Nanosized Particles of Synthetic Silicon Dioxide Delay the Regeneration of Gastric Ulcers Created by N-Methyl-N′-Nitro-N-Nitrosoguanidine and Induce Hyper-Trophic Gastritis-like Symptoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silica Particles
2.2. Animals
2.3. MNNG and Silica Particle Injection into the Submucosa of the Stomach
2.4. Oral Gavage Administration of Silica Particles
2.5. Preparation and Observation of the HE- and Azan-Stained Specimens
2.6. Experimental Frequency and Statistical Analysis
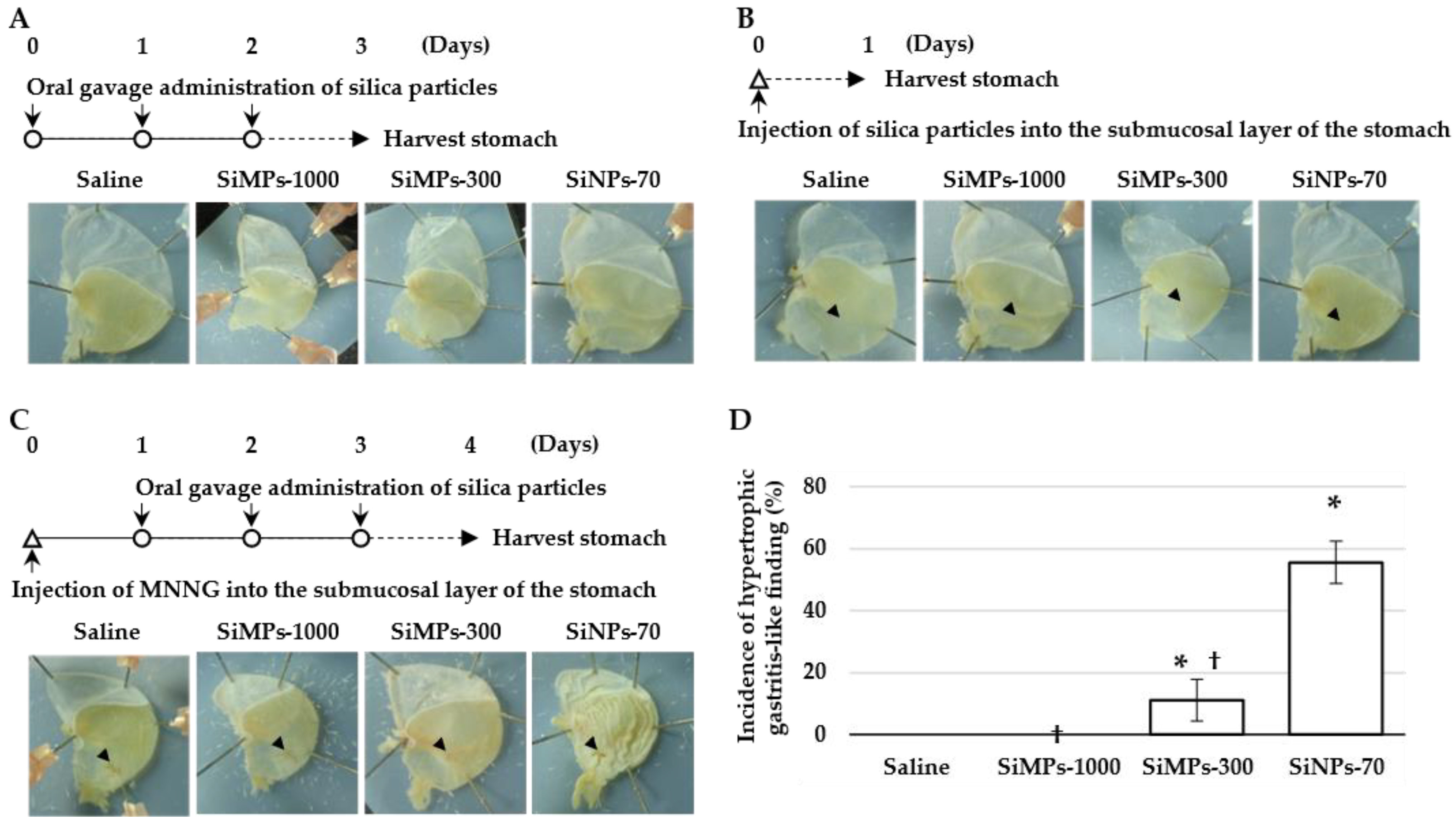
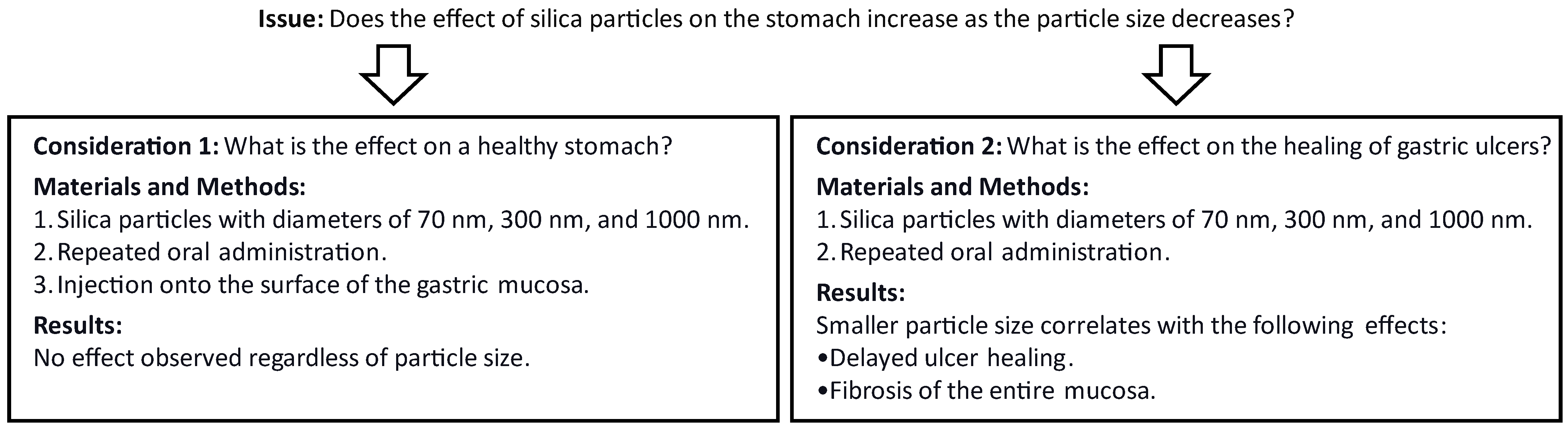
3. Results and Discussion
3.1. Hypertrophic Gastritis-like Lesions form Due to Exposure to Amorphous Nanosilica during the Healing Process of Gastric Ulcers
3.2. Amorphous Nanosilica Not Only Delays the Healing of Gastric ulcers but also Induces Fibrosis on the Surface of the Gastric Mucosa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fruijtier-Pölloth, C. The toxicological mode of action and the safety of synthetic amorphous silica—A nanostructured material. Toxicology 2012, 294, 61–79. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA J. 2018, 16, e05088. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. The safety of nanostructured synthetic amorphous silica (SAS) as a food additive (E 551). Arch. Toxicol. 2016, 90, 2885–2916. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, S.; Krystek, P.; Peters, R.J.B.; Lankveld, D.P.K.; Bokkers, B.G.H.; van Hoeven-Arentzen, P.H.; Bouwmeester, H.; Oomen, A.G. Presence and risks of nanosilica in food products. Nanotoxicology 2011, 5, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles an update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ma, R.; Li, X.; Lv, S.; Liu, X.; Abulikemu, A.; Zhao, X.; Li, Y.; Guo, C.; Sun, Z. Disturbed mitochondrial quality control involved in hepatocytotoxicity induced by silica nanoparticles. Nanoscale 2020, 12, 13034–13045. [Google Scholar] [CrossRef]
- De Matteis, V. Exposure to inorganic nanoparticles: Routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Jha, S. Amorphous nanosilica induced toxicity, inflammation and innate immune responses: A critical review. Toxicology 2020, 441, 152519. [Google Scholar] [CrossRef]
- Rosenstock, S.J.; Jørgensen, T. Prevalence and incidence of peptic ulcer disease in a Danish County a prospective cohort study. Gut 1995, 36, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shetty, S.; Mutalik, S.; Nandakumar, K.; Mathew, E.M.; Jha, A.; Mishra, B.; Rajpurohit, S.; Ravi, G.; Saha, M.; et al. Treatment of H. pylori infection and gastric ulcer: Need for novel Pharmaceutical formulation. Heliyon 2023, 9, e20406. [Google Scholar] [CrossRef] [PubMed]
- Dall, M.; Schaffalitzky de Muckadell, O.B.; Lassen, A.T.; Hallas, J. There is an association between selective serotonin reuptake inhibitor use and uncomplicated peptic ulcers: A populationbased case-control study. Aliment. Pharmacol. Ther. 2010, 32, 1383–1391. [Google Scholar] [CrossRef]
- Nguyen, T.N.M.; Sha, S.; Chen, L.-J.; Holleczek, B.; Brenner, H.; Schöttker, B. Strongly increased risk of gastric and duodenal ulcers among new users of low-dose aspirin: Results from two large cohorts with new-user design. Aliment. Pharmacol. Ther. 2022, 56, 251–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, L.; Yu, D.; Gao, C. Influence of silica particle internalization on adhesion and migration of human dermal fibroblasts. Biomaterials 2010, 31, 8465–8474. [Google Scholar] [CrossRef]
- Cornu, R.; Chrétien, C.; Pellequer, Y.; Martin, H.; Béduneau, A. Small silica nanoparticles transiently modulate the intestinal permeability by actin cytoskeleton disruption in both Caco-2 and Caco-2HT29-MTX models. Arch. Toxicol. 2020, 94, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Guth, P.H.; Mendick, R. The effect of chronic restraint stress on gastric ulceration in the rat. Gastroenterology 1964, 46, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Okabe, S.; Saziki, R. A new method for the production of chronic gastric ulcer in rats and the effect of several drugs on its healing. Jpn. J. Pharmacol. 1969, 19, 418–426. [Google Scholar] [CrossRef]
- Okabe, S.; Amagase, K. An overview of acetic acid ulcer models—The history and state of the art of peptic ulcer research. Biological and Pharmaceutical Bulletin. Biol. Pharm. Bull. 2005, 28, 1321–1341. [Google Scholar] [CrossRef]
- Onodera, A.; Yayama, K.; Tanaka, A.; Morosawa, H.; Furuta, T.; Takeda, N.; Kakiguchi, K.; Yonemura, S.; Yanagihara, I.; Tsutsumi, Y.; et al. Amorphous nanosilica particles evoke vascular relaxation through PI3K/Akt/eNOS signaling. Fundam. Clin. Pharmacol. 2016, 5, 419–428. [Google Scholar] [CrossRef]
- Fuentes, C.; Ruiz-Rico, M.; Fuentes, A.; Ruiz, M.J.; Barat, J.M. Degradation of silica particles functionalised with essential oil components under simulated physiological conditions. J. Hazard. Mater. 2020, 399, 123120. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Yoshihashi, K.; Suzuki, H.; Tsutsumi, S.; Mutoh, H.; Maeda, S.; Yamagata, Y.; Seto, Y.; Aburatani, H.; Hatakeyama, M. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc. Natl Acad. Sci. USA 2012, 109, 20584–20589. [Google Scholar] [CrossRef] [PubMed]
- Vomero, N.D.; Colpo, E. Nutritional care in peptic ulcer. Arq. Bras. Cir. Dig. 2014, 27, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S. Experimental studies on the presumption of the time after food intake from stomach contents. Forensic Sci. Int. 1987, 35, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Okumura, R.; Nagano, K.; Minemura, T.; Izumi, M.; Motooka, D.; Nakamura, S.; Iida, T.; Maeda, Y.; Kumanogoh, A.; et al. Oral intake of silica nanoparticles exacerbates intestinal inflammation. Biochem. Biophys. Res. Commun. 2021, 534, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Onodera, A.; Kawai, Y.; Kashimura, A.; Ogita, F.; Tsutsumi, Y.; Itoh, N. Suppression of alkylating agent induced cell transformation and gastric ulceration by low-dose alkylating agent pretreatment. Biochem. Biophys. Res. Commun. 2013, 435, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.; Konturek, J.W.; Pohle, T.; Schuppan, D.; Herbst, H.; Domschke, W. Remodeling of extracellular matrix in gastric ulceration. Microsc. Res. Tech. 2001, 53, 396–408. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Yoshida, N.; Takanashi, M.; Ito, Y.; Fukami, K.; Yanagihara, K.; Yashiro, M.; Sakai, R. Stromal fibroblasts mediate extracellular matrix remodeling and invasion of scirrhous gastric carcinoma cells. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Brenmoehl, J.; Leeb, S.; Schölmerich, J.; Rogler, G. Wound healing and fibrosis in intestinal disease. Gut 2007, 56, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Hermenean, A.; Oatis, D.; Herman, H.; Ciceu, A.; D’Amico, G.; Trotta, M.C. Galectin 1-A key player between tissue repair and fibrosis. Int. J. Mol. Sci. 2022, 23, 5548. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Wang, T.C. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007, 117, 60–69. [Google Scholar] [CrossRef]
- Genta, R.M. Gastric atrophy and atrophic gastritis—Nebulous concepts in search of a definition. Aliment. Pharmacol. Ther. 1998, 1 (Suppl. S1), 17–23. [Google Scholar] [CrossRef]
- Gómez-Ferrer, M.; Amaro-Prellezo, E.; Dorronsoro, A.; Sánchez-Sánchez, R.; Vicente, Á.; Cosín-Roger, J.; Barrachina, M.D.; Baquero, M.C.; Valencia, J.; Sepúlveda, P. HIF-overexpression and pro-inflammatory priming in human mesenchymal stromal cells improves the healing properties of extracellular vesicles in experimental Crohn’s disease. Int. J. Mol. Sci. 2021, 22, 11269. [Google Scholar] [CrossRef] [PubMed]
- Hani, U.; Osmani, R.A.M.; Yasmin, S.; Gowda, B.H.J.; Ather, H.; Ansari, M.Y.; Siddiqua, A.; Ghazwani, M.; Fatease, A.A.; Alamri, A.H.; et al. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics 2022, 14, 1576. [Google Scholar] [CrossRef]
- Laroui, H.; Sitaraman, S.V.; Merlin, D. Chapter six—Gastrointestinal Delivery of Anti-inflammatory Nanoparticles. Methods Enzymol. 2012, 509, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Wilson, D.S.; Dalmasso, G.; Salaita, K.; Murthy, N.; Sitaraman, S.V.; Merlin, D. Nanomedicine in GI. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G371–G383. [Google Scholar] [CrossRef]
- Kanaoujiya, R.; Porwal, D.; Srivastava, S. Applications of nanomaterials for gastrointestinal tumors: A review. Front. Med. Technol. 2022, 4, 997123. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, A.; Kawai, Y.; Onodera, A. Nanosized Particles of Synthetic Silicon Dioxide Delay the Regeneration of Gastric Ulcers Created by N-Methyl-N′-Nitro-N-Nitrosoguanidine and Induce Hyper-Trophic Gastritis-like Symptoms. Sci. Pharm. 2024, 92, 20. https://doi.org/10.3390/scipharm92020020
Iwasaki A, Kawai Y, Onodera A. Nanosized Particles of Synthetic Silicon Dioxide Delay the Regeneration of Gastric Ulcers Created by N-Methyl-N′-Nitro-N-Nitrosoguanidine and Induce Hyper-Trophic Gastritis-like Symptoms. Scientia Pharmaceutica. 2024; 92(2):20. https://doi.org/10.3390/scipharm92020020
Chicago/Turabian StyleIwasaki, Ayaka, Yuichi Kawai, and Akira Onodera. 2024. "Nanosized Particles of Synthetic Silicon Dioxide Delay the Regeneration of Gastric Ulcers Created by N-Methyl-N′-Nitro-N-Nitrosoguanidine and Induce Hyper-Trophic Gastritis-like Symptoms" Scientia Pharmaceutica 92, no. 2: 20. https://doi.org/10.3390/scipharm92020020
APA StyleIwasaki, A., Kawai, Y., & Onodera, A. (2024). Nanosized Particles of Synthetic Silicon Dioxide Delay the Regeneration of Gastric Ulcers Created by N-Methyl-N′-Nitro-N-Nitrosoguanidine and Induce Hyper-Trophic Gastritis-like Symptoms. Scientia Pharmaceutica, 92(2), 20. https://doi.org/10.3390/scipharm92020020






