Neonatal Hypoxic-Ischemic Brain Injury Alters Brain Acylcarnitine Levels in a Mouse Model
Abstract
:1. Introduction
1.1. Hypoxic-Ischemic Brain Injury
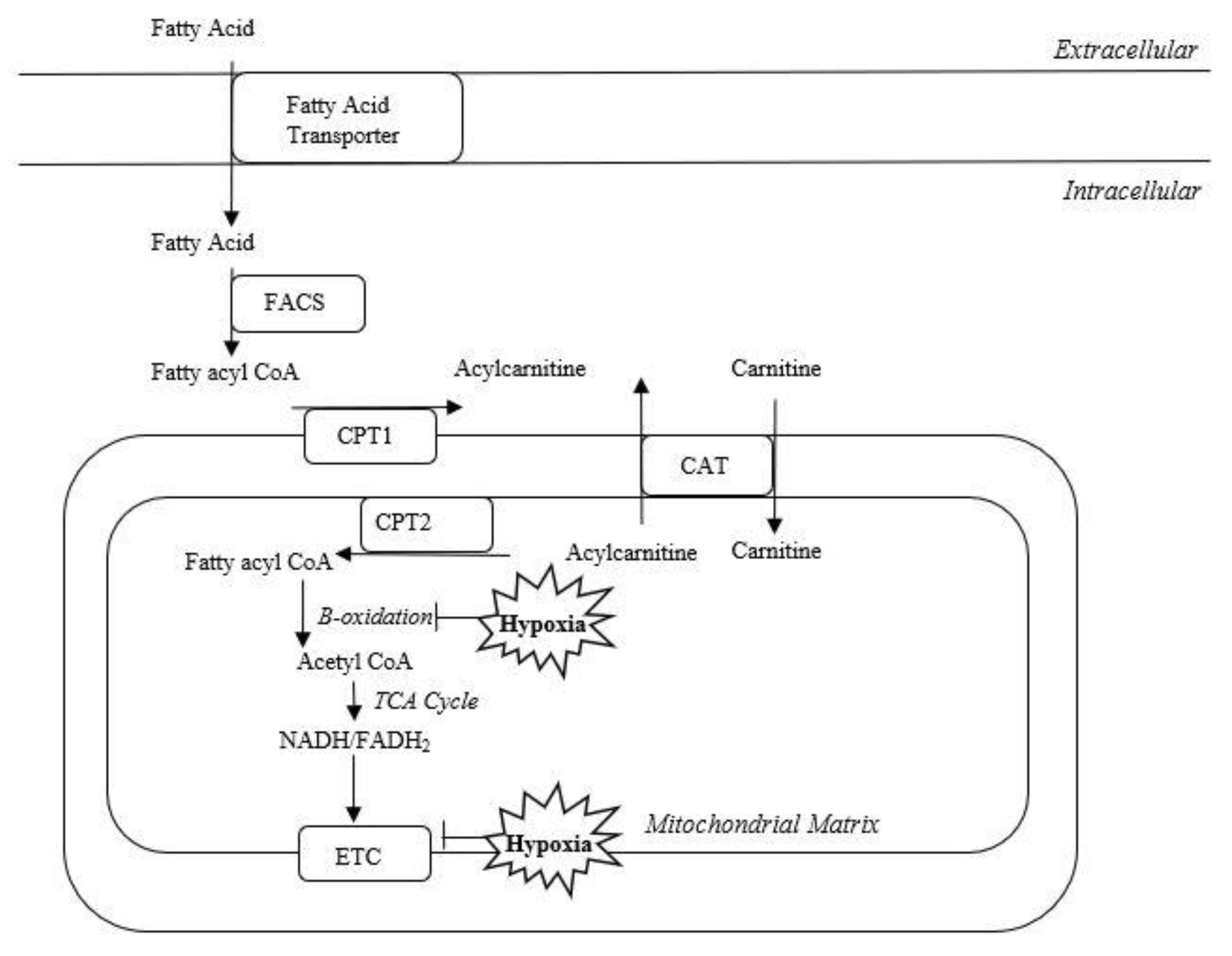
1.2. Acylcarnitines
1.3. Role of Acylcarnitines in Hypoxic-Ischemic Brain Injury
2. Results
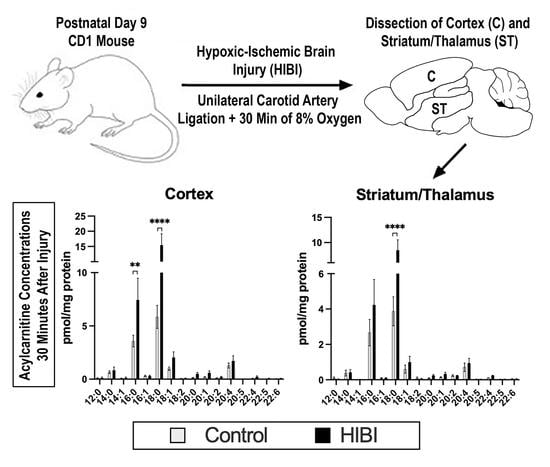
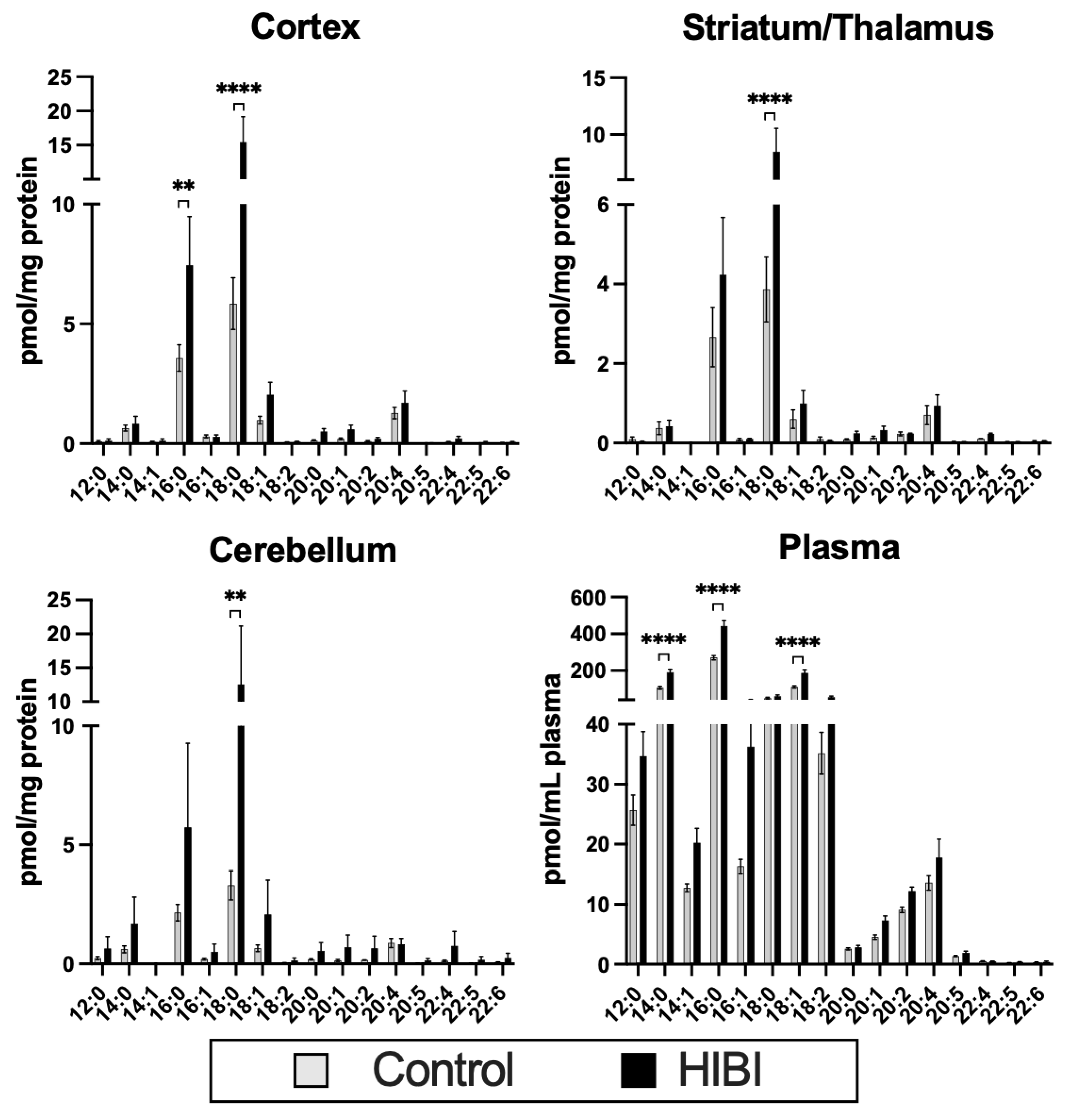
2.1. Individual Brain Acylcarnitine Changes: HIBI vs. Control
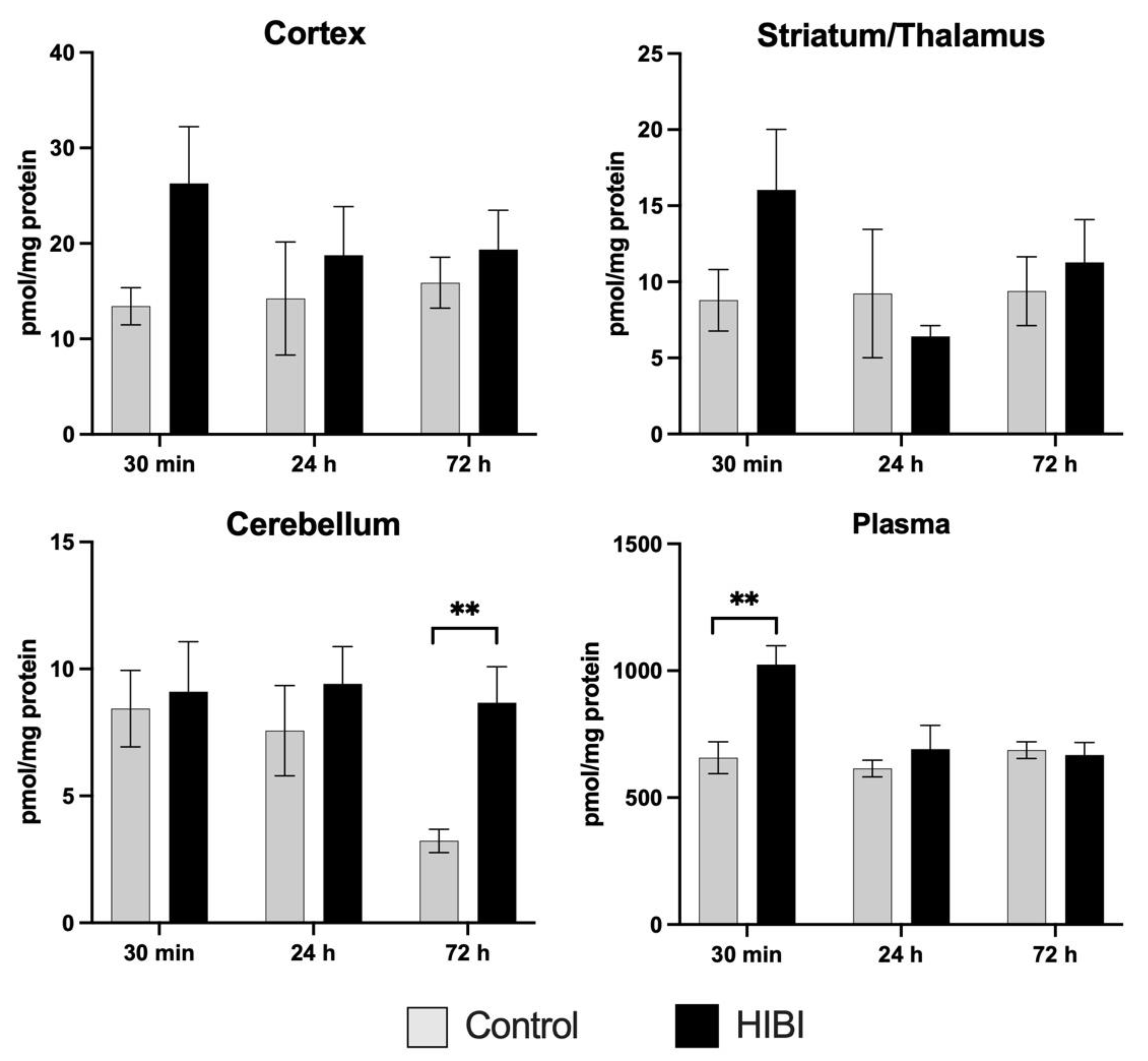
2.2. Total Brain Acylcarnitine Changes: HIBI vs. Control
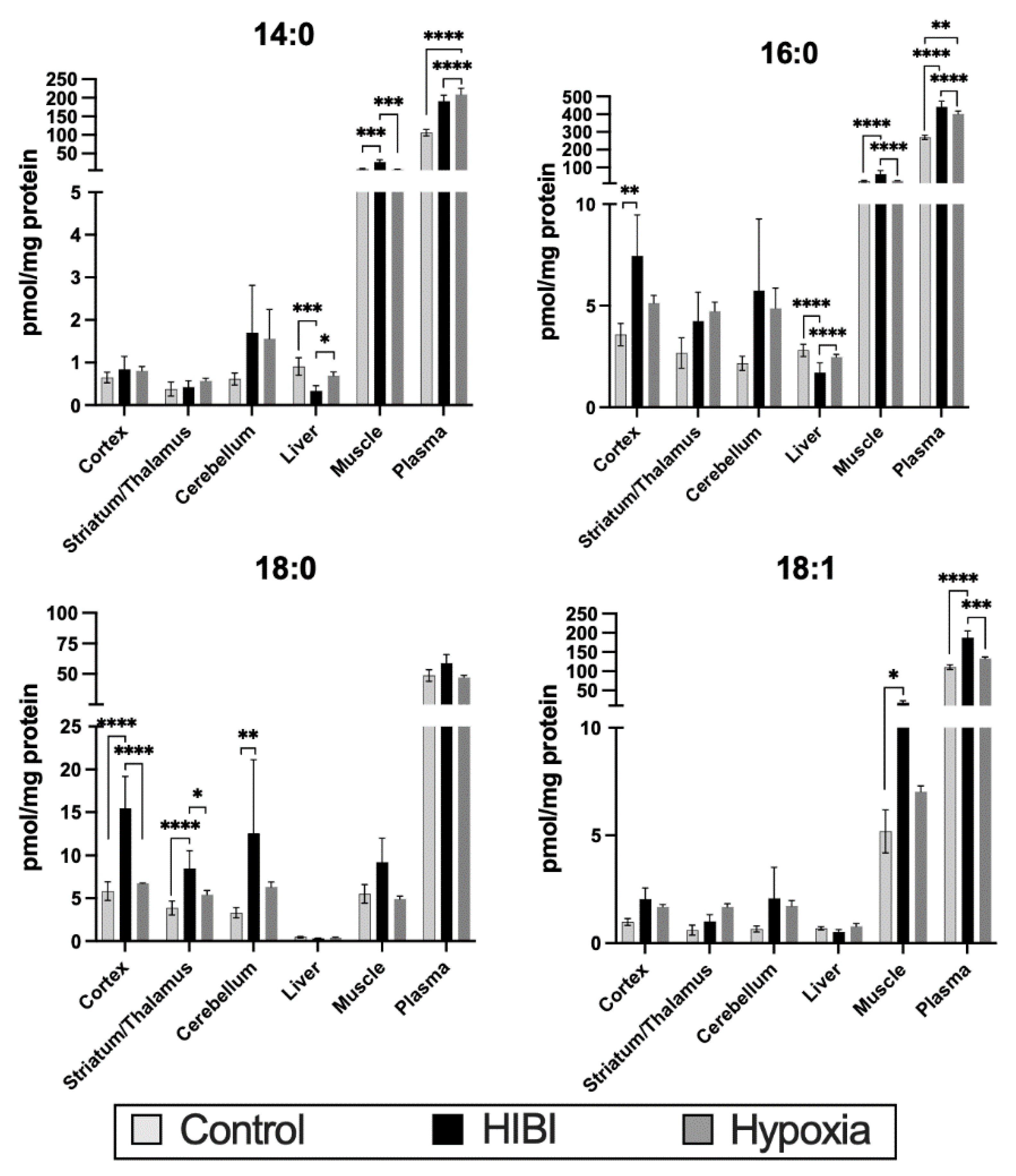
2.3. Systemic Acylcarnitine Changes: HIBI vs. Hypoxia vs. Control
3. Discussion
3.1. Acylcarnitine Function
3.2. Plasma Acylcarnitine Changes
3.3. Systemic Acylcarnitine Changes
4. Materials and Methods
4.1. IACUC Statement
4.2. Animal Model of Hypoxia-Ischemia
4.3. Tissue Preservation and Analysis
4.4. Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, G.; Pappas, A.; Shankaran, S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE). Semin. Perinatol. 2016, 40, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.Y.; Castillo, M. Hypoxic-ischemic brain injury: Imaging findings from birth to adulthood. Radiographics 2008, 28, 417–439; discussion 617. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef]
- Gnoni, A.; Longo, S.; Gnoni, G.V.; Giudetti, A.M. Carnitine in Human Muscle Bioenergetics: Can Carnitine Supplementation Improve Physical Exercise? Molecules 2020, 25, 182. [Google Scholar] [CrossRef] [Green Version]
- Wassink, G.; Gunn, E.R.; Drury, P.P.; Bennet, L.; Gunn, A.J. The mechanisms and treatment of asphyxial encephalopathy. Front. Neurosci. 2014, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M.S.; Mannix, M.K.; Brown, J.; Stumpf, D.A. L-carnitine reduces brain injury after hypoxia-ischemia in newborn rats. Pediatr. Res. 2003, 54, 688–695. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Xu, S.; Lu, X.; Gullapalli, R.P.; McKenna, M.C.; Waddell, J. Neuroprotective Effects of Acetyl-L-Carnitine on Neonatal Hypoxia Ischemia-Induced Brain Injury in Rats. Dev. Neurosci. 2016, 38, 384–396. [Google Scholar] [CrossRef] [Green Version]
- Walsh, B.H.; Broadhurst, D.I.; Mandal, R.; Wishart, D.S.; Boylan, G.B.; Kenny, L.C.; Murray, D.M. The metabolomic profile of umbilical cord blood in neonatal hypoxic ischaemic encephalopathy. PLoS One 2012, 7, e50520. [Google Scholar] [CrossRef] [Green Version]
- Meyburg, J.; Schulze, A.; Kohlmueller, D.; Linderkamp, O.; Mayatepek, E. Postnatal changes in neonatal acylcarnitine profile. Pedatric Res. 2001, 49, 125–129. [Google Scholar] [CrossRef] [Green Version]
- López-Suárez, O.; Concheiro-Guisán, A.; Sánchez-Pintos, P.; Cocho, J.A.; Fernández Lorenzo, J.R.; Couce, M.L. Acylcarnitine profile in neonatal hypoxic-ischemic encephalopathy: The value of butyrylcarnitine as a prognostic marker. Medicine 2019, 98, e15221. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Thornton, C. Mitochondrial dynamics in the neonatal brain—A potential target following injury? Biosci. Rep. 2022, 42, BSR20211696. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Valez, V.; Cimarra, C.; Blasina, F.; Radi, R. Hypoxic-Ischemic Encephalopathy and Mitochondrial Dysfunction: Facts, Unknowns, and Challenges. Antioxid. Redox Signal. 2020, 33, 247–262. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; De la Rosa, M.V.G.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Aitken-Buck, H.M.; Krause, J.; Zeller, T.; Jones, P.P.; Lamberts, R.R. Long-Chain Acylcarnitines and Cardiac Excitation-Contraction Coupling: Links to Arrhythmias. Front. Physiol. 2020, 11, 577856. [Google Scholar] [CrossRef]
- Roussel, J.; Thireau, J.; Brenner, C.; Saint, N.; Scheuermann, V.; Lacampagne, A.; Le Guennec, J.Y.; Fauconnier, J. Palmitoyl-carnitine increases RyR2 oxidation and sarcoplasmic reticulum Ca2+ leak in cardiomyocytes: Role of adenine nucleotide translocase. Biochim. Biophys. Acta 2015, 1852, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.J.; Cohen, D.W.; Gupte, S.; Johnson, J.D.; Wallick, E.T.; Wang, T.; Schwartz, A. In vitro effects of palmitylcarnitine on cardiac plasma membrane Na,K-ATPase, and sarcoplasmic reticulum Ca2+-ATPase and Ca2+ transport. J. Biol. Chem. 1979, 254, 12404–12410. [Google Scholar] [CrossRef]
- Mutomba, M.C.; Yuan, H.; Konyavko, M.; Adachi, S.; Yokoyama, C.B.; Esser, V.; McGarry, J.D.; Babior, B.M.; Gottlieb, R.A. Regulation of the activity of caspases by L-carnitine and palmitoylcarnitine. FEBS Lett. 2000, 478, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.A.; Kanter, E.M.; Newatia, A. Long-chain acylcarnitine induces Ca2+ efflux from the sarcoplasmic reticulum. J. Cardiovasc. Pharmacol. 2000, 36, 14–21. [Google Scholar] [CrossRef]
- El-Farghali, O.G.; El-Chimi, M.S.; El-Abd, H.S.; El-Desouky, E. Amino acid and acylcarnitine profiles in perinatal asphyxia: A case-control study. J. Matern. Fetal. Neonatal Med. 2018, 31, 1462–1469. [Google Scholar] [CrossRef]
- Wainwright, M.S.; Kohli, R.; Whitington, P.F.; Chace, D.H. Carnitine treatment inhibits increases in cerebral carnitine esters and glutamate detected by mass spectrometry after hypoxia-ischemia in newborn rats. Stroke 2006, 37, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Türkyilmaz, C.; Türkyilmaz, Z.; Onal, E.; Atalay, Y.; Söylemezoğlu, F.; Celasun, B. L-carnitine pre-treatment reduces apoptotic cell death in seven-day-old rats hypoxia ischemia. Restor. Neurol. Neurosci. 2010, 28, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, J.N.; Bowman, C.E.; Wolfgang, M.J.; Scafidi, S. Developmental regulation and localization of carnitine palmitoyltransferases (CPTs) in rat brain. J. Neurochem. 2017, 142, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.; Freed, A.; Bornstein, R.; Su, K.; Snell, J.; Pan, A.; Sun, G.; Park, K.; Jung, S.; Worstman, H.; et al. Mechanisms underlying neonate-specific metabolic effects of volatile anesthetics. Elife 2021, 13, e65400. [Google Scholar] [CrossRef]
- Xu, G.; Hansen, X.; Zhao, S.; Chen, M.; Hoene, X.; Wang, J.; Clemmesen, N.; Secher, H.; Haring, B.; Pedersen, R.; et al. Liver and Muscle Contribute Differently to the Plasma Acylcarnitine Pool During Fasting and Exercise in Humans. J. Clin. Endocrinol. Metab. 2016, 101, 5044–5052. [Google Scholar] [CrossRef]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- de Vries, L.S.; Jongmans, M.J. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F220–F224. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.; Genaro-Mattos, T.C.; Allen, L.B.; Koczok, K.; Korade, Z.; Mirnics, K. Interaction of maternal immune activation and genetic interneuronal inhibition. Brain Res. 2021, 1759, 147370. [Google Scholar] [CrossRef]
- Brittain, E.; Talati, M.; Fessel, J.; Zhu, H.; Penner, N.; Calcutt, M.; West, J.; Funke, M.; Lewis, G.; Gerszten, R.; et al. Fatty Acid Metabolic Defects and Right Ventricular Lipotoxicity in Human Pulmonary Arterial Hypertension. Circulation 2016, 133, 1936–1944. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dave, A.M.; Genaro-Mattos, T.C.; Korade, Z.; Peeples, E.S. Neonatal Hypoxic-Ischemic Brain Injury Alters Brain Acylcarnitine Levels in a Mouse Model. Metabolites 2022, 12, 467. https://doi.org/10.3390/metabo12050467
Dave AM, Genaro-Mattos TC, Korade Z, Peeples ES. Neonatal Hypoxic-Ischemic Brain Injury Alters Brain Acylcarnitine Levels in a Mouse Model. Metabolites. 2022; 12(5):467. https://doi.org/10.3390/metabo12050467
Chicago/Turabian StyleDave, Amanda M., Thiago C. Genaro-Mattos, Zeljka Korade, and Eric S. Peeples. 2022. "Neonatal Hypoxic-Ischemic Brain Injury Alters Brain Acylcarnitine Levels in a Mouse Model" Metabolites 12, no. 5: 467. https://doi.org/10.3390/metabo12050467
APA StyleDave, A. M., Genaro-Mattos, T. C., Korade, Z., & Peeples, E. S. (2022). Neonatal Hypoxic-Ischemic Brain Injury Alters Brain Acylcarnitine Levels in a Mouse Model. Metabolites, 12(5), 467. https://doi.org/10.3390/metabo12050467