The Risks of “Getting High” on Over-the-Counter Drugs during Pregnancy
Abstract
:1. Introduction
2. Getting High on Over-the-Counter Opioid Drugs
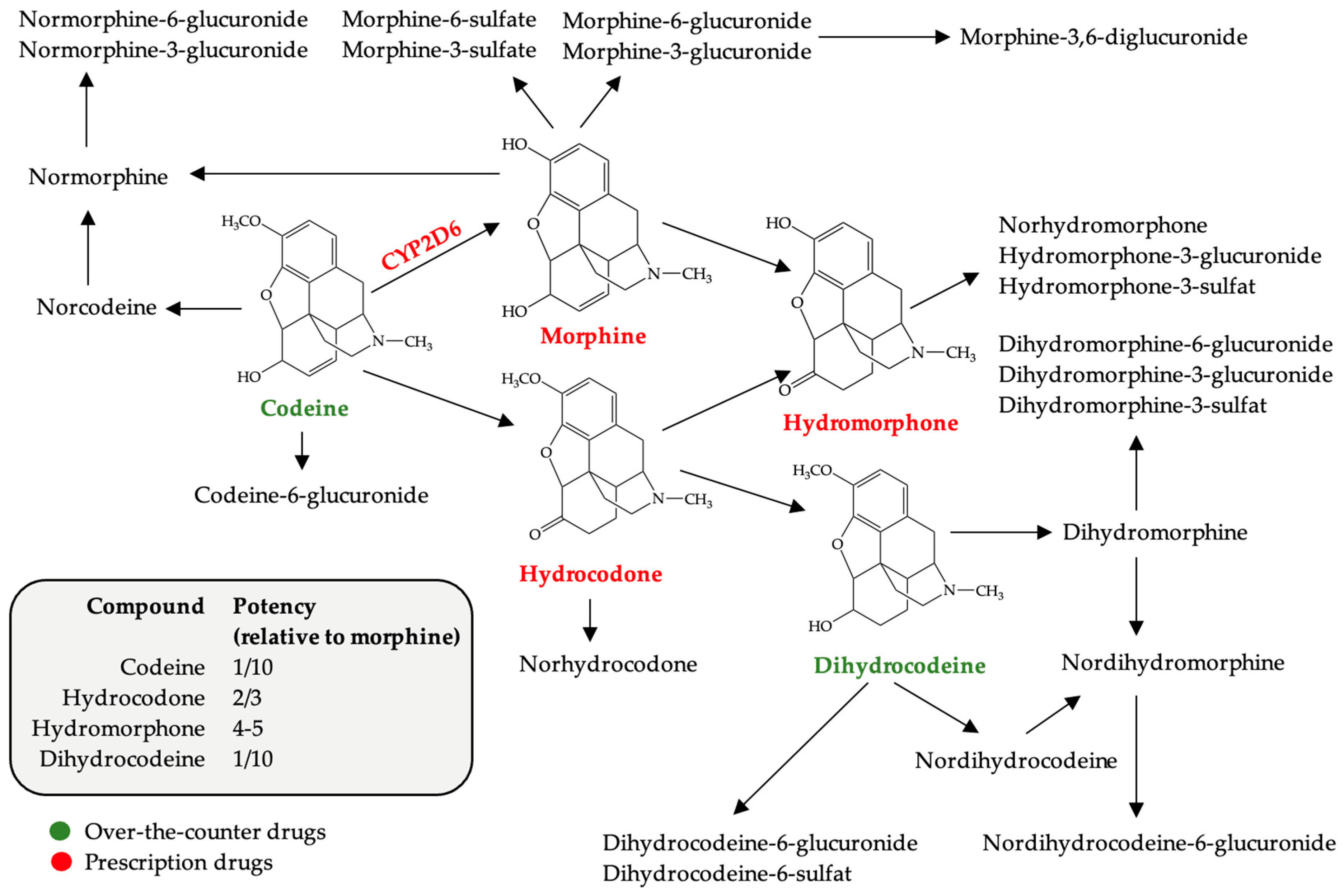
2.1. Codeine
2.1.1. Pharmacological Features of Codeine
2.1.2. Over-the-Counter Codeine Drugs and Pregnancy Risks
2.2. Loperamide
2.2.1. The Pharmacological Features of Loperamide
2.2.2. Loperamide and Pregnancy Risks
3. Getting High on Over-the-Counter Pseudoephedrine
3.1. The Pharmacological Features of Pseudoephedrine
3.2. Over-the-Counter Pseudoephedrine Drugs and Pregnancy Risks
4. Getting High on Over-the-Counter Antihistamines
5. Getting High on Over-the-Counter Local Antiseptic (Benzydamine)
6. Additional Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chautrakarn, S.; Khumros, W.; Phutrakool, P. Self-Medication with Over-the-Counter Medicines Among the Working Age Population in Metropolitan Areas of Thailand. Front. Pharmacol. 2021, 12, 726643. [Google Scholar] [CrossRef] [PubMed]
- Heil, S.H.; Jones, H.E.; Arria, A.; Kaltenbach, K.; Coyle, M.; Fischer, G.; Stine, S.; Selby, P.; Martin, P.R. Unintended Pregnancy in Opioid-Abusing Women. J. Subst. Abuse Treat. 2011, 40, 199–202. [Google Scholar] [CrossRef]
- Black, K.I.; Day, C.A. Improving Access to Long-Acting Contraceptive Methods and Reducing Unplanned Pregnancy Among Women with Substance Use Disorders. Subst. Abus. 2016, 10, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Drosdzol-Cop, A.; Staniczek, J.; Orszulak, D.; Kowalczyk, K.; Fuchs, A.; Sieroszewski, P.; Wielgos, M.; Kalinka, J.; Huras, H.; Wegrzyn, P.; et al. The Polish Society of Gynecologists and Obstetricians’ Expert Group Recommendations regarding adolescent pregnancy. Ginekol. Pol. 2023, 94, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, A. Misuse of prescription and over-the-counter drugs to obtain illicit highs: How pharmacists can prevent abuse. Pharm. J. 2020, 305, 7943. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S.; Miuli, A.; Mosca, A.; Santovito, M.C.; Corkery, J.M.; Guirguis, A.; Pettorruso, M.; Di Giannantonio, M.; Martinotti, G. Focus on Over-the-Counter Drugs’ Misuse: A Systematic Review on Antihistamines, Cough Medicines, and Decongestants. Front. Psychiatry 2021, 7, 657397. [Google Scholar] [CrossRef]
- Buckley, N.A.; Whyte, I.M.; Dawson, A.H.; Cruickshank, D.A. Pheniramine-a Much Abused Drug. Med. J. Aust. 1994, 160, 188–192. [Google Scholar] [CrossRef]
- Delphin-Rittmon, M.E. 2022 The National Survey on Drug Use and Health. 2020. Available online: https://www.samhsa.gov/data/sites/default/files/reports/slides-2020-nsduh/2020NSDUHNationalSlides072522.pdf (accessed on 27 December 2023).
- Pathan, H.; Williams, J. Basic Opioid Pharmacology: An Update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Merrer, J.L.; Becker, J.A.J.; Befort, K.; Kieffer, B.L. Reward Processing by the Opioid System in the Brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, Ł.; Goryński, K. Pharmacological Aspects of Over-the-Counter Opioid Drugs Misuse. Molecules 2020, 25, 3905. [Google Scholar] [CrossRef]
- Hout, M.C.V.; Horan, A.; Santlal, K.; Rich, E.; Bergin, M. ‘Codeine Is My Companion’: Misuse and Dependence on Codeine Containing Medicines in Ireland. Ir. J. Psychol. Med. 2018, 35, 275–288. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-92-4-155039-0.
- Lyngstad, G.; Skjelbred, P.; Swanson, D.M.; Skoglund, L.A. Analgesic Effect of Oral Paracetamol 1000 Mg/Ibuprofen 400 Mg, Paracetamol 1000 Mg/Codeine 60 Mg, Paracetamol 1000 Mg/Ibuprofen 400 Mg/Codeine 60 Mg, or Placebo on Acute Postoperative Pain: A Single-Dose, Randomized, and Double-Blind Study. Eur. J. Clin. Pharmacol. 2023, 79, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Akande-Sholabi, W.; Adisa, R.; Ilesanmi, O.S.; Bello, A.E. Extent of misuse and dependence of codeine-containing products among medical and pharmacy students in a Nigerian University. BMC Public. Health 2019, 19, 1709. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.C.; Aronson, J.K.; MacKenna, B.; Goldacre, B.; Hobbs, F.R.; Heneghan, C. Sales of Over-the-Counter Products Containing Codeine in 31 Countries, 2013–2019: A Retrospective Observational Study. Drug Saf. 2022, 45, 237–247. [Google Scholar] [CrossRef]
- Nielsen, S.; Van Hout, M.C. Over-the-Counter Codeine—From Therapeutic Use to Dependence, and the Grey Areas in Between. In Non-Medical and Illicit Use of Psychoactive Drugs; Nielsen, S., Bruno, R., Schenk, S., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2017; pp. 59–75. ISBN 978-3-319-60016-1. [Google Scholar]
- Hockenhull, J.; Wood, D.M.; Fonseca, F.; Guareschi, M.; Scherbaum, N.; Iwanicki, J.L.; Dart, R.C.; Dargan, P.I. The Association between the Availability of over the Counter Codeine and the Prevalence of Non-Medical Use. Eur. J. Clin. Pharmacol. 2022, 78, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- UNODC. Drug Use in Nigeria 2018, United Nations Office on Drugs and Crime, Vienna. 2018. Available online: https://www.unodc.org/documents/data-and-analysis/statistics/Drugs/Drug_Use_Survey_Nigeria_2019_BOOK.pdf (accessed on 20 October 2023).
- Shah, S.; Banh, E.T.; Koury, K.; Bhatia, G.; Nandi, R.; Gulur, P. Pain Management in Pregnancy: Multimodal Approaches. Pain. Res. Treat. 2015, 2015, 987483. [Google Scholar] [CrossRef]
- Brogly, S.B.; Bowie, A.C.; Li, W.; Camden, A.; Velez, M.P.; Guttmann, A.; Werler, M.M. Safety of Prenatal Opioid Analgesics: Do Results Differ between Public Health Insurance Beneficiary and Population-Based Cohorts? Birth Defects Res. 2023, 115, 555–562. [Google Scholar] [CrossRef]
- Werler, M.M.; Kerr, S.M.; Ailes, E.C.; Reefhuis, J.; Gilboa, S.M.; Browne, M.L.; Kelley, K.E.; Hernandez-Diaz, S.; Smith-Webb, R.S.; Garcia, M.H.; et al. Patterns of Prescription Medication Use during the First Trimester of Pregnancy in the United States, 1997–2018. Clin. Pharmacol. Ther. 2023, 114, 836–844. [Google Scholar] [CrossRef]
- Fishman, B.; Daniel, S.; Koren, G.; Lunenfeld, E.; Levy, A. Pregnancy outcome following opioid exposure: A cohort study. PLoS ONE 2019, 14, e0219061. [Google Scholar] [CrossRef]
- Bowie, A.C.; Werler, M.M.; Velez, M.P.; Li, W.; Camden, A.; Guttmann, A.; Brogly, S.B. Prescribed Opioid Analgesics in Early Pregnancy and the Risk of Congenital Anomalies: A Population-Based Cohort Study. CMAJ 2022, 194, E152–E162. [Google Scholar] [CrossRef]
- Nezvalová-Henriksen, K.; Spigset, O.; Nordeng, H. Effects of Codeine on Pregnancy Outcome: Results from a Large Population-Based Cohort Study. Eur. J. Clin. Pharmacol. 2011, 67, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.; Kane, M. Codeine Therapy and CYP2D6 Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Galligan, J.J.; Sternini, C. Insights into the Role of Opioid Receptors in the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.E. Loperamide: A Pharmacological Review. Rev. Gastroenterol. Disord. 2007, 7 (Suppl. 3), S11–S18. [Google Scholar]
- Rungsiprakarn, P.; Laopaiboon, M.; Sangkomkamhang, U.S.; Lumbiganon, P.; Pratt, J.J. Interventions for Treating Constipation in Pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD011448. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Soraisham, A.S.; Akierman, A. Neonatal Withdrawal Syndrome Due to Maternal Codeine Use. Paediatr. Child Health 2012, 17, e40-1. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Jang, F.F.; Teng, C.; Tai-Zhen, H. Apoptosis may involve in prenatally heroin exposed neurobehavioral teratogenicity? Med. Hypotheses 2009, 73, 976–977. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Seidler, F.J.; Yanai, J. Heroin Neuroteratogenicity: Targeting Adenylyl Cyclase as an Underlying Biochemical Mechanism. Dev. Brain Res. 2001, 132, 69–79. [Google Scholar] [CrossRef]
- Little, B.; Sud, N.; Nobile, Z.; Bhattacharya, D. Teratogenic Effects of Maternal Drug Abuse on Developing Brain and Underlying Neurotransmitter Mechanisms. Neurotoxicology 2021, 86, 172–179. [Google Scholar] [CrossRef]
- Monnelly, V.J.; Anblagan, D.; Quigley, A.; Cabez, M.B.; Cooper, E.S.; Mactier, H.; Semple, S.I.; Bastin, M.E.; Boardman, J.P. Prenatal Methadone Exposure Is Associated with Altered Neonatal Brain Development. Neuroimage Clin. 2018, 18, 9–14. [Google Scholar] [CrossRef]
- Vestal-Laborde, A.A.; Eschenroeder, A.C.; Bigbee, J.W.; Robinson, S.E.; Sato-Bigbee, C. The Opioid System and Brain Development: Methadone Effects on the Oligodendrocyte Lineage and the Early Stages of Myelination. Dev. Neurosci. 2014, 36, 409–421. [Google Scholar] [CrossRef]
- Sahi, N.; Nguyen, R.; Santos, C. Loperamide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wandel, C.; Kim, R.; Wood, M.; Wood, A. Interaction of Morphine, Fentanyl, Sufentanil, Alfentanil, and Loperamide with the Efflux Drug Transporter P-Glycoprotein. Anesthesiology 2002, 96, 913–920. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S. Is There Such a Thing as a “lope” Dope? Analysis of Loperamide-Related European Medicines Agency (EMA) Pharmacovigilance Database Reports. PLoS ONE 2018, 13, e0204443. [Google Scholar] [CrossRef] [PubMed]
- Doser, K.; Meyer, B.; Nitsche, V.; Binkert-Graber, P. Bioequivalence Evaluation of Two Different Oral Formulations of Loperamide (Diarex Lactab vs Imodium Capsules). Int. J. Clin. Pharmacol. Ther. 1995, 33, 431–436. [Google Scholar] [PubMed]
- Yu, J.H.; Kim, H.J.; Lee, S.; Hwang, S.-J.; Kim, W.; Moon, C.J. LC-MS Determination and Bioavailability Study of Loperamide Hydrochloride after Oral Administration of Loperamide Capsule in Human Volunteers. J. Pharm. Biomed. Anal. 2004, 36, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kapaganti, S.; Anwar Ansari, S.; Saba, R.; Elkhouly, A.; Hassib, M. A Rare Case of Loperamide-Induced Cardiac Arrest. Cureus 2020, 12, e9396. [Google Scholar] [CrossRef]
- Wu, P.E.; Juurlink, D.N. Clinical Review: Loperamide Toxicity. Ann. Emerg. Med. 2017, 70, 245–252. [Google Scholar] [CrossRef]
- Lasoff, D.R.; Koh, C.H.; Corbett, B.; Minns, A.B.; Cantrell, F.L. Loperamide Trends in Abuse and Misuse over 13 Years: 2002–2015. Pharmacotherapy 2017, 37, 249–253. [Google Scholar] [CrossRef]
- Eggleston, W.; Clark, K.H.; Marraffa, J.M. Loperamide Abuse Associated With Cardiac Dysrhythmia and Death. Ann. Emerg. Med. 2017, 69, 83–86. [Google Scholar] [CrossRef]
- Borron, S.W.; Watts, S.H.; Tull, J.; Baeza, S.; Diebold, S.; Barrow, A. Intentional Misuse and Abuse of Loperamide: A New Look at a Drug with “Low Abuse Potential”. J. Emerg. Med. 2017, 53, 73–84. [Google Scholar] [CrossRef]
- Lee, V.R.; Vera, A.; Alexander, A.; Ruck, B.; Nelson, L.S.; Wax, P.; Campleman, S.; Brent, J.; Calello, D.P. Loperamide Misuse to Avoid Opioid Withdrawal and to Achieve a Euphoric Effect: High Doses and High Risk. Clin. Toxicol. 2019, 57, 175–180. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Drug Safety Communication: FDA Warns about Serious Heart Problems with High Doses of the Antidiarrheal Medicine Loperamide (Imodium), Including from Abuse and Misuse; FDA: Silver Spring, MD, USA, 2021.
- Rasla, S.; Parikh, P.; Hoffmeister, P.; Amand, A.S.; Garas, M.K.; Meligy, A.E.; Minami, T.; Shah, N.R. Unexpected Serious Cardiac Arrhythmias in the Setting of Loperamide Abuse. Rhode Isl. Med. J. 2017, 100, 33–36. [Google Scholar]
- Kang, J.; Compton, D.R.; Vaz, R.J.; Rampe, D. Proarrhythmic Mechanisms of the Common Anti-Diarrheal Medication Loperamide: Revelations from the Opioid Abuse Epidemic. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Einarson, A.; Mastroiacovo, P.; Arnon, J.; Ornoy, A.; Addis, A.; Malm, H.; Koren, G. Prospective, Controlled, Multicentre Study of Loperamide in Pregnancy. Can. J. Gastroenterol. 2000, 14, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Källén, B.; Nilsson, E.; Otterblad Olausson, P. Maternal Use of Loperamide in Early Pregnancy and Delivery Outcome. Acta Paediatr. 2008, 97, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Nguyen, M.; Diaz, J.; Smith, T. Loperamide toxicity mimicking peripartum cardiomyopathy. Am. J. Emerg. Med. 2020, 38, 693.e5–693.e6. [Google Scholar] [CrossRef] [PubMed]
- Ceckova-Novotna, M.; Pavek, P.; Staud, F. P-Glycoprotein in the Placenta: Expression, Localization, Regulation and Function. Reprod. Toxicol. 2006, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Statler, A.K.; Maani, C.V.; Kohli, A. Ephedrine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kanfer, I.; Dowse, R.; Vuma, V. Pharmacokinetics of Oral Decongestants. Pharmacotherapy 1993, 13, 116S–128S. [Google Scholar] [CrossRef]
- Alevizos, B. Dependence and Chronic Psychosis with D-nor-Pseudoephedrine. Eur. Psychiatry 2003, 18, 423–425. [Google Scholar] [CrossRef]
- Leighton, K.M. Paranoid Psychosis after Abuse of Actifed. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 789–790. [Google Scholar] [CrossRef]
- Pugh, C.R.; Howie, S.M. Dependence on Pseudoephedrine. Br. J. Psychiatry 1986, 149, 798. [Google Scholar] [CrossRef]
- Dalton, R. Mixed Bipolar Disorder Precipitated by Pseudoephedrine Hydrochloride. South. Med. J. 1990, 83, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G. Acute Psychosis Following Intravenous Abuse of Pseudoephedrine: A Case Report. J. Psychopharmacol. 1996, 10, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.; Woods, D. Pseudoephedrine Causing Mania-like Symptoms. N. Z. Med. J. 2002, 115, 86. [Google Scholar] [PubMed]
- Mäki-Marttunen, V.; Andreassen, O.A.; Espeseth, T. The Role of Norepinephrine in the Pathophysiology of Schizophrenia. Neurosci. Biobehav. Rev. 2020, 118, 298–314. [Google Scholar] [CrossRef]
- European Medicines Agency: EMA/535476/2023—PRAC Recommends Measures to Minimise the Risk of Serious Side Effects with Medicines Containing Pseudoephedrine. 1 December 2023. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/pseudoephedrine-containing-medicinal-products (accessed on 1 October 2023).
- Werler, M.M. Teratogen Update: Pseudoephedrine. Birth Defects Res. Part. A Clin. Mol. Teratol. 2006, 76, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiev, M.D.; Hosseini, F.; Moran, J.; Cooper, C.E. Effects of Pseudoephedrine on Parameters Affecting Exercise Performance: A Meta-Analysis. Sports Med. Open. 2018, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Sikk, K.; Haldre, S.; Aquilonius, S.-M.; Taba, P. Manganese-Induced Parkinsonism Due to Ephedrone Abuse. Parkinsons Dis. 2011, 2011, 865319. [Google Scholar] [CrossRef]
- How Is Methamphetamine Manufactured? Available online: https://nida.nih.gov/publications/research-reports/methamphetamine/how-methamphetamine-manufactured (accessed on 1 October 2023).
- Munafò, A.; Frara, S.; Perico, N.; Di Mauro, R.; Cortinovis, M.; Burgaletto, C.; Cantarella, G.; Remuzzi, G.; Giustina, A.; Bernardini, R. In Search of an Ideal Drug for Safer Treatment of Obesity: The False Promise of Pseudoephedrine. Rev. Endocr. Metab. Disord. 2021, 22, 1013–1025. [Google Scholar] [CrossRef]
- Werler, M.M.; Mitchell, A.A.; Hernandez-Diaz, S.; Honein, M.A. Use of over-the-counter medications during pregnancy. Am. J. Obstet. Gynecol. 2005, 193 Pt 1, 771–777. [Google Scholar] [CrossRef]
- Werler, M.M.; Mitchell, A.A.; Shapiro, S. First Trimester Maternal Medication Use in Relation to Gastroschisis. Teratology 1992, 45, 361–367. [Google Scholar] [CrossRef]
- Werler, M.M.; Sheehan, J.E.; Mitchell, A.A. Maternal Medication Use and Risks of Gastroschisis and Small Intestinal Atresia. Am. J. Epidemiol. 2002, 155, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Torfs, C.P.; Katz, E.A.; Bateson, T.F.; Lam, P.K.; Curry, C.J.R. Maternal medications and environmental exposures as risk factors for gastroschisis. Teratology 1996, 54, 84–92. [Google Scholar] [CrossRef]
- Werler, M.M.; Sheehan, J.E.; Hayes, C.; Mitchell, A.A.; Mulliken, J.B. Vasoactive Exposures, Vascular Events, and Hemifacial Microsomia. Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Rentea, R.M.; Gupta, V. Gastroschisis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hoyme, H.E.; Higginbottom, M.C.; Jones, K.L. The Vascular Pathogenesis of Gastroschisis: Intrauterine Interruption of the Omphalomesenteric Artery. J. Pediatr. 1981, 98, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Dyhrfjeld-Johnsen, J.; Attali, P. Management of Peripheral Vertigo with Antihistamines: New Options on the Horizon. Br. J. Clin. Pharmacol. 2019, 85, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.G.; Berning, S.; Dudden, R.; Milgrom, H.; Tran, Z.V. Sedation and Performance Impairment of Diphenhydramine and Second-Generation Antihistamines: A Meta-Analysis. J. Allergy Clin. Immunol. 2003, 111, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.E.; Pinnick, R.V.; Terhes, J.M. Life-Threatening Diphenhydramine Overdose Treated With Charcoal Hemoperfusion and Hemodialysis. Ann. Emerg. Med. 1999, 33, 104–107. [Google Scholar] [CrossRef]
- Saran, J.S.; Barbano, R.L.; Schult, R.; Wiegand, T.J.; Selioutski, O. Chronic Diphenhydramine Abuse and Withdrawal. Neurol. Clin. Pract. 2017, 7, 439–441. [Google Scholar] [CrossRef]
- Shenai, N.; Shulman, J.; Gopalan, P.; Cheng, E.; Cerimele, J.M. Fetal Outcomes in Intentional Over-the-Counter Medication Overdoses in Pregnancy. Psychosomatics 2018, 59, 400–404. [Google Scholar] [CrossRef]
- Brost, B.C.; Scardo, J.A.; Newman, R.B. Diphenhydramine Overdose during Pregnancy: Lessons from the Past. Am. J. Obstet. Gynecol. 1996, 175, 1376–1377. [Google Scholar] [CrossRef]
- Miller, A.A. Diphenhydramine Toxicity in a Newborn: A Case Report. J. Perinatol. 2000, 20, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Stellpflug, S.J.; Bangh, S.A.; Cole, J.B. The Treatment of Maternal and Fetal Anticholinergic Toxicity with Physostigmine. Toxicol. Commun. 2018, 2, 35–38. [Google Scholar] [CrossRef]
- Nguyen, P.; Einarson, A. Managing Nausea and Vomiting of Pregnancy with Pharmacological and Nonpharmacological Treatments. Womens Health 2006, 2, 763–770. [Google Scholar] [CrossRef]
- Watt, L.O. Oxytocic Effects of Dimenhydrinate in Obstetrics. Can. Med. Assoc. J. 1961, 84, 533–534. [Google Scholar]
- Ősz, B.-E.; Jîtcă, G.; Sălcudean, A.; Rusz, C.M.; Vari, C.-E. Benzydamine—An Affordable Over-the-Counter Drug with Psychoactive Properties—From Chemical Structure to Possible Pharmacological Properties. Pharmaceuticals 2023, 16, 566. [Google Scholar] [CrossRef]
- Stefania, C.; Andrea, M.; Alessio, M.; Mauro, P.; Amira, G.; Martin, C.J.; Giovanni, M.; Massimo, D.G.; Fabrizio, S. The Benzydamine Experience: A Systematic Review of Benzydamine Abuse. Curr. Neuropharmacol. 2021, 19, 1728–1737. [Google Scholar] [CrossRef]
- Zaprutko, T.; Koligat, D.; Michalak, M.; Wieczorek, M.; Józiak, M.; Ratajczak, M.; Szydłowska, K.; Miazek, J.; Kus, K.; Nowakowska, E. Misuse of OTC Drugs in Poland. Health Policy 2016, 120, 875–881. [Google Scholar] [CrossRef]
- Opaleye, E.S.; Noto, A.R.; van der Meer Sanchez, Z.; de Moura, Y.G.; Galduróz, J.C.F.; Carlini, E.A. Recreational Use of Benzydamine as a Hallucinogen among Street Youth in Brazil. Braz. J. Psychiatry 2009, 31, 208–213. [Google Scholar] [CrossRef]
- Gürü, M.; Şafak, Y.; Cengiz, G.F.; Kuru, E.; Örsel, S. Chronic Psychosis Related to Benzydamine Hydrochloride Abuse. Neurocase 2019, 25, 156–158. [Google Scholar] [CrossRef]
- Can, B.; Oz, I.; Ozer, H.; Simsek, T. Hallucinations after Ingesting a High Dose of Benzydamine Hydrochloride. Clin. Psychopharmacol. Neurosci. 2016, 14, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Acar, Y.A.; Kalkan, M.; Çetin, R.; Çevik, E.; Çınar, O. Acute Psychotic Symptoms Due to Benzydamine Hydrochloride Abuse with Alcohol. Case Rep. Psychiatry 2014, 2014, 290365. [Google Scholar] [CrossRef] [PubMed]
- Balaban, O.D.; Atagun, M.I.; Yilmaz, H.; Yazar, M.S.; Alpkan, L.R. Benzydamine Abuse as a Hallucinogen: A Case Report. Bull. Clin. Psychopharmacol. 2013, 23, 276–279. [Google Scholar] [CrossRef]
- Howell, L.L.; Cunningham, K.A. Serotonin 5-HT2 Receptor Interactions with Dopamine Function: Implications for Therapeutics in Cocaine Use Disorder. Pharmacol. Rev. 2015, 67, 176–197. [Google Scholar] [CrossRef] [PubMed]
- Mankes, R.; Abraham, R.; LeFevre, R. Reproductive Inhibition Caused by the Anti-Inflammatory Analgesic Benzydamine in the Rat. Ecotoxicol. Environ. Saf. 1981, 5, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Krzeszowski, W.; Wilczyński, J.; Grzesiak, M.; Nowakowska, D. Prenatal Sonographic Diagnosis of Premature Constriction of the Fetal Ductus Arteriosus after Maternal Self-Medication with Benzydamine Hydrochloride: Report of 3 Cases and Review of the Literature. J. Ultrasound Med. 2015, 34, 531–535. [Google Scholar] [CrossRef]
- Bakas, A.M.; Healy, H.M.; Bell, K.A.; Brown, D.W.; Mullen, M.; Scheid, A. Prenatal Duct Closure Leading to Severe Pulmonary Hypertension in a Preterm Neonate—A Case Report. Cardiovasc. Diagn. Ther. 2020, 10, 1691–1695. [Google Scholar] [CrossRef]
- Lancaster, E.M.; Hiatt, J.R.; Zarrinpar, A. Acetaminophen Hepatotoxicity: An Updated Review. Arch. Toxicol. 2015, 89, 193–199. [Google Scholar] [CrossRef]
- Harnett, J.T.; Dines, A.M.; Wood, D.M.; Archer, J.R.H.; Dargan, P.I. Cold Water Extraction of Codeine/Paracetamol Combination Products: A Case Series and Literature Review. Clin. Toxicol. 2020, 58, 107–111. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dureja, G.P.; Kadhe, G.; Mane, A.; Phansalkar, A.A.; Sawant, S.; Kapatkar, V. Cross-Sectional Study for Prevalence of Non-Steroidal Anti-Inflammatory Drug-Induced Gastrointestinal, Cardiac and Renal Complications in India: Interim Report. Gastroenterol. Res. 2015, 8, 216–221. [Google Scholar] [CrossRef]
- Panchal, N.K.; Prince Sabina, E. Non-steroidal anti-inflammatory drugs (NSAIDs): A current insight into its molecular mechanism eliciting organ toxicities. Food Chem. Toxicol. 2023, 172, 113598. [Google Scholar] [CrossRef]
| Dose | Symptoms | References |
|---|---|---|
| 52.5 mg/day |
| [56] |
| 80 mg/day |
| |
| Approx. 600 mg/day (weekends) |
| [57] |
| 300–1800 mg/day |
| [58] |
| 480 mg (one dose) |
| [59] |
| 60 mg (IV) |
| [60] |
| Type of Study | Time of Exposure | Risk | References |
|---|---|---|---|
| Case-control (surveillance program) | First quarter | Gastroschisis | [70] |
| Case-control study | Early pregnancy | Gastroschisis Small intestinal atresia (alone or in combination with acetaminophen) | [71] |
| Case-control study | First quarter | Gastroschisis | [72] |
| Case-control study (multicenter) | First quarter | Hemifacial microsomia | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ősz, B.-E.; Ștefănescu, R.; Sălcudean, A.; Jîtcă, G.; Vari, C.-E. The Risks of “Getting High” on Over-the-Counter Drugs during Pregnancy. Sci. Pharm. 2024, 92, 7. https://doi.org/10.3390/scipharm92010007
Ősz B-E, Ștefănescu R, Sălcudean A, Jîtcă G, Vari C-E. The Risks of “Getting High” on Over-the-Counter Drugs during Pregnancy. Scientia Pharmaceutica. 2024; 92(1):7. https://doi.org/10.3390/scipharm92010007
Chicago/Turabian StyleŐsz, Bianca-Eugenia, Ruxandra Ștefănescu, Andreea Sălcudean, George Jîtcă, and Camil-Eugen Vari. 2024. "The Risks of “Getting High” on Over-the-Counter Drugs during Pregnancy" Scientia Pharmaceutica 92, no. 1: 7. https://doi.org/10.3390/scipharm92010007
APA StyleŐsz, B. -E., Ștefănescu, R., Sălcudean, A., Jîtcă, G., & Vari, C. -E. (2024). The Risks of “Getting High” on Over-the-Counter Drugs during Pregnancy. Scientia Pharmaceutica, 92(1), 7. https://doi.org/10.3390/scipharm92010007








