Nanotechnology, Inflammation and the Skin Barrier: Innovative Approaches for Skin Health and Cosmesis
Abstract
:1. Introduction
2. The Skin Barrier—Architecture, Function and the Impact of Inflammation

3. The Aesthetic Impact of Inflammation

4. Nanomaterials—A Primer
5. Nanotherapeutics as a Treatment for Inflammation
5.1. Prevention and Direct Inhibition of Inflammation
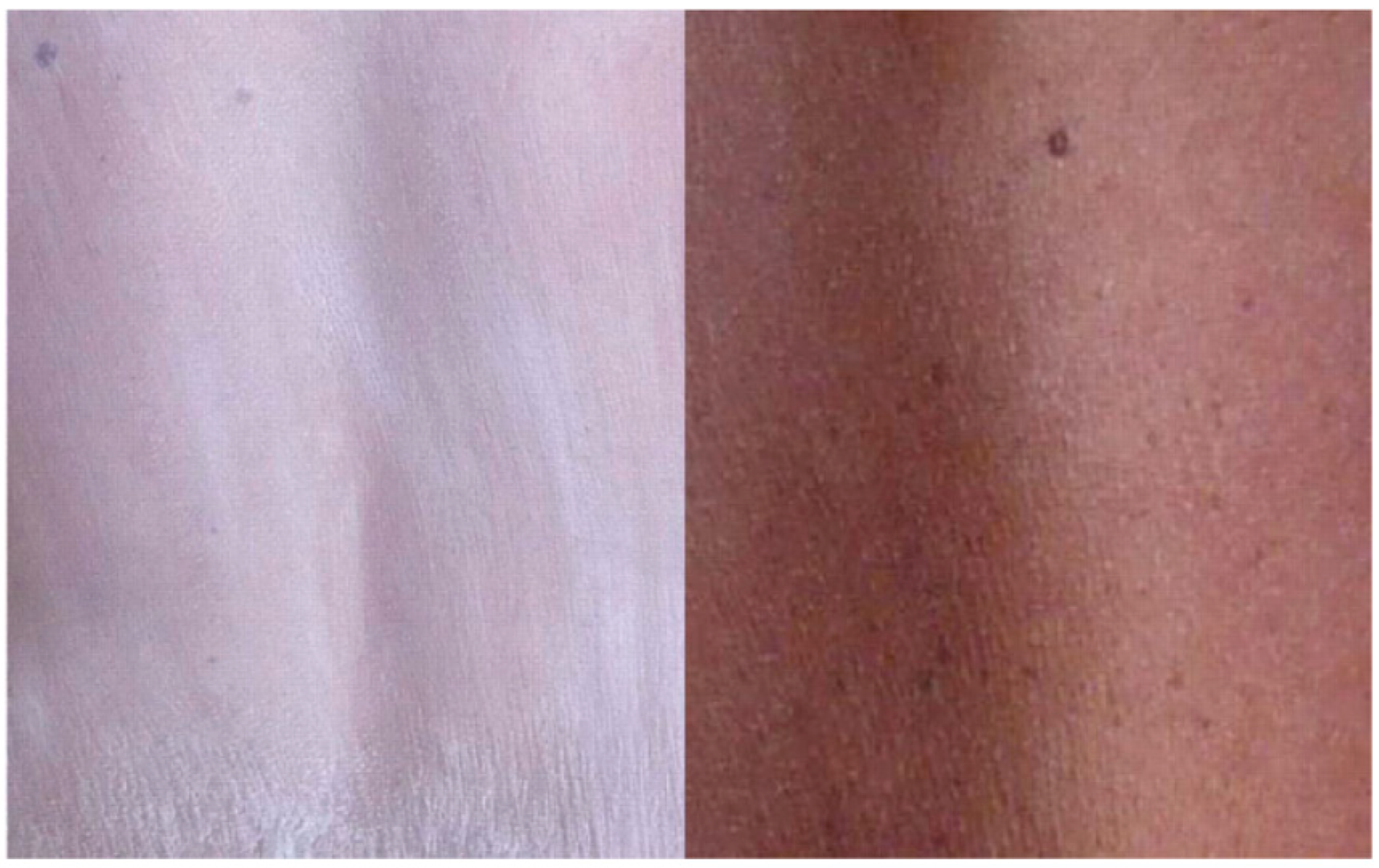
5.2. Moisturization and Fortification of the Skin Barrier
5.3. Color Correction
6. Conclusion
Author Contributions
Conflicts of Interest
References
- Hvid, M.; Johansen, C.; Deleuran, B.; Kemp, K.; Deleuran, M.; Vestergaard, C. Regulation of caspase 14 expression in keratinocytes by inflammatory cytokines—A possible link between reduced skin barrier function and inflammation? Exp. Dermatol. 2011, 20, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Hvid, M.; Vestergaard, C.; Kemp, K.; Christensen, G.B.; Deleuran, B.; Deleuran, M. Il-25 in atopic dermatitis: A possible link between inflammation and skin barrier dysfunction&quest. J. Investig. Dermatol. 2011, 131, 150–157. [Google Scholar] [PubMed]
- McDade, T.W. Early environments and the ecology of inflammation. Proc. Natl. Acad. Sci. 2012, 109, 17281–17288. [Google Scholar] [CrossRef] [PubMed]
- Mihranyan, A.; Ferraz, N.; Strømme, M. Current status and future prospects of nanotechnology in cosmetics. Prog. Mater. Sci. 2012, 57, 875–910. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L.; Gooris, G.S.; Ponec, M. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Nijhawan, R.; Alexis, A. Post-inflammatory pigment alteration. In Acneiform Eruptions in Dermatology; Zeichner, J., Ed.; Springer: New York, NY, USA, 2014; pp. 279–287. [Google Scholar]
- Hsu, C.-C.; Lee, J.Y.-Y. Pronounced facial flushing and persistent erythema of rosacea effectively treated by carvedilol, a nonselective β-adrenergic blocker. J. Am. Acad. Dermatol. 2012, 67, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Haveli, S.D.; Walter, P.; Patriarche, G.; Ayache, J.; Castaing, J.; Van Elslande, E.; Tsoucaris, G.; Wang, P.-A.; Kagan, H.B. Hair fiber as a nanoreactor in controlled synthesis of fluorescent gold nanoparticles. Nano Lett. 2012, 12, 6212–6217. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. New Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Friedman, A.J.; Friedman, J.M. Nitric oxide releasing nanoparticle synthesis and characterization. In Nitric oxide; Springer: New York, NY, USA, 2011; pp. 187–195. [Google Scholar]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Karadzovska, D.; Brooks, J.D.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting skin permeability from complex vehicles. Adv. Drug Deliv. Rev. 2013, 65, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Schlupp, P.; Blaschke, T.; Kramer, K.D.; Holtje, H.D.; Mehnert, W.; Schafer-Korting, M. Drug release and skin penetration from solid lipid nanoparticles and a base cream: A systematic approach from a comparison of three glucocorticoids. Skin Pharmacol. Physiol. 2011, 24, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Santos Maia, C.; Mehnert, W.; Schaller, M.; Korting, H.C.; Gysler, A.; Haberland, A.; Schafer-Korting, M. Drug targeting by solid lipid nanoparticles for dermal use. J. Drug Target. 2002, 10, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.S.; Mehnert, W.; Schäfer-Korting, M. Solid lipid nanoparticles as drug carriers for topical glucocorticoids. Int. J. Pharm. 2000, 196, 165–167. [Google Scholar] [CrossRef]
- Wiechers, J.W.; Musee, N. Engineered inorganic nanoparticles and cosmetics: Facts, issues, knowledge gaps and challenges. J. Biomed. Nanotechnol. 2010, 6, 408–431. [Google Scholar] [CrossRef] [PubMed]
- Filipe, P.; Silva, J.N.; Silva, R.; Cirne de Castro, J.L.; Marques Gomes, M.; Alves, L.C.; Santus, R.; Pinheiro, T. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol. Physiol. 2009, 22, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytam, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L.; et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Maibach, H.I. Barrier creams—Skin protectants: Can you protect skin? J. Cosmet. Dermatol. 2002, 1, 20–23. [Google Scholar] [PubMed]
- De Fine Olivarius, F.; Hansen, A.B.; Karlsmark, T.; Wulf, H.C. Water protective effect of barrier creams and moisturizing creams: A new in vivo test method. Contact Dermat. 1996, 35, 219–225. [Google Scholar] [CrossRef]
- Wissing, S.A.; Muller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity—in vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- Wissing, S.; Lippacher, A.; Muller, R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–324. [Google Scholar] [PubMed]
- Kato, S.; Taira, H.; Aoshima, H.; Saitoh, Y.; Miwa, N. Clinical evaluation of fullerene-C60 dissolved in squalane for anti-wrinkle cosmetics. J. Nanosci. Nanotechnol. 2010, 10, 6769–6774. [Google Scholar] [CrossRef] [PubMed]
- Alfano, R.; Ni, X.; Zevallos, M. Changing Skin-Color Perception Using Quantum and Optical Principles in Cosmetic Preparations. Patent US 11/656,738, 27 August 2007. [Google Scholar]
- Ha, T.H.; Jeong, J.Y.; Jung, B.H.; Kim, J.K.; Lim, Y.T. Cosmetic Pigment Composition Containing Gold or Silver Nano-Particles. Patents US20090022765 A1, 22 January 2009. [Google Scholar]
- Cassin, G.; Simonnet, J.T. Cosmetic compositions comprising photoluminescent nanoparticles and at least one rare-earth metal. Patent WO2006054202 A1, 26 May 2006. [Google Scholar]
- Yoshimura, K.; Tsukamoto, K.; Okazaki, M.; Virador, V.M.; Lei, T.C.; Suzuki, Y.; Uchida, G.; Kitano, Y.; Harii, K. Effects of all-trans retinoic acid on melanogenesis in pigmented skin equivalents and monolayer culture of melanocytes. J. Dermatol. Sci. 2001, 27, 68–75. [Google Scholar] [CrossRef]
- Schafer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Gatti, R.; Gioia, M.G.; Cavrini, V. Analysis and stability study of retinoids in pharmaceuticals by LC with fluorescence detection. J. Pharm. Biomed. Anal. 2000, 23, 147–159. [Google Scholar] [CrossRef]
- Morganti, P.; del Ciotto, P.; Carezzi, F.; Guarneri, F.; Yeo, Y.J. Skin lightening efficacy of new formulations enhanced by chitin nanoparticles delivery system. Note I. J. Appl. Cosmetol. 2014, 32, 57–71. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landriscina, A.; Rosen, J.; Friedman, A.J. Nanotechnology, Inflammation and the Skin Barrier: Innovative Approaches for Skin Health and Cosmesis. Cosmetics 2015, 2, 177-186. https://doi.org/10.3390/cosmetics2020177
Landriscina A, Rosen J, Friedman AJ. Nanotechnology, Inflammation and the Skin Barrier: Innovative Approaches for Skin Health and Cosmesis. Cosmetics. 2015; 2(2):177-186. https://doi.org/10.3390/cosmetics2020177
Chicago/Turabian StyleLandriscina, Angelo, Jamie Rosen, and Adam J. Friedman. 2015. "Nanotechnology, Inflammation and the Skin Barrier: Innovative Approaches for Skin Health and Cosmesis" Cosmetics 2, no. 2: 177-186. https://doi.org/10.3390/cosmetics2020177




