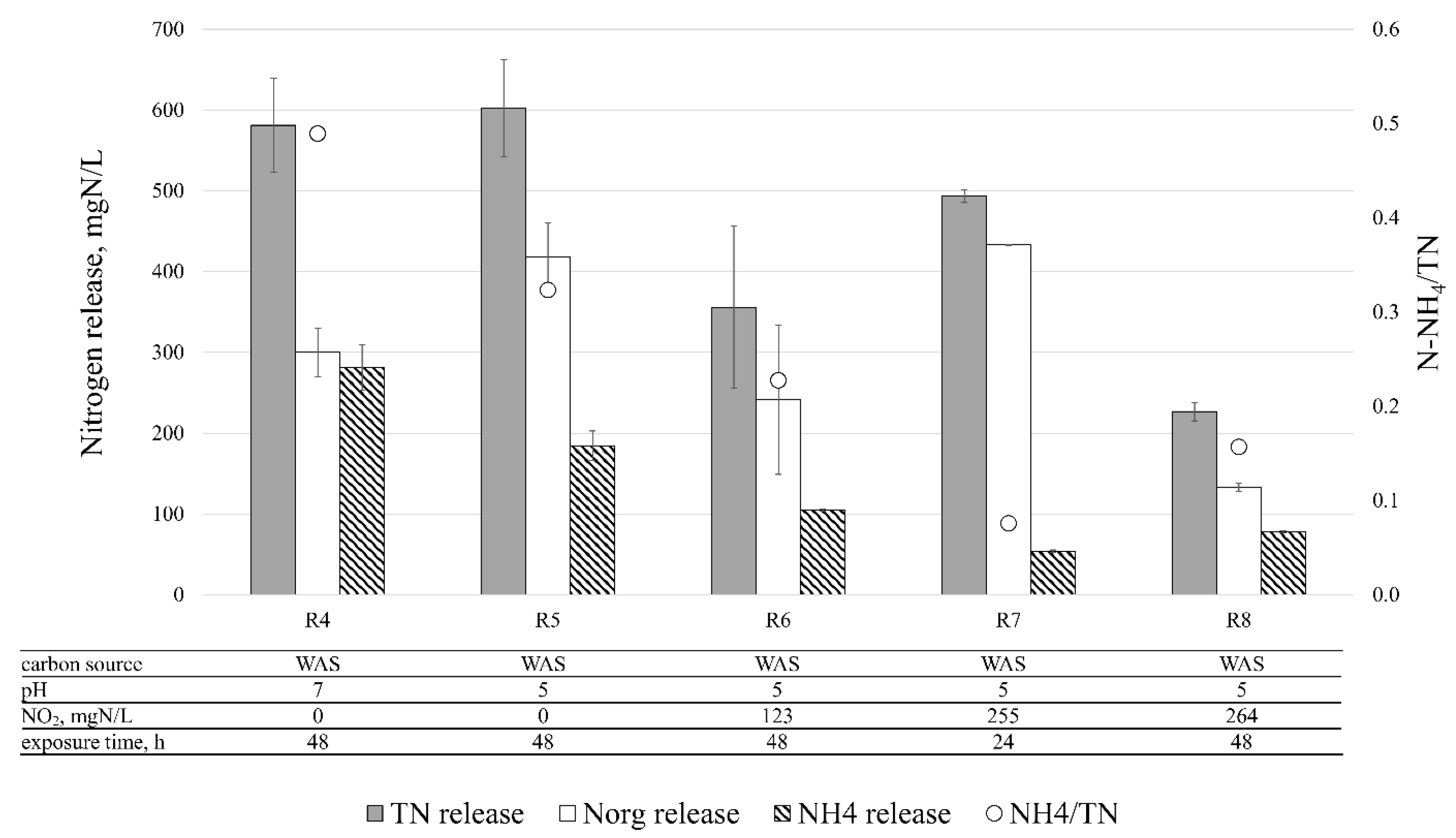
3.3. Disintegration Efficiency—Nitrogen
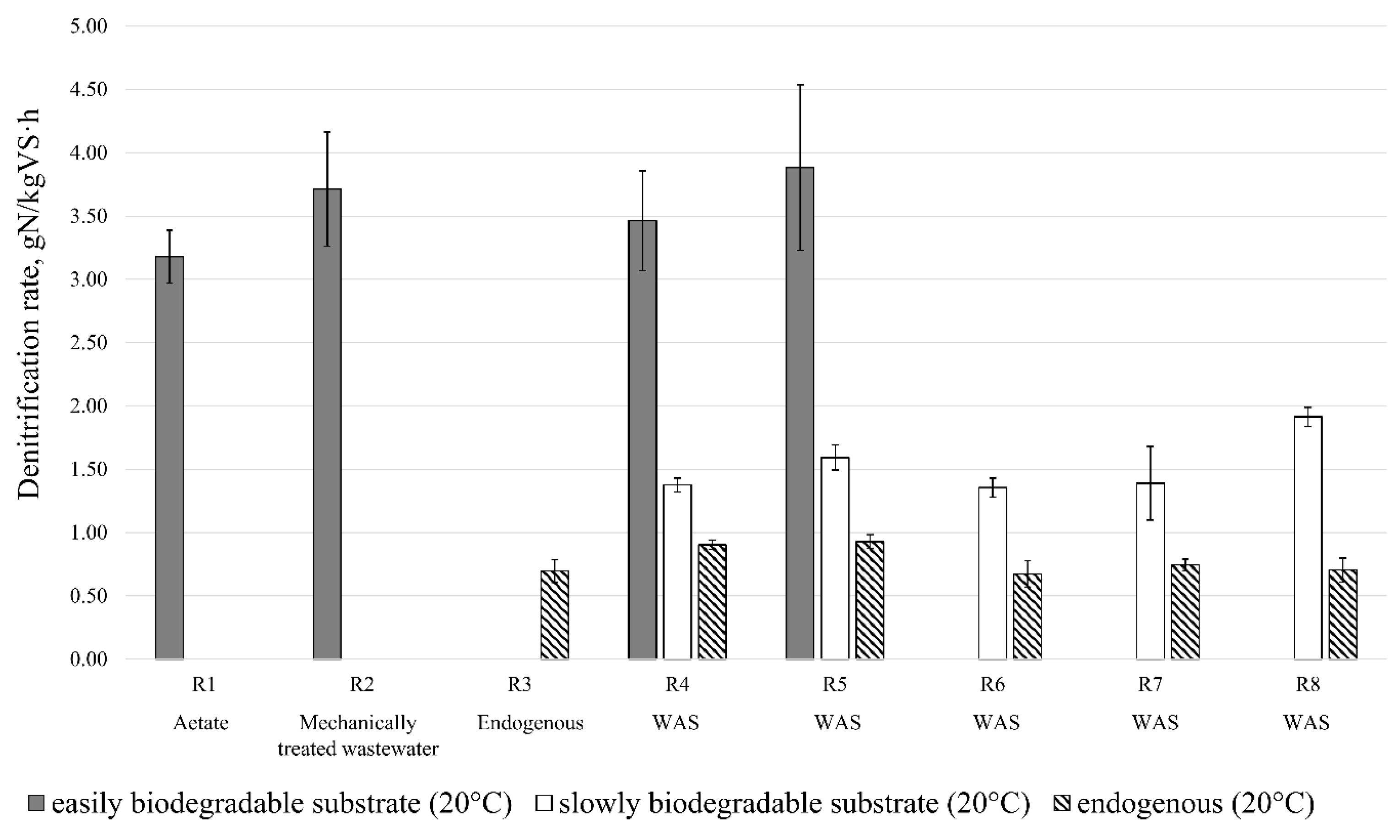
3.4. Denitrification—Process Rates
3.4.1. Reference Substrates
3.4.2. Disintegration Products
3.5. Denitrification—The Biodegradability of Disintegration Products
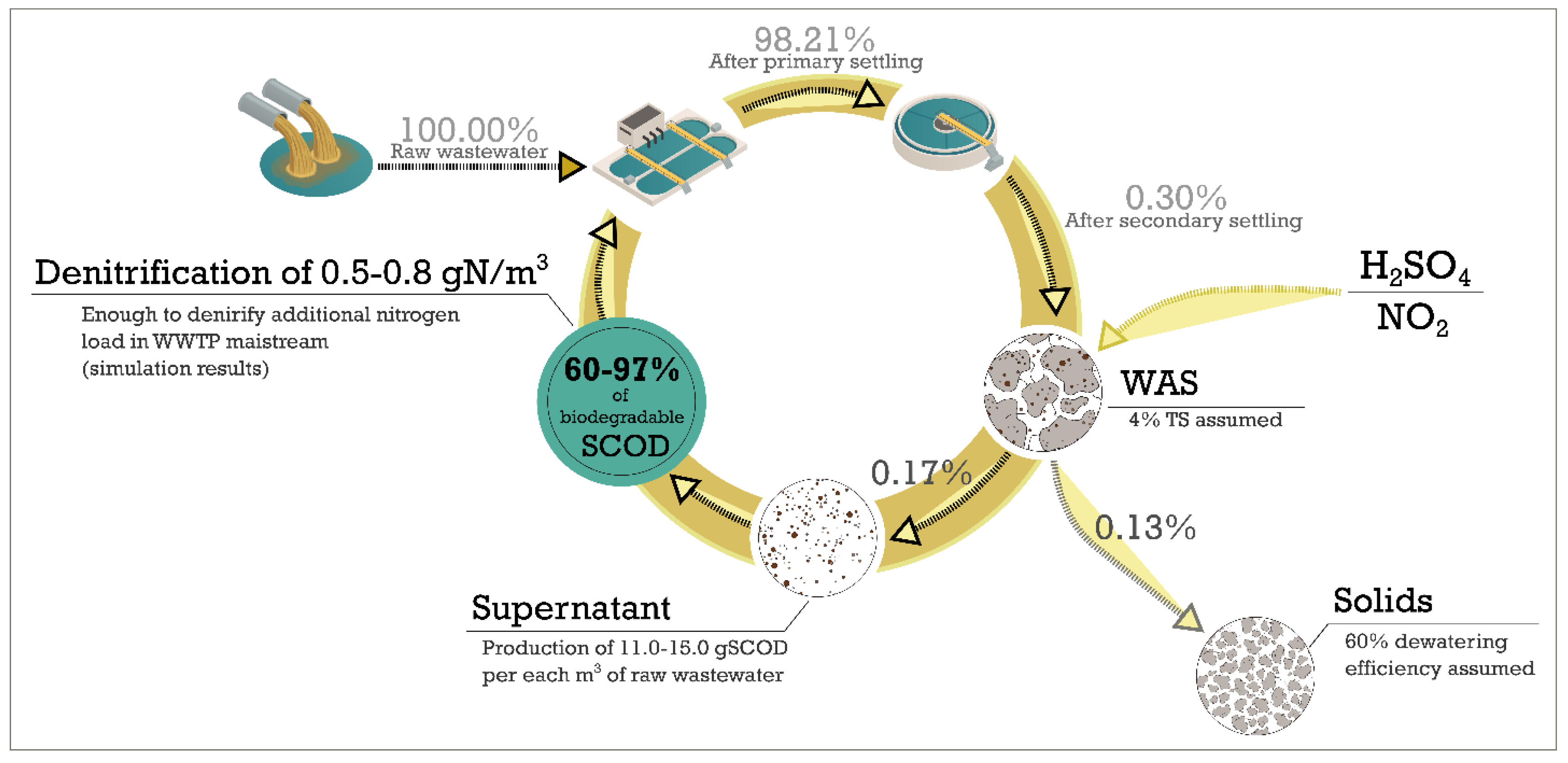
3.6. Assessment of Full-Scale Potential
Simulation Results
3.7. Summary
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Liu, S.; Ma, T.; Zheng, M.; Ni, J. Simultaneous nitri fi cation, denitri fi cation and phosphorus removal in a sequencing batch reactor (SBR) under low temperature. Chemosphere 2019, 229, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, G.; Zhang, H.; Li, F.; Li, L. Enhancement of nitrogen and phosphorus removal, sludge reduction and microbial community structure in an anaerobic/anoxic/oxic process coupled with composite ferrate solution disintegration. Environ. Res. 2020, 190, 110006. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.K.H.; Straka, L. New directions in biological nitrogen removal and recovery from wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-P.; Carles, P.I.N.; Merkey, B.; Zhou, Q.; Xia, S.-Q.; Yang, D.-H.; Sun, J.-H.; Smets, B.F. Effective Biological Nitrogen Removal Treatment Processes for Domestic Wastewaters with Low C/N Ratios: A Review. Environ. Eng. Sci. 2010, 27, 111–126. [Google Scholar] [CrossRef]
- Kim, E.; Gu, S.; Hanifa, A.; Vincent, J. Use of food waste-recycling wastewater as an alternative carbon source for denitri fi cation process: A full-scale study. Bioresour. Technol. 2017, 245, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yuan, S.; Wang, W.; Xiao, L.; Zhan, X.; Hu, Z. Effect of solid-liquid separation on food waste fermentation products as external carbon source for denitri fi cation. J. Clean. Prod. 2021, 284, 124687. [Google Scholar] [CrossRef]
- Shao, M.; Guo, L.; She, Z.; Gao, M.; Zhao, Y.; Sun, M.; Guo, Y. Enhancing denitrification efficiency for nitrogen removal using waste sludge alkaline fermentation liquid as external carbon source. Environ. Sci. Pollut. Res. 2019, 26, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A. Mini review: Update on bioaugmentation in anaerobic processes for biogas production. Anaerobe 2017, 46, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, S.; Choolaei, Z.; Meyer, T.; Edwards, E.A.; Yakunin, A.F.; Allen, D.G. Evaluating the effect of enzymatic pretreatment on the anaerobic digestibility of pulp and paper biosludge. Biotechnol. Rep. 2018, 17, 77–85. [Google Scholar] [CrossRef]
- Sun, D.; Qiao, M.; Xu, Y.; Ma, C.; Zhang, X. Pretreatment of waste activated sludge by peracetic acid oxidation for enhanced anaerobic digestion. Environ. Prog. Sustain. Energy. 2018, 37, 2058–2062. [Google Scholar] [CrossRef]
- Lippert, T.; Bandelin, J.; Vogl, D.; Tesieh, Z.A.; Wild, T.; Drewes, J.E.; Koch, K. Full-Scale Assessment of Ultrasonic Sewage Sludge Pretreatment Using a Novel Double-Tube Reactor. ACS ES T Eng. 2020, 1, 298–309. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, L.; Jiang, G.; Jensen, P.D.; Batstone, D.J.; Yuan, Z. Free nitrous acid (FNA)-based pretreatment enhances methane production from waste activated sludge, Environ. Sci. Technol. 2013, 47, 11897–11904. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.; Icaran, P.; Yuan, Z.; Pijuan, M. Assessment of free nitrous acid pre-treatment on a mixture of primary sludge and waste activated sludge: Effect of exposure time and concentration. Bioresour. Technol. 2016, 216, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Hallaji, S.M.; Siami, S.; Torabian, A.; Aminzadeh, B.; Eshtiaghi, N.; Zahedi, S. Synergy of combined free nitrous acid and Fenton technology in enhancing anaerobic digestion of actual sewage waste activated sludge. Sci. Rep. 2020, 10, 5027. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Peng, Y.; Wei, Y.; Li, B.; Bao, P.; Wang, Y. Free nitrous acid pretreatment of wasted activated sludge to exploit internal carbon source for enhanced denitrification. Bioresour. Technol. 2015, 179, 20–25. [Google Scholar] [CrossRef]
- Szypulska, D.; Miodoński, S.; Muszyński-Huhajło, M.; Zięba, B.; Janiak, K. Determination of the major factor responsible for soluble organic matter release during nitrite/free nitrous acid pre-treatment of waste activated sludge. Bioresour. Technol. 2021, 329, 124917. [Google Scholar] [CrossRef]
- Szypulska, D.; Miodoński, S.; Janiak, K.; Muszyński-Huhajło, M.; Zięba, B.; Cema, G. Waste activated sludge disintegration with free nitrous acid—Comprehensive analysis of the process parameters. Renew. Energy 2021, 172, 112–120. [Google Scholar] [CrossRef]
- Park, S.; Bae, W. Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid. Process Biochem. 2009, 6, 631–640. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Reddy, K.R. Temperature Effects in Treatment Wetlands. Water Environ. Res. 2001, 73, 543–557. [Google Scholar] [CrossRef]
- APHA. 2540 G. Total, fixed, and volatile solids in solid and semisolid samples. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- APHA. 5220 Chemical Oxygen Demand (COD). In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2000. [Google Scholar]
- Hauduc, H.; Takács, I.; Smith, S.; Szabo, A.; Murthy, S.; Daigger, G.T.; Spérandio, M. A dynamic physicochemical model for chemical phosphorus removal. Water Res. 2015, 73, 157–170. [Google Scholar] [CrossRef]
- Varga, E.; Hauduc, H.; Barnard, J.; Dunlap, P.; Jimenez, J.; Menniti, A.; Schauer, P.; Vazquez, C.M.L.; Gu, A.Z.; Sperandio, M.; et al. Recent advances in bio-P modelling—A new approach verified by full-scale observations. Water Sci. Technol. 2018, 78, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Atay, Ş.; Akbal, F. Classification and Effects of Sludge Disintegration Technologies Integrated into Sludge Handling Units: An Overview. Clean-Soil Air Water 2016, 44, 1198–1213. [Google Scholar] [CrossRef]
- de Sousa, T.A.T.; Monte, F.P.D.; Silva, J.V.D.N.; Lopes, W.S.; Leite, V.D.; van Lier, J.B.; de Sousa, J.T. Alkaline and acid solubilisation of waste activated sludge. Water Sci. Technol. 2021, 83, 2980–2996. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, Y.; Dong, B.; Dai, X. Effects of free nitrous acid and nitrite on two-phase anaerobic digestion of waste activated sludge: A preliminary study. Sci. Total Environ. 2019, 654, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Walczak, J.; Zubrowska-Sudol, M. The rate of denitrification using hydrodynamically disintegrated excess sludge as an organic carbon source. Water Sci. Technol. 2018, 77, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, V.; Urbain, V.; Buckley, C.A. Characterization of wastewater and activated sludge from European municipal wastewater treatment plants using the NUR test. Water Sci. Technol. 1998, 38, 303–305. [Google Scholar] [CrossRef]
- Rodríguez, L.; Villaseñor, J.; Fernández, F.J.J. Use of agro-food wastewaters for the optimisation of the denitrification process. Water Sci. Technol. 2007, 55, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kowal, P.; Ciesielski, S.; Otieno, J.; Majtacz, J.B.; Czerwionka, K.; Mąkinia, J. Denitrification process enhancement and diversity of the denitrifying community in the full scale activated sludge system after adaptation to fusel oil. Energies 2021, 14, 5225. [Google Scholar] [CrossRef]
- Sage, M.; Daufin, G.; Ge, G. Denitrification potential and rates of complex carbon source from dairy effluents in activated sludge system. Water Res. 2006, 40, 2747–2755. [Google Scholar] [CrossRef]
- Świniarski, M. The Effect of External Carbon Cources on Enhancing the Denitrification Process in Activated Sludge Systems. Ph.D. Thesis, Gdańsk University of Technology, Gdańsk, Poland, 2011. [Google Scholar]
- Henze, M.; Gujer, W.; Mino, T.; Van Loosdrecht, M.C.M. Activated Sludge Models ASM1, ASM2, ASM2d and ASM3; IWA Publishing: London, UK, 2000. [Google Scholar]
- Guo, Y.; Guo, L.; Sun, M.; Zhao, Y.; Gao, M.; She, Z. Effects of hydraulic retention time (HRT) on denitrification using waste activated sludge thermal hydrolysis liquid and acidogenic liquid as carbon sources. Bioresour. Technol. 2017, 224, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Langone, M.; Andreottola, G. Ultrasonics Sonochemistry A swirling jet-induced cavitation to increase activated sludge solubilisation and aerobic sludge biodegradability. Ultrason.-Sonochem. 2017, 35, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Duan, H.; Chen, X.; Wu, Z.; Yu, W.; Silva, C.E.; Li, Y.; Shrestha, S.; Wang, Z.; Keller, J.; et al. Enhancing anaerobic digestion using free nitrous acid: Identifying the optimal pre-treatment condition in continuous operation. Water Res. 2021, 205, 117694. [Google Scholar] [CrossRef] [PubMed]
- Pijuan, M.; Wang, Q.; Ye, L.; Yuan, Z. Improving secondary sludge biodegradability using free nitrous acid treatment. Bioresour. Technol. 2012, 116, 92–98. [Google Scholar] [CrossRef] [PubMed]
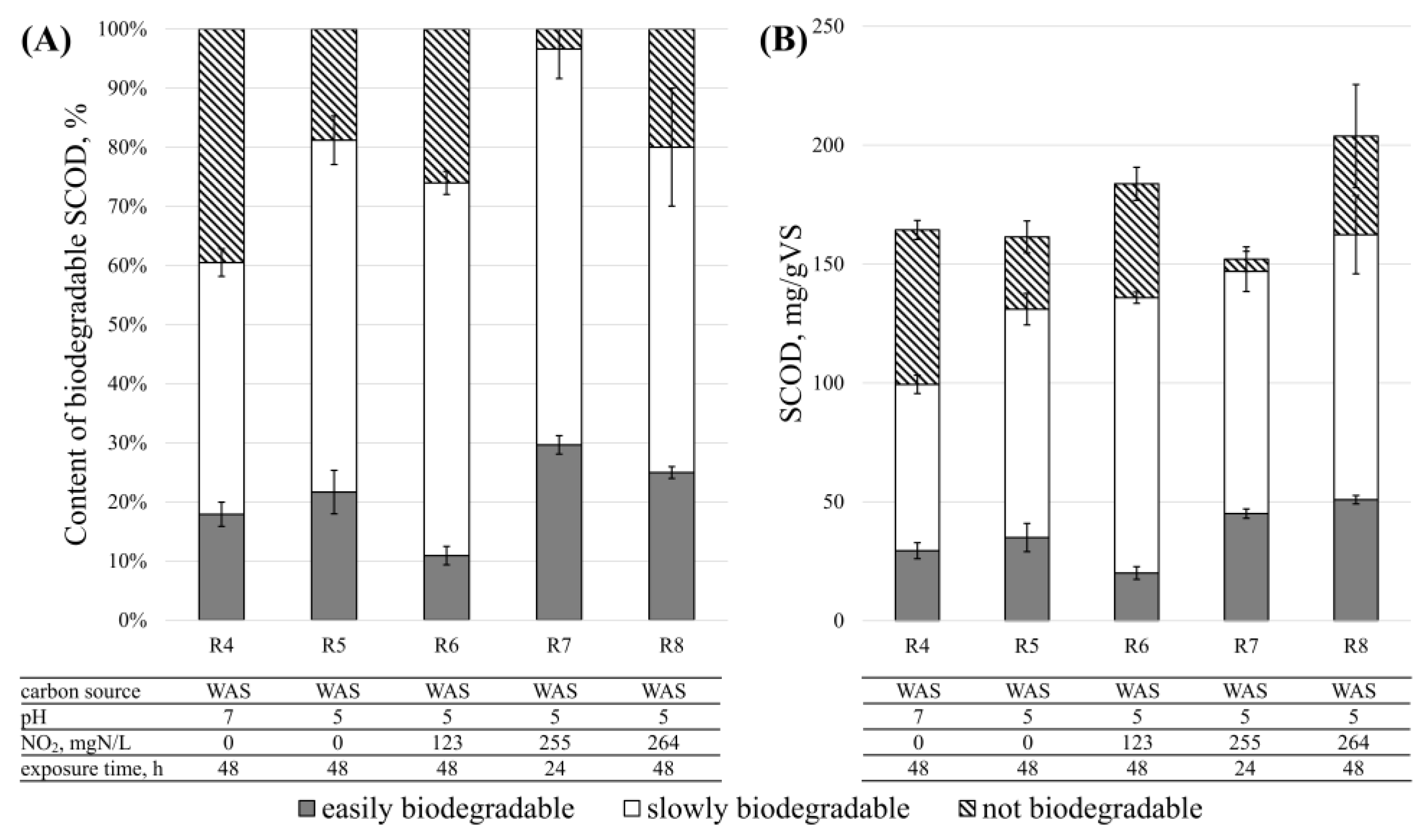
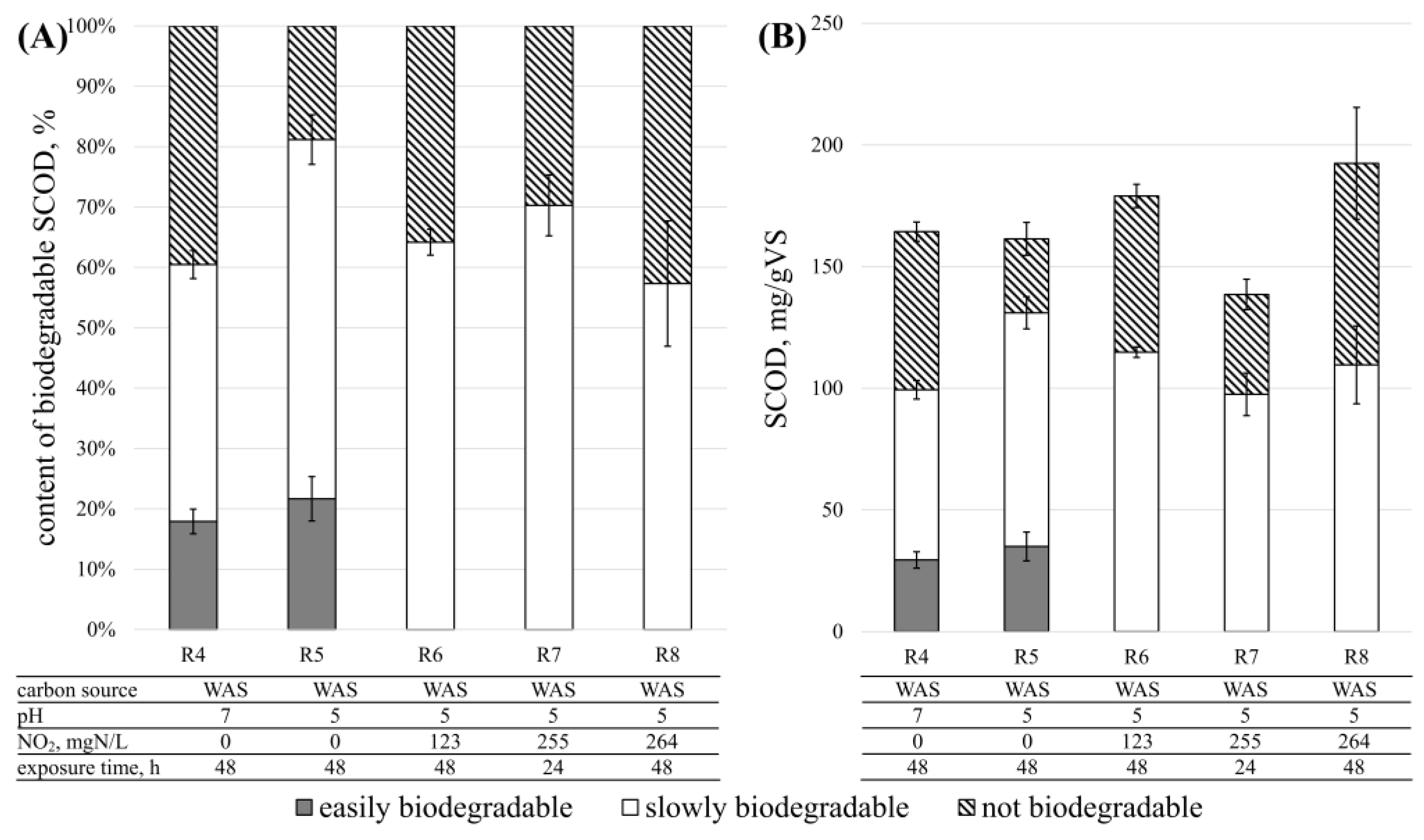

| Substrate | TSgTS/kg | VSgVS/kg | TCODmgO2/L | SCODmgO2/L | TN mgN/L |
|---|---|---|---|---|---|
| WAS | 60.2 ± 1.0 | 44.2 ± 1.7 | 75 650 ± 1 774 | 276 ± 24 | 34 ± 11 |
| Activated sludge | 5.9 ± 0.5 | 4.1 ± 0.4 | not determined | 38 ± 7 | not determined |
| Mechanically treated wastewater | 0.3 ± 0.1 | 0.2 ± 0.1 | 700 ± 147 | 229 ± 31 | 62 ± 10 |
| Carbon Source | Disintegration Parameters before Denitrification | Number of Repetitions | ||||
|---|---|---|---|---|---|---|
| NO2, mg N-NO2/L | FNA, mg N-HNO2/L | pH | Exposure Time, h | |||
| R1 | Acetate | Not applicated | 3 | |||
| R2 | Mechanically treated wastewater | Not applicated | 3 | |||
| R3 | Endogenous | Not applicated | 3 | |||
| R4 | WAS | 0.0 ± 0.0 | 0.0 ± 0.0 | 7.0 ± 0.0 | 48 | 3 |
| R5 | WAS | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.1 ± 0.0 | 48 | 3 |
| R6 | WAS | 123.0 ± 29.7 | 2.8 ± 0.1 | 5.1 ± 0.0 | 48 | 2 |
| R7 | WAS | 254.6 ± 25.2 | 5.8 ± 0.9 | 5.1 ± 0.1 | 24 | 2 |
| R8 | WAS | 263.5 ± 17.0 | 5.8 ± 0.0 | 5.1 ± 0.2 | 48 | 2 |
| Scenarios | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Unit | Reference | R4 | R5 | R6 | R7 | R8 |
| Total ammonia (NHx) | g N/m3 | 0.64 | 0.64 | 0.64 | 0.64 | 0.64 | 0.64 |
| Nitrate (NO3) | g N/m3 | 7.98 | 7.39 | 7.48 | 7.23 | 7.19 | 7.15 |
| Total nitrogen | g N/m3 | 9.81 | 9.25 | 9.33 | 9.08 | 9.03 | 9.00 |
| Orthophosphate (PO4) | g P/m3 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Air flow rate | m3/d | 1,785,649 | 1,785,649 | 1,783,686 | 1,784,468 | 1,773,731 | 1,789,684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szypulska, D.; Janiak, K.; Zięba, B.; Wizimirska, A.; Mołczan, M.; Szerzyna, S.; Tomczak-Wandzel, R. Disintegrated Waste-Activated Sludge (NO2/FNA Method) as a Source of Carbon for Denitrification in the Mainstream of a WWTP. Resources 2024, 13, 80. https://doi.org/10.3390/resources13060080
Szypulska D, Janiak K, Zięba B, Wizimirska A, Mołczan M, Szerzyna S, Tomczak-Wandzel R. Disintegrated Waste-Activated Sludge (NO2/FNA Method) as a Source of Carbon for Denitrification in the Mainstream of a WWTP. Resources. 2024; 13(6):80. https://doi.org/10.3390/resources13060080
Chicago/Turabian StyleSzypulska, Dorota, Kamil Janiak, Bartosz Zięba, Anna Wizimirska, Marek Mołczan, Sławomir Szerzyna, and Renata Tomczak-Wandzel. 2024. "Disintegrated Waste-Activated Sludge (NO2/FNA Method) as a Source of Carbon for Denitrification in the Mainstream of a WWTP" Resources 13, no. 6: 80. https://doi.org/10.3390/resources13060080







